The Impact of Rhizospheric and Endophytic Bacteria on the Germination of Carajasia cangae: A Threatened Rubiaceae of the Amazon Cangas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Isolation of Rhizospheric Bacteria
2.3. Isolation and Suspension of Endophytic Bacteria
2.4. DNA Extraction and Sequencing
2.5. Bioinformatics
2.6. Selection and Inoculation of Isolates and Germination Test
2.7. Data Analysis
3. Results
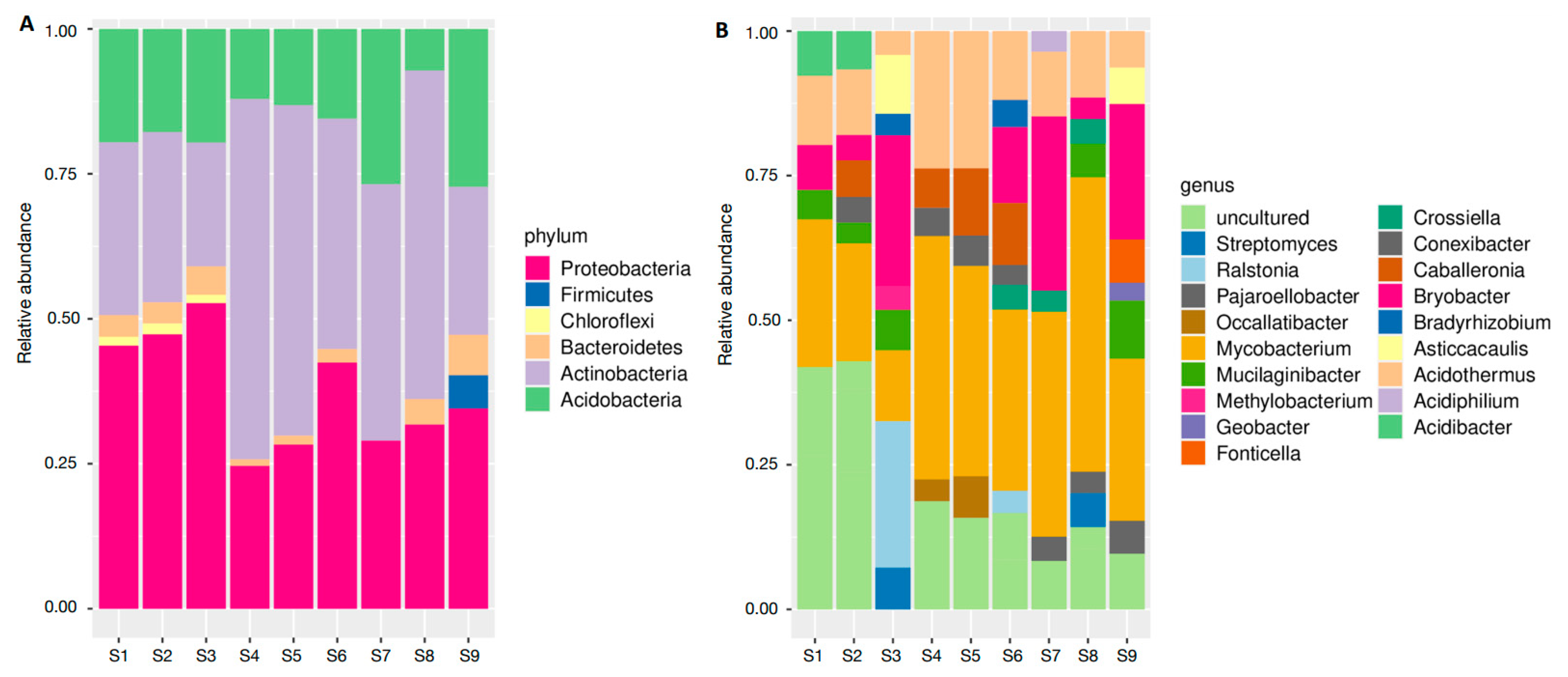
3.1. Soil Bacterial Communities
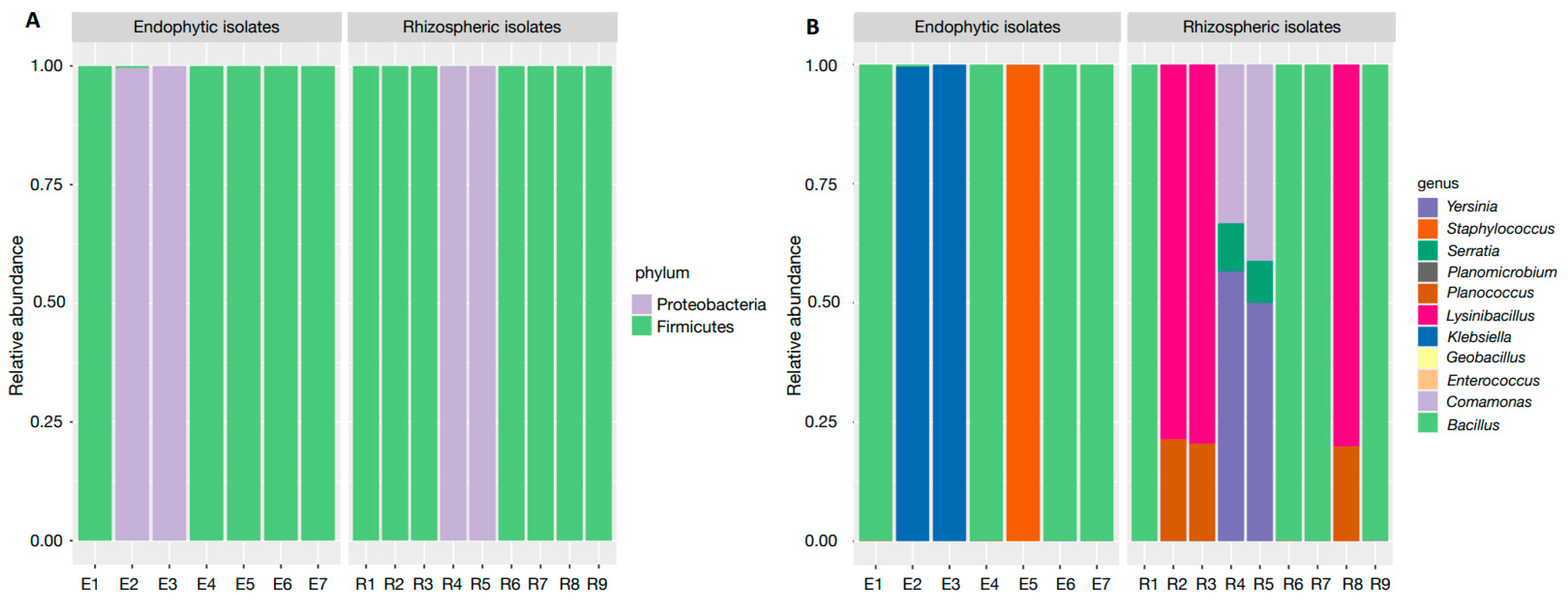
3.2. Rhizospheric and Endophytic Isolates Isolation and Molecular Identification
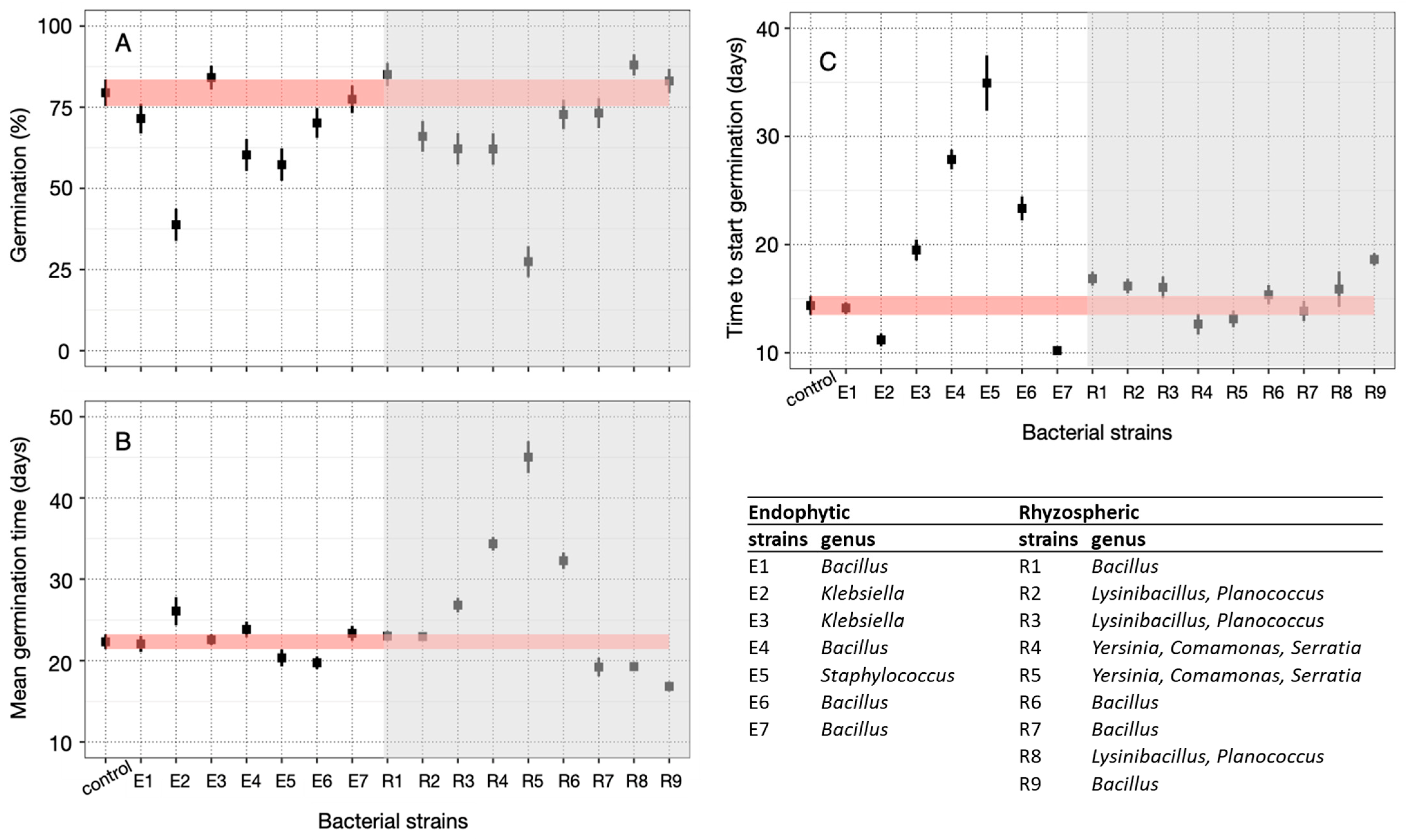
3.3. Seed Germination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devecchi, M.F.; Lovo, J.; Moro, M.F.; Andrino, C.O.; Barbosa-Silva, R.G.; Viana, P.L.; Giulietti, A.M.; Antar, G.; Watanabe, M.T.C.; Zappi, D.C. Beyond Forests in the Amazon: Biogeography and Floristic Relationships of the Amazonian Savannas. Bot. J. Linn. Soc. 2020, 193, 478–503. [Google Scholar] [CrossRef]
- Zappi, D.C.; Moro, M.F.; Walker, B.; Meagher, T.; Viana, P.L.; Mota, N.F.O.; Watanabe, M.T.C.; Nic Lughadha, E. Plotting a Future for Amazonian Canga Vegetation in a Campo Rupestre Context. PLoS ONE 2019, 14, e0219753. [Google Scholar] [CrossRef]
- Petchey, O.L. Species Diversity, Species Extinction, and Ecosystem Function. Am. Nat. 2000, 155, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Mota, N.F.D.O.; Watanabe, M.T.C.; Zappi, D.C.; Hiura, A.L.; Pallos, J.; Viveros, R.S.; Giulietti, A.M.; Viana, P.L. Amazon Canga: The Unique Vegetation of Carajás Revealed by the List of Seed Plants. Rodriguésia 2018, 69, 1435–1488. [Google Scholar] [CrossRef]
- Giulietti, A.M.; Giannini, T.C.; Mota, N.F.O.; Watanabe, M.T.C.; Viana, P.L.; Pastore, M.; Silva, U.C.S.; Siqueira, M.F.; Pirani, J.R.; Lima, H.C.; et al. Edaphic Endemism in the Amazon: Vascular Plants of the Canga of Carajás, Brazil. Bot. Rev. 2019, 85, 357–383. [Google Scholar] [CrossRef]
- Jacobi, C.M.; Do Carmo, F.F.; Vincent, R.C.; Stehmann, J.R. Plant Communities on Ironstone Outcrops: A Diverse and Endangered Brazilian Ecosystem. Biodivers. Conserv. 2007, 16, 2185–2200. [Google Scholar] [CrossRef]
- Viana, P.L.; Mota, N.F.D.O.; Gil, A.D.S.B.; Salino, A.; Zappi, D.C.; Harley, R.M.; Ilkiu-Borges, A.L.; Secco, R.D.S.; Almeida, T.E.; Watanabe, M.T.C.; et al. Flora Das Cangas Da Serra Dos Carajás, Pará, Brasil: História, Área de Estudos e Metodologia. Rodriguésia 2016, 67, 1107–1124. [Google Scholar] [CrossRef]
- Zappi, D.C.; Moro, M.F.; Meagher, T.R.; Nic Lughadha, E. Plant Biodiversity Drivers in Brazilian Campos Rupestres: Insights from Phylogenetic Structure. Front. Plant Sci. 2017, 8, 2141. [Google Scholar] [CrossRef]
- Skirycz, A.; Castilho, A.; Chaparro, C.; Carvalho, N.; Tzotzos, G.; Siqueira, J.O. Canga Biodiversity, a Matter of Mining. Front. Plant Sci. 2014, 5, 653. [Google Scholar] [CrossRef]
- Souza-Filho, P.W.M.; De Souza, E.B.; Silva Júnior, R.O.; Nascimento, W.R.; Versiani De Mendonça, B.R.; Guimarães, J.T.F.; Dall’Agnol, R.; Siqueira, J.O. Four Decades of Land-Cover, Land-Use and Hydroclimatology Changes in the Itacaiúnas River Watershed, Southeastern Amazon. J. Environ. Manag. 2016, 167, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Salas, R.M.; Viana, P.L.; Cabral, E.L.; Dessein, S.; Janssens, S. Carajasia (Rubiaceae), a New and Endangered Genus from CarajáS Mountain Range, Pará Brazil. Phytotaxa 2015, 206, 14–29. [Google Scholar] [CrossRef]
- Pinto, C.E.; Awade, M.; Watanabe, M.T.C.; Brito, R.M.; Costa, W.F.; Maia, U.M.; Imperatriz-Fonseca, V.L.; Giannini, T.C. Size and Isolation of Naturally Isolated Habitats Do Not Affect Plant-Bee Interactions: A Case Study of Ferruginous Outcrops within the Eastern Amazon Forest. PLoS ONE 2020, 15, e0238685. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, Z.G.; Laux, J.H.; Teixeira, J.B.G. Considerações Sobre a Origem Das Formações Ferríferas Da Formação Carajás, Serra Dos Carajás. RBG 2001, 31, 21–28. [Google Scholar] [CrossRef]
- Spier, C.A.; Levett, A.; Rosière, C.A. Geochemistry of Canga (Ferricrete) and Evolution of the Weathering Profile Developed on Itabirite and Iron Ore in the Quadrilátero Ferrífero, Minas Gerais, Brazil. Min. Depos. 2019, 54, 983–1010. [Google Scholar] [CrossRef]
- Amorim, E.; Bicalho, M. Carajasia cangae (Rubiaceae); Lista Vermelha da Flora Brasileira; Centro Nacional de Conservação da Flora/Instituto de Pesquisas Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2022.
- Caldeira, C.F.; Abranches, C.B.; Gastauer, M.; Ramos, S.J.; Guimarães, J.T.F.; Pereira, J.B.S.; Siqueira, J.O. Sporeling Regeneration and Ex Situ Growth of Isoëtes Cangae (Isoetaceae): Initial Steps towards the Conservation of a Rare Amazonian Quillwort. Aquat. Bot. 2019, 152, 51–58. [Google Scholar] [CrossRef]
- Dalapicolla, J.; Alves, R.; Jaffé, R.; Vasconcelos, S.; Pires, E.S.; Nunes, G.L.; Pereira, J.B.D.S.; Guimarães, J.T.F.; Dias, M.C.; Fernandes, T.N.; et al. Conservation Implications of Genetic Structure in the Narrowest Endemic Quillwort from the Eastern Amazon. Ecol. Evol. 2021, 11, 10119–10132. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, W.P.; Dalapicolla, J.; Carvalho, C.S.; Costa Veiga, J.; Vasconcelos, S.; Ramos, S.J.; Gastauer, M.; Jaffé, R.; Caldeira, C.F. Genetic Diversity and Structure of an Endangered Medicinal Plant Species (Pilocarpus microphyllus) in Eastern Amazon: Implications for Conservation. Conserv. Genet. 2022, 23, 745–758. [Google Scholar] [CrossRef]
- Caldeira, C.F.; Giannini, T.C.; Ramos, S.J.; Vasconcelos, S.; Mitre, S.K.; Pires, J.P.D.A.; Ferreira, G.C.; Ohashi, S.; Mota, J.A.; Castilho, A.; et al. Sustainability of Jaborandi in the Eastern Brazilian Amazon. Perspect. Ecol. Conserv. 2017, 15, 161–171. [Google Scholar] [CrossRef]
- Zappi, D.C.; Miguel, L.M.; Sobrado, S.V.; Salas, R.M. Flora Das Cangas Da Serra Dos Carajás, Pará, Brasil: Rubiaceae. Rodriguésia 2017, 68, 1091–1137. [Google Scholar] [CrossRef]
- Bairu, M.W.; Kulkarni, M.G.; Street, R.A.; Mulaudzi, R.B.; Van Staden, J. Studies on Seed Germination, Seedling Growth, and In Vitro Shoot Induction of Aloe Ferox Mill., a Commercially Important Species. horts 2009, 44, 751–756. [Google Scholar] [CrossRef]
- Zanetti, M.; Dayrell, R.L.C.; Wardil, M.V.; Damasceno, A.; Fernandes, T.; Castilho, A.; Santos, F.M.G.; Silveira, F.A.O. Seed Functional Traits Provide Support for Ecological Restoration and Ex Situ Conservation in the Threatened Amazon Ironstone Outcrop Flora. Front. Plant Sci. 2020, 11, 599496. [Google Scholar] [CrossRef] [PubMed]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y. Effect of Exogenous Nitric Oxide on Sulfur and Nitrate Assimilation Pathway Enzymes in Maize (Zea mays L.) under Drought Stress. Acta Physiol. Plant 2018, 40, 206. [Google Scholar] [CrossRef]
- Dutta, S.; Sarkar, A.; Dutta, S. Characterization of Pseudomonas Aeruginosa MCC 3198 and Its Potential for Growth Promotion of Seedlings of the Medicinal Plant Celosia cristata L. Int. J. Curr. Microbiol. App. Sci 2019, 8, 985–997. [Google Scholar] [CrossRef]
- Pavlova, A.S.; Leontieva, M.R.; Smirnova, T.A.; Kolomeitseva, G.L.; Netrusov, A.I.; Tsavkelova, E.A. Colonization Strategy of the Endophytic Plant Growth-Promoting Strains of Pseudomonas fluorescens and Klebsiella oxytoca on the Seeds, Seedlings and Roots of the Epiphytic Orchid, Dendrobium nobile Lindl. J. Appl. Microbiol. 2017, 123, 217–232. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Egorova, M.A.; Leontieva, M.R.; Malakho, S.G.; Kolomeitseva, G.L.; Netrusov, A.I. Dendrobium Nobile Lindl. Seed Germination in Co-Cultures with Diverse Associated Bacteria. Plant Growth Regul. 2016, 80, 79–91. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Cherdyntseva, T.A.; Klimova, S.Y.; Shestakov, A.I.; Botina, S.G.; Netrusov, A.I. Orchid-Associated Bacteria Produce Indole-3-Acetic Acid, Promote Seed Germination, and Increase Their Microbial Yield in Response to Exogenous Auxin. Arch. Microbiol. 2007, 188, 655–664. [Google Scholar] [CrossRef]
- Zhao, K.; Li, J.; Zhang, X.; Chen, Q.; Liu, M.; Ao, X.; Gu, Y.; Liao, D.; Xu, K.; Ma, M.; et al. Actinobacteria Associated with Glycyrrhiza Inflata Bat. Are Diverse and Have Plant Growth Promoting and Antimicrobial Activity. Sci. Rep. 2018, 8, 13661. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. metz 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Kado, C.; Heskett, M. Selective Media for Isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas and Xanthomonas. Phytopathology 1970, 60, 969–979. [Google Scholar] [CrossRef]
- Bircher, L.; Schwab, C.; Geirnaert, A.; Lacroix, C. Cryopreservation of Artificial Gut Microbiota Produced with In Vitro Fermentation Technology. Microb. Biotechnol. 2018, 11, 163–175. [Google Scholar] [CrossRef]
- Mendes, R.; Pizzirani-Kleiner, A.A.; Araujo, W.L.; Raaijmakers, J.M. Diversity of Cultivated Endophytic Bacteria from Sugarcane: Genetic and Biochemical Characterization of Burkholderia cepacia Complex Isolates. Appl. Environ. Microbiol. 2007, 73, 7259–7267. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.C.C.; Da Silva, G.B.; Silva-Lobo, V.L.; Côrtes, M.V.C.B.; Moraes, A.J.G.; Prabhu, A.S. Leaf Blast (Magnaporthe oryzae) Suppression and Growth Promotion by Rhizobacteria on Aerobic Rice in Brazil. Biol. Control 2011, 58, 160–166. [Google Scholar] [CrossRef]
- Costa, P.H.D.O.; Nascimento, S.V.D.; Herrera, H.; Gastauer, M.; Ramos, S.J.; Caldeira, C.F.; Oliveira, G.; Valadares, R.B.D.S. Non-Specific Interactions of Rhizospheric Microbial Communities Support the Establishment of Mimosa acutistipula Var. Ferrea in an Amazon Rehabilitating Mineland. Processes 2021, 9, 2079. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.R.M.; Silva, R.; Nunes, G.L.; Oliveira, G. PIMBA: A PIpeline for MetaBarcoding Analysis. In Advances in Bioinformatics and Computational Biology; Stadler, P.F., Walter, M.E.M.T., Hernandez-Rosales, M., Brigido, M.M., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 13063, pp. 106–116. ISBN 978-3-030-91813-2. [Google Scholar]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A Fast and Accurate Illumina Paired-End ReAd MergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus Subtilis: A Plant-Growth Promoting Rhizobacterium That Also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Tiwari, S.; Prasad, V.; Lata, C. Bacillus: Plant Growth Promoting Bacteria for Sustainable Agriculture and Environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–55. ISBN 978-0-444-64191-5. [Google Scholar]
- Walia, A.; Mehta, P.; Chauhan, A.; Shirkot, C.K. Effect of Bacillus Subtilis Strain CKT1 as Inoculum on Growth of Tomato Seedlings Under Net House Conditions. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 145–155. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of Plant Growth-Promoting Rhizobacteria (PGPR) in Stimulating Salinity Stress Defense in Plants: A Review. IJMS 2021, 22, 3154. [Google Scholar] [CrossRef] [PubMed]
- Tsukanova, K.A.; Chebotar, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of Plant Growth-Promoting Rhizobacteria on Plant Hormone Homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Yuan, F.; Chen, M. Exogenous Salicylic Acid Improves the Germination of Limonium bicolor Seeds under Salt Stress. Plant Signal. Behav. 2019, 14, e1644595. [Google Scholar] [CrossRef]
- Zulfiqar, F. Effect of Seed Priming on Horticultural Crops. Sci. Hortic. 2021, 286, 110197. [Google Scholar] [CrossRef]
- Bourak, K.; Oulkhir, F.E.; Maghnia, F.Z.; Massart, S.; Biskri, L.; Jijakli, M.H.; Allaoui, A. A Comprehensive Approach Combining Short-Chain Polyphosphate and Bacterial Biostimulants for Effective Nutrient Solubilization and Enhanced Wheat Growth. Microorganisms 2024, 12, 1423. [Google Scholar] [CrossRef]
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Ali, S.A.; Chaudhary, H.J.; Solieman, T.H.I.; Ibrahim, A.A. Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-Deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Kumar, S.; Saxena, A.K.; Suman, A. Molecular Diversity and Multifarious Plant Growth Promoting Attributes of Bacilli Associated with Wheat (Triticum aestivum L.) Rhizosphere from Six Diverse Agro-Ecological Zones of India: Diversity and Plant Growth Promoting Attributes of Bacilli. J. Basic. Microbiol. 2016, 56, 44–58. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Inoculation with Lysinibacillus Fusiformis Strain YJ4 and Lysinibacillus Sphaericus Strain YJ5 Alleviates the Effects of Cold Stress in Maize Plants. Gesunde Pflanz. 2023, 75, 77–95. [Google Scholar] [CrossRef]
- Hashmi, I.; Bindschedler, S.; Junier, P. Firmicutes. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 363–396. ISBN 978-0-12-823414-3. [Google Scholar]
- Thatoi, H.; Mishra, R.R.; Behera, B.C. Biotechnological Potentials of Halotolerant and Halophilic Bacteria from Mangrove Ecosystems. In Biotechnological Utilization of Mangrove Resources; Elsevier: Amsterdam, The Netherlands, 2020; pp. 413–433. ISBN 978-0-12-819532-1. [Google Scholar]
- Bouteau, H.E.-M.; Job, C.; Job, D.; Corbineau, F.; Bailly, C. ROS Signaling in Seed Dormancy Alleviation. Plant Signal. Behav. 2007, 2, 362–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Maarouf-Bouteau, H.; Sajjad, Y.; Bazin, J.; Langlade, N.; Cristescu, S.M.; Balzergue, S.; Baudouin, E.; Bailly, C. Reactive Oxygen Species, Abscisic Acid and Ethylene Interact to Regulate Sunflower Seed Germination: ROS, Ethylene and ABA in Seed Germination. Plant Cell Environ. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Gani, M.; Kaur, J.J.; Godara, L.C.; Singh, C.; Chauhan, S.S.; Francies, R.M.; Bhardwaj, A.; Bharat Kumar, N.; Ghosh, M.K. Reactive Oxygen Species (ROS): A Way to Stress Survival in Plants. In Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective; Zargar, S.M., Zargar, M.Y., Eds.; Springer Singapore: Singapore, 2018; pp. 127–153. ISBN 978-981-10-7478-3. [Google Scholar]
- Fouts, D.E.; Tyler, H.L.; DeBoy, R.T.; Daugherty, S.; Ren, Q.; Badger, J.H.; Durkin, A.S.; Huot, H.; Shrivastava, S.; Kothari, S.; et al. Complete Genome Sequence of the N2-Fixing Broad Host Range Endophyte Klebsiella Pneumoniae 342 and Virulence Predictions Verified in Mice. PLoS Genet. 2008, 4, e1000141. [Google Scholar] [CrossRef] [PubMed]
- Rajkumari, J.; Paikhomba Singha, L.; Pandey, P. Genomic Insights of Aromatic Hydrocarbon Degrading Klebsiella Pneumoniae AWD5 with Plant Growth Promoting Attributes: A Paradigm of Soil Isolate with Elements of Biodegradation. 3 Biotech. 2018, 8, 118. [Google Scholar] [CrossRef]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Zhou, W.; Dai, Y.; Qi, Y.; Du, J.; Yang, F.; Liu, J.; et al. Exogenous Auxin Represses Soybean Seed Germination through Decreasing the Gibberellin/Abscisic Acid (GA/ABA) Ratio. Sci. Rep. 2017, 7, 12620. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Puente, E.O.; García-Hernández, J.L.; Preciado-Rangel, P.; Murillo-Amador, B.; Tarazón-Herrera, M.A.; Flores-Hernández, A.; Holguin-Peña, J.; Aybar, A.N.; Barrón Hoyos, J.M.; Weimers, D.; et al. Germination of Salicornia bigelovii Ecotypes under Stressing Conditions of Temperature and Salinity and Ameliorative Effects of Plant Growth-promoting Bacteria. J. Agron. Crop Sci. 2007, 193, 167–176. [Google Scholar] [CrossRef]
- Fiodor, A.; Ajijah, N.; Dziewit, L.; Pranaw, K. Biopriming of Seed with Plant Growth-Promoting Bacteria for Improved Germination and Seedling Growth. Front. Microbiol. 2023, 14, 1142966. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Maitra, S.S.; Lakkaboyana, S.K. In Vivo Removal of Profenofos in Agricultural Soil and Plant Growth Promoting Activity on Vigna radiata by Efficient Bacterial Formulation. Int. J. Phytoremediation 2020, 22, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effects of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Bektaş, İ.; Küsek, M. The Isolation and Characterization of Phosphate Solubilizing Bacteria from the Onion Rhizosphere and Their Effect on Onion Growth. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg. 2021, 24, 1084–1092. [Google Scholar] [CrossRef]
- Bahadir, P.S.; Liaqat, F.; Eltem, R. Plant Growth Promoting Properties of Phosphate Solubilizing Bacillus Species Isolated from the Aegean Region of Turkey. Turk. J. Bot. 2018, 42, 183–196. [Google Scholar] [CrossRef]
- Mohamed, E.A.H.; Farag, A.G.; Youssef, S.A. Phosphate Solubilization by Bacillus subtilis and Serratia marcescens Isolated from Tomato Plant Rhizosphere. JEP 2018, 9, 266–277. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, M.; Hussain, A.; Jamil, M. Integrated Use of Phosphate-Solubilizing Bacillus subtilis Strain IA6 and Zinc-Solubilizing Bacillus Sp. Strain IA16: A Promising Approach for Improving Cotton Growth. Folia Microbiol. 2021, 66, 115–125. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boanares, D.; Cardoso, A.F.; Escobar, D.F.E.; Costa, K.J.A.; Bitencourt, J.A.; Costa, P.H.O.; Ramos, S.; Gastauer, M.; Caldeira, C.F. The Impact of Rhizospheric and Endophytic Bacteria on the Germination of Carajasia cangae: A Threatened Rubiaceae of the Amazon Cangas. Microorganisms 2024, 12, 1843. https://doi.org/10.3390/microorganisms12091843
Boanares D, Cardoso AF, Escobar DFE, Costa KJA, Bitencourt JA, Costa PHO, Ramos S, Gastauer M, Caldeira CF. The Impact of Rhizospheric and Endophytic Bacteria on the Germination of Carajasia cangae: A Threatened Rubiaceae of the Amazon Cangas. Microorganisms. 2024; 12(9):1843. https://doi.org/10.3390/microorganisms12091843
Chicago/Turabian StyleBoanares, Daniela, Aline Figueiredo Cardoso, Diego Fernando Escobar Escobar, Keila Jamille Alves Costa, José Augusto Bitencourt, Paulo Henrique O. Costa, Silvio Ramos, Markus Gastauer, and Cecilio Frois Caldeira. 2024. "The Impact of Rhizospheric and Endophytic Bacteria on the Germination of Carajasia cangae: A Threatened Rubiaceae of the Amazon Cangas" Microorganisms 12, no. 9: 1843. https://doi.org/10.3390/microorganisms12091843
APA StyleBoanares, D., Cardoso, A. F., Escobar, D. F. E., Costa, K. J. A., Bitencourt, J. A., Costa, P. H. O., Ramos, S., Gastauer, M., & Caldeira, C. F. (2024). The Impact of Rhizospheric and Endophytic Bacteria on the Germination of Carajasia cangae: A Threatened Rubiaceae of the Amazon Cangas. Microorganisms, 12(9), 1843. https://doi.org/10.3390/microorganisms12091843






