Bacillus subtilis and Rhizophagus intraradices Improve Vegetative Growth, Yield, and Fruit Quality of Fragaria × ananassa var. San Andreas
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area Characteristics
2.2. Experimental Design and Treatments
2.3. Microbial Inoculation
2.4. Fertilization Treatments
2.5. Biometric Parameters
2.6. Root Staining and Quantification of Mycorrhizal Colonization
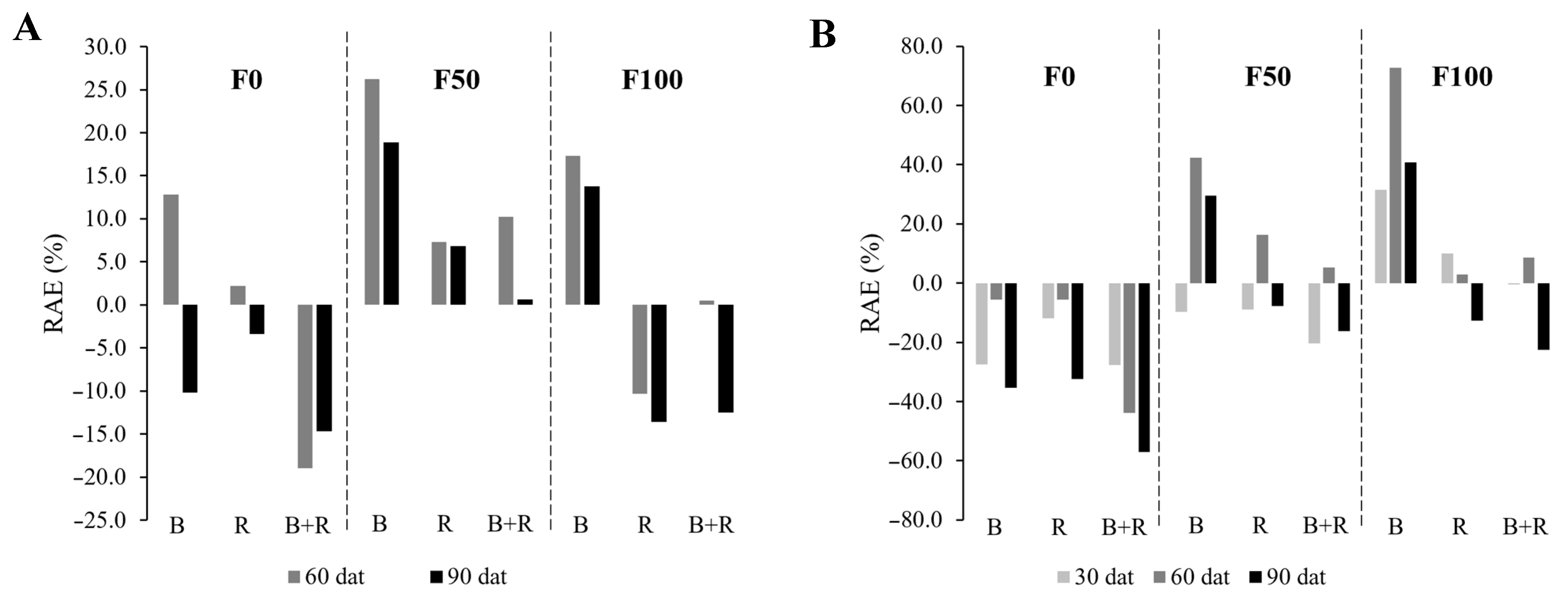
2.7. Relative Agronomic Efficiency Percentage (RAE%)
2.8. Yield Components
2.9. Fruit Quality Components
2.10. Statistical Analysis
3. Results
3.1. Mycorrhizal Colonization
3.2. Plant Height and Leaf Area
3.3. Fresh and Dry Aerial Weight
3.4. Plant Coverage
3.5. Fruit Quality
3.6. Yield
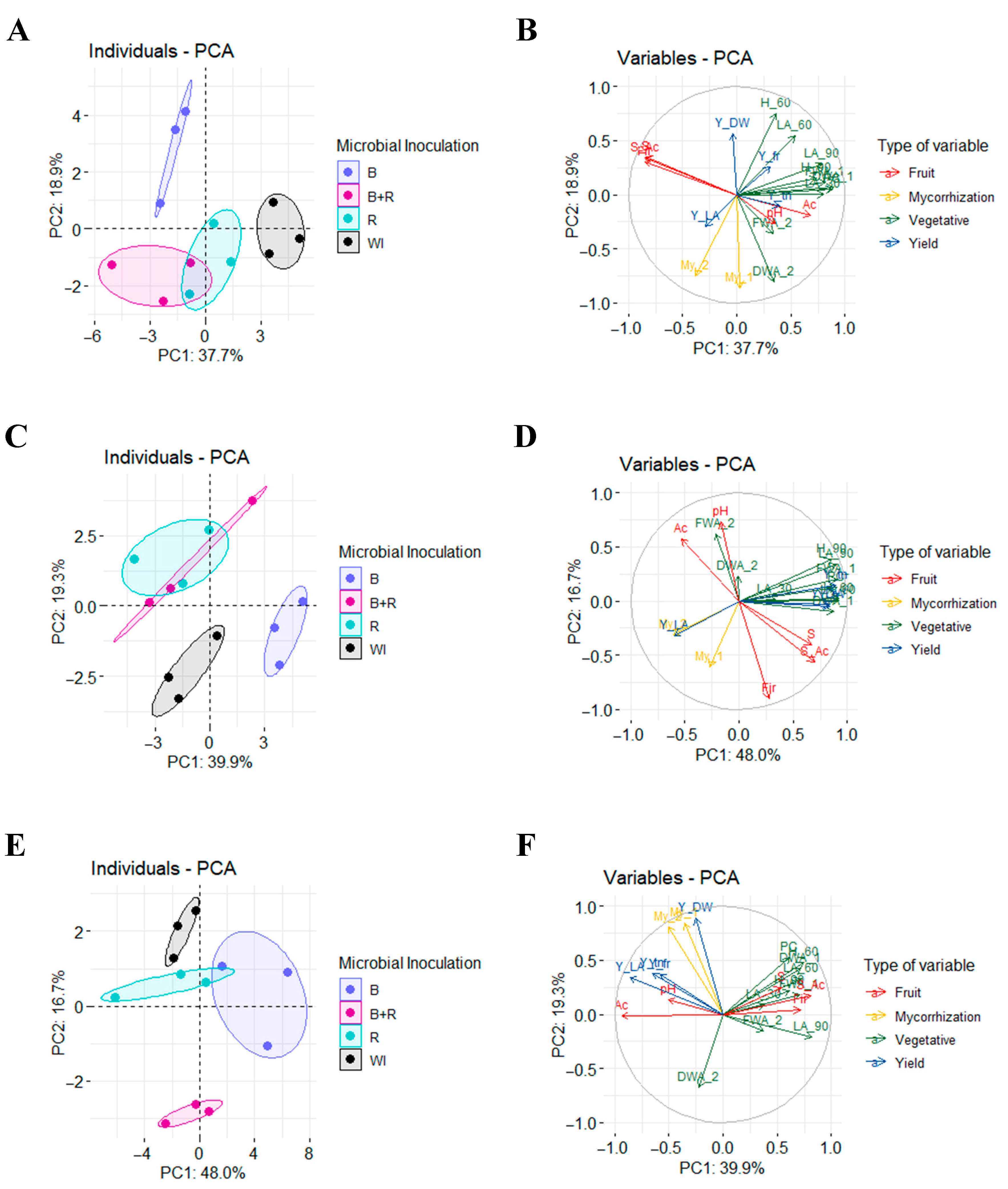
3.7. Principal Component Analysis
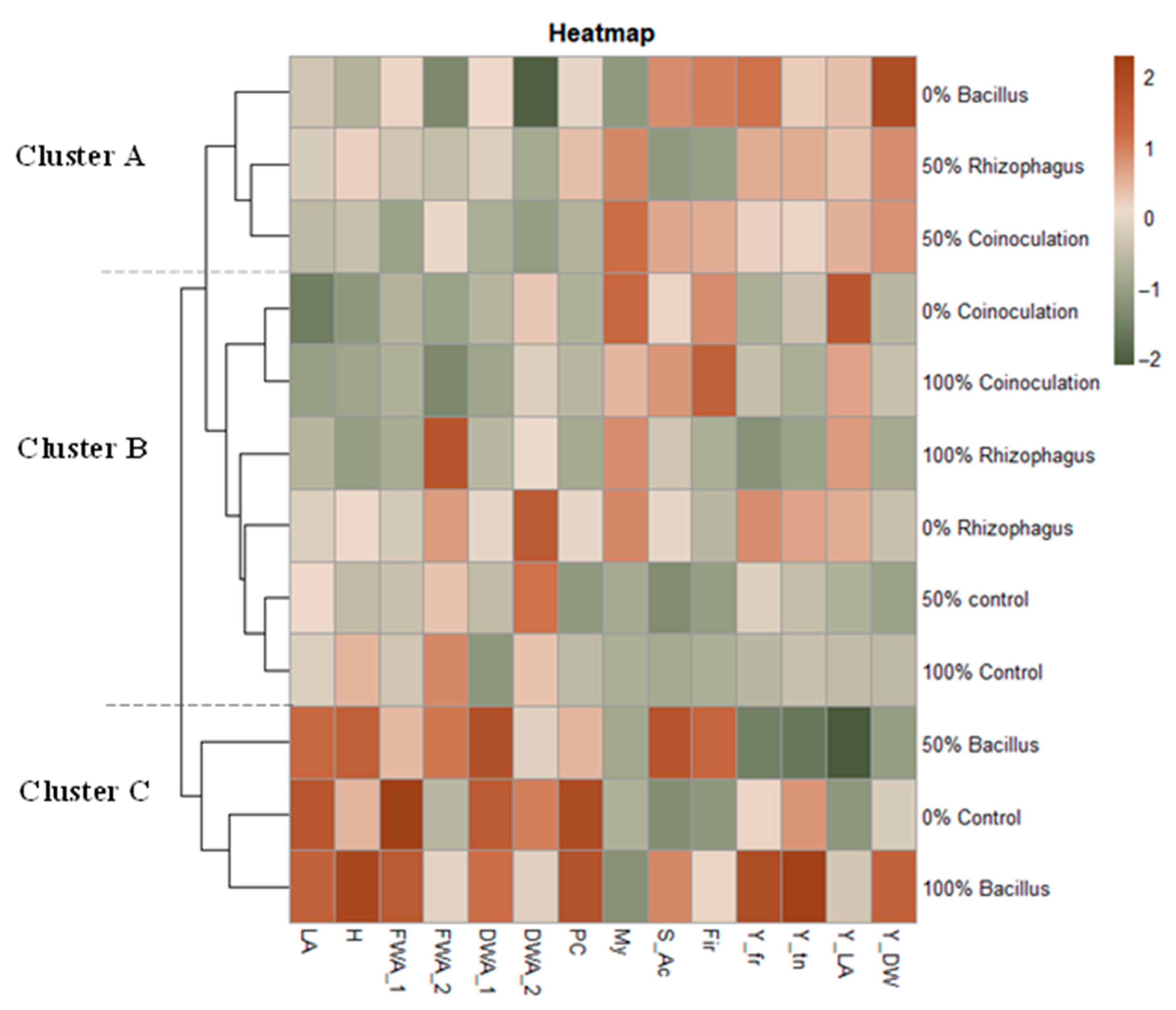
3.8. Heatmap Graph Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández-Martinez, N.R.; Blanchard, C.; Wells, D.; Salazar-Gutiérrez, M.R. Current state and future perspective of commercial strawberry production: A reviw. Sci. Hortic. 2023, 312, 111893. [Google Scholar] [CrossRef]
- FAO. Agricultural Data/Agricultural Production/Crops Primary [WWW Document]. FAOSTAT. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 14 August 2024).
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive Use of Nitrogenous Fertilizers: An Unawareness Causing Serious Threats to Environment and Human Health. Environ. Sci. Pollut. Res. 2017, 24, 26983–26987. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C. Environmental Risk Assessment of Soil Contamination; IntechOpen: Rijeka, Croatia, 2014. [Google Scholar]
- Singh, K.; Bhattacharya, S.; Sharma, P. Assessment of Heavy Metal Contents of Some Indian Medicinal Plants. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 1125–1129. [Google Scholar]
- Salem, M.A.; Bedade, D.K.; Al-Ethawi, L.; Al-waleed, S.M. Assessment of Physiochemical Properties and Concentration of Heavy Metals in Agricultural Soils Fertilized with Chemical Fertilizers. Heliyon 2020, 6, e05224. [Google Scholar] [CrossRef] [PubMed]
- Ai, P.; Jin, K.; Alengebawy, A.; Elsayed, M.; Meng, L.; Chen, M.; Ran, Y. Effect of application of different biogas fertilizer on eggplant production: Analysis of fertilizer value and risk assessment. Environ. Technol. Innov. 2020, 19, 101019. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Alamri, S.A. Fertilizers and Their Contaminants in Soils, Surface and Groundwater. Encycl. Anthr. 2018, 1–5, 225–240. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, X.; Wei, X.; Kai, Z.; Xu, Y. Excessive Application of Chemical Fertilizer and Organophosphorus Pesticides Induced Total Phosphorus Loss from Planting Causing Surface Water Eutrophication. Sci. Rep. 2021, 11, 23015. [Google Scholar] [CrossRef]
- Prashar, P.; Shah, S. Impact of Fertilizers and Pesticides on Soil Microflora in Agriculture. In Sustainable Agriculture Reviews. Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2016; pp. 331–361. [Google Scholar] [CrossRef]
- Savci, S. An Agricultural Pollutant: Chemical Fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 73–80. [Google Scholar] [CrossRef]
- O’Callaghan, M. Microbial Inoculation of Seed for Improved Crop Performance: Issues and Opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Nader, A.A.; Hauka, F.I.; Afify, A.H.; El-Sawah, A.M. Drought-Tolerant Bacteria and Arbuscular Mycorrhizal Fungi Mitigate the Detrimental Effects of Drought Stress Induced by Withholding Irrigation at Critical Growth Stages of Soybean (Glycine max, L.). Microorganisms 2024, 12, 1123. [Google Scholar] [CrossRef]
- Santoro, M.; Cappellari, L.; Giordano, W.; Banchio, E. Production of Volatile Organic Compounds in PGPR. In Handbook for Azospirillum; Cassán, F., Okon, Y., Creus, C., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Velasco, J.; Aguirre, G.; Ortuño, N. Humus líquido y microorganismos para favorecer la producción de lechuga (Lactuca sativa var. Crespa) en cultivo de hidroponía. J. Selva Andin. Biosph. 2016, 4, 71–83. [Google Scholar] [CrossRef]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next Generation of Microbial Inoculants for Agriculture and Bioremediation. Microb. Biotechnol. 2017, 10, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Creus, C.M. Inoculantes Microbianos: Piezas de Un Rompecabezas Que Aún Requiere Ser Ensamblado. Rev. Argent. Microbiol. 2017, 49, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Hernández Flores, L.; Munive Hernández, J.A.; Sandoval Castro, E.; Martínez Carrera, D.; Villegas Hernández, C. Efecto de Las Prácticas Agrícolas Sobre Las Poblaciones Bacterianas Del Suelo En Sistemas de Cultivo En Chihuahua, México. Rev. Mex. Cienc. Agrícolas 2013, 4, 353–365. [Google Scholar]
- NOM-021-RECNAT-2000; Norma Oficial Mexicana Que Establece Las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreo y Análisis. 2002. Available online: https://faolex.fao.org/docs/pdf/mex50674.pdf (accessed on 13 April 2024).
- USEPA. METHOD 9045D. SOIL AND WASTE pH 2004; USEPA: Washington, DC, USA, 2004. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/9045d.pdf (accessed on 13 April 2024).
- ISO 11265:1994; Soil Quality—Determination of the Specific Electrical Conductivity. ISO (International Organization for Standardization): Geneva, Switzerland, 1994. Available online: https://www.iso.org/standard/19243.html (accessed on 13 April 2024).
- Solórzano-Acosta, R.A.; Quispe, K.R. Assessing the role of field isolated Pseudomonas and Bacillus as growth-promoting rizobacteria on avocado (Persea americana) seedlings. J. Sustain. Agric. Environ. 2024, 3, e12114. [Google Scholar] [CrossRef]
- Ortiz, J.A.; Delgadillo, J.; Rodríguez, M.; Calderón, G. Bacterial Inoculation of Five Strawberry Varieties in Contrasting Soil PH: Effect on Growth and Fruit Quality. Terra Latinoam. 2016, 34, 177–185. Available online: https://www.scielo.org.mx/scielo.php?pid=S0187-57792016000200177&script=sci_abstract&tlng=en (accessed on 22 July 2024).
- Castañeda, W.; Toro, M.; Solorzano, A.; Zúñiga-Dávila, D.; Castañeda, W.; Toro, M.; Solorzano, A.; Zúñiga-Dávila, D. Production and Nutritional Quality of Tomatoes (Solanum lycopersicum Var. Cerasiforme) Are Improved in the Presence of Biochar and Inoculation with Arbuscular Mycorrhizae. Am. J. Plant Sci. 2020, 11, 426–436. [Google Scholar] [CrossRef]
- Olivera, J. Cultivo de fresa (Fragaria x ananassa Duch.), 1st ed.; Instituto Nacional de Innovación Agraria: La Molina, Peru, 2012; Volume 1. [Google Scholar]
- Serret-López, M.; Espinosa Victoria, D.; Gómez-Rodríguez, O.; Delgadillo Martínez, J. Tolerancia de Plantas de Fresa (“Fragaria × Ananassa” Duch.) Premicorrizadas Con Rhizophagus Intraradices e Inoculadas Con PGPR’s a Phytophthora Capsici. Agrociencia 2016, 50, 1107–1121. [Google Scholar]
- Koske, R.E.; Gemma, J.N. A Modified Procedure for Staining Roots to Detect VA Mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Léon, L.A.; Fenster, W.E.; Hammond, L.L. Agronomic Potential of Eleven Phosphate Rocks from Brazil, Colombia, Perú, and Venezuela. Soil Sci. Soc. Am. J. 1986, 50, 798–802. [Google Scholar] [CrossRef]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, D.L. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Sarkar, D.; Rakshit, A.; Al-Turki, A.I.; Sayyed, R.Z.; Datta, R. Connecting bio-priming approach with integrated nutrient management for improved nutrient use efficiency in crop species. Agriculture 2021, 11, 372. [Google Scholar] [CrossRef]
- Bueno, C.B.; dos Santos, R.M.; de Souza Buzo, F.; de Andrade da Silva, M.S.R.; Rigobelo, E.C. Effects of Chemical Fertilization and Microbial Inoculum on Bacillus Subtilis Colonization in Soybean and Maize Plants. Front. Microbiol. 2022, 13, 901157. [Google Scholar] [CrossRef] [PubMed]
- Agbodjato, N.A.; Adoko, M.Y.; Babalola, O.O.; Amogou, O.; Badé, F.T.; Noumavo, P.A.; Adjanohoun, A.; Baba-Moussa, L. Efficacy of Biostimulants Formulated With Pseudomonas Putida and Clay, Peat, Clay-Peat Binders on Maize Productivity in a Farming Environment in Southern Benin. Front. Sustain. Food Syst. 2021, 5, 666718. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Abd-Allah, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Hang, N.T.T.; Oh, S.O.; Kim, G.H.; Hur, J.S.; Koh, Y.J. Bacillus Subtilis S1-0210 as a Biocontrol Agent against Botrytis Cinerea in Strawberries. Plant Pathol. J. 2005, 21, 59–63. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Zhang, Y.; Li, R.; Liu, L.; Deng, J. Biocontrol Ability and Action Mechanism of Bacillus Halotolerans against Botrytis Cinerea Causing Grey Mould in Postharvest Strawberry Fruit. Postharvest Biol. Technol. 2021, 174, 111456. [Google Scholar] [CrossRef]
- Abd-El-Kareem, F.; Elshahawy, I.E.; Abd-Elgawad, M.M.M. Application of Bacillus pumilus Isolates for Management of Black Rot Disease in Strawberry. Egypt. J. Biol. Pest Control 2021, 31, 25. [Google Scholar] [CrossRef]
- Zhang, Y. Identification and Characterization of a Bacillus Subtilis Strain TS06 as Bio-Control Agent of Strawberry Replant Disease (Fusarium and Verticilium Wilts). Afr. J. Biotechnol. 2012, 11, 570–580. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Qi, D.; Zhou, D.; Qi, C.; Li, C.; Liu, S.; Xiang, D.; Zhang, L.; Xie, J.; et al. Biocontrol Ability and Mechanism of a Broad-Spectrum Antifungal Strain Bacillus safensis Sp. QN1NO-4 Against Strawberry Anthracnose Caused by Colletotrichum fragariae. Front. Microbiol. 2021, 12, 735732. [Google Scholar] [CrossRef] [PubMed]
- Chebotar, V.K.; Chizhevskaya, E.P.; Vorobyov, N.I.; Bobkova, V.V.; Pomyaksheva, L.V.; Khomyakov, Y.V.; Konovalov, S.N. The Quality and Productivity of Strawberry (Fragaria × Ananassa Duch.) Improved by the Inoculation of PGPR Bacillus Velezensis BS89 in Field Experiments. Agronomy 2022, 12, 2600. [Google Scholar] [CrossRef]
- de Lima, B.C.; Moro, A.L.; Santos, A.C.P.; Bonifacio, A.; Araujo, A.S.F.; de Araujo, F.F. Bacillus Subtilis Ameliorates Water Stress Tolerance in Maize and Common Bean. J. Plant Interact. 2019, 14, 432–439. [Google Scholar] [CrossRef]
- Braga, G.M.; Chagas, L.F.B.; Amaral, L.R.O.; Miller, L.O.; Chagas, A.F. Eficiência Da Inoculação Por Bacillus Subtilis Sobre Biomassa e Produtividade de Soja. Rev. Bras. Ciências Agrárias 2018, 13, 1–6. [Google Scholar] [CrossRef]
- de Oliveira, J.R.G.; e Silva, E.M.; Teixeira-Rios, T.; de Melo, N.F.; Yano-Melo, A.M. Response of an Endangered Tree Species from Caatinga to Mycorrhization and Phosphorus Fertilization. Acta Bot. Bras. 2015, 29, 94–102. [Google Scholar] [CrossRef][Green Version]
- Mitova, I.; Nenova, L.; Stancheva, I.; Geneva, M.; Hristozkova, M.; Mincheva, J. Lettuce Response to Nitrogen Fertilizers and Root Mycorrhization. Bulg. J. Agric. Sci. 2017, 23, 260–264. [Google Scholar]
- Püschel, D.; Kolaříková, Z.; Šmilauer, P.; Rydlová, J. Survival and Long-Term Infectivity of Arbuscular Mycorrhizal Fungi in Peat-Based Substrates Stored under Different Temperature Regimes. Appl. Soil Ecol. 2019, 140, 98–107. [Google Scholar] [CrossRef]
- Sun, J.; Jia, Q.; Li, Y.; Zhang, T.; Chen, J.; Ren, Y.; Dong, K.; Xu, S.; Shi, N.; Fu, S. Effects of Arbuscular Mycorrhizal Fungi and Biochar on Growth, Nutrient Absorption, and Physiological Properties of Maize (Zea mays L.). J. Fungi 2022, 8, 1275. [Google Scholar] [CrossRef]
- Barna, G.; Makó, A.; Takács, T.; Skic, K.; Füzy, A.; Horel, Á. Biochar alters soil physical characteristics, Arbuscular mycorrhizal fungi colonization, and glomalin production. Agronomy 2020, 10, 1933. [Google Scholar] [CrossRef]
- Mishra, R.; Kar, A.; Ebihara, K.; Ferrante, A.; Turrini, A. Effect of Storage on the Physicochemical and Flavour Attributes of Two Cultivars of Strawberry Cultivated in Northern India. Sci. World J. 2014, 2014, 794926. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ji, M.L.; Chen, M.; Sun, M.Y.; Fu, X.L.; Li, L.; Gao, D.S.; Zhu, C.Y. The Flavor and Nutritional Characteristic of Four Strawberry Varieties Cultured in Soilless System. Food Sci. Nutr. 2016, 4, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Dung, C.D.; Wallace, H.M.; Bai, S.H.; Ogbourne, S.M.; Trueman, S.J. Cross-Pollination Affects Fruit Colour, Acidity, Firmness and Shelf Life of Self-Compatible Strawberry. PLoS ONE 2021, 16, e0256964. [Google Scholar] [CrossRef] [PubMed]
- Abraham Juárez, M.d.R.; Espitia Vázquez, I.; Guzmán Mendoza, R.; Olalde Portugal, V.; Ruiz Aguilar, G.M.L.; García Hernández, J.L.; Herrera Isidrón, L.; Núñez Palenius, H.G. Development, Yield, and Quality of Melon Fruit (Cucumis melo L.) Inoculated with Mexican Native Strains of Bacillus subtilis (Ehrenberg). Agrociencia 2018, 52, 91–102. [Google Scholar]
- Qiu, F.; Liu, W.; Chen, L.; Wang, Y.; Ma, Y.; Lyu, Q.; Yi, S.; Xie, R.; Zheng, Y. Bacillus Subtilis Biofertilizer Application Reduces Chemical Fertilization and Improves Fruit Quality in Fertigated Tarocco Blood Orange Groves. Sci. Hortic. 2021, 281, 110004. [Google Scholar] [CrossRef]
- Moreno Reséndez, A.; García Mendoza, V.; Reyes Carrillo, J.L.; Vásquez Arroyo, J.; Cano Ríos, P. Rizobacterias promotoras del crecimiento vegetal: Una alternativa de biofertilización para la agricultura sustentable. Rev. Colomb. Biotecnol. 2018, 20, 68–83. [Google Scholar] [CrossRef]
- Bejarano-Herrera, W.F.; Marcillo-Paguay, C.A.; Rojas-Tapias, D.F.; Estrada-Bonilla, G.A. Effect of Mineral Fertilization and Microbial Inoculation on Cabbage Yield and Nutrition: A Field Experiment. Agronomy 2024, 14, 210. [Google Scholar] [CrossRef]




| Treat. | Factor 1. Fertilization | Factor 2. Microbial Inoculation |
|---|---|---|
| 1 | Without fertilization (F0) | Without inoculation (WI) |
| 2 | Bacillus subtilis (B) | |
| 3 | Rhizophagus intraradices (R) | |
| 4 | Co-inoculation (B + R) | |
| 5 | Half dose of fertilization (F50) | Without inoculation (WI) |
| 6 | Bacillus subtilis (B) | |
| 7 | Rhizophagus intraradices (R) | |
| 8 | Co-inoculation (B + R) | |
| 9 | Full dose of fertilization (F100) | Without inoculation (WI) |
| 10 | Bacillus subtilis (B) | |
| 11 | Rhizophagus intraradices (R) | |
| 12 | Co-inoculation (B + R) |
| Fertilizers | N° Fertilization | Total (g 4.8 m−2) | |||
|---|---|---|---|---|---|
| 1° | 2° | 3° | 4° | ||
| Full dose (F100) | |||||
| Urea (N) | 58.18 | 45.25 | 45.25 | 45.25 | 193.93 |
| Diammonium phosphate (P) | 62.61 | 13.91 | 13.91 | 13.91 | 104.35 |
| Potassium sulfate (K) | 72 | 56 | 56 | 56 | 240.00 |
| Half dose (F50) | |||||
| Urea (N) | 29.09 | 22.62 | 22.62 | 22.62 | 96.97 |
| Diammonium phosphate (P) | 31.31 | 6.95 | 6.95 | 6.95 | 52.18 |
| Potassium sulfate (K) | 36.00 | 28.00 | 28.00 | 28.00 | 120.00 |
| Fert. | Inoc. | Fresh Weight | RAE% * | Dry Weight | RAE% * | ||
|---|---|---|---|---|---|---|---|
| 90 dat | Harvest | 90 dat | Harvest | ||||
| F0 | WI | 61.0 ± 10.3 a | 91.1 ± 26.4 n.s. | 14.2 ± 2.0 a | 39.6 ± 8.2 n.s. | ||
| B | 40.4 ± 2.6 b | 78.1 ± 15.0 | −14.3 | 9.7 ± 0.3 b | 24.0 ± 6.3 | −39.3 | |
| R | 36.6 ± 3.5 b | 112.9 ± 13.9 | 23.9 | 9.3 ± 1.0 b | 42.7 ± 8.9 | 7.8 | |
| B + R | 32.5 ± 9.2 b | 84.5 ± 23.7 | −7.3 | 7.5 ± 2.1 b | 36.0 ± 10.1 | −8.9 | |
| F50 | WI | 34.9 ± 7.9 n.s. | 106.3 ± 21.0 n.s. | 7.9 ± 2.3n.s. | 40.2 ± 7.0 n.s. | ||
| B | 43.1 ± 11.8 | 118.7 ± 22.1 | 11.6 | 14.8 ± 6.4 | 33.8 ± 10.5 | −15.9 | |
| R | 36.0 ± 5.4 | 93.3 ± 30.4 | −12.3 | 8.9 ± 1.5 | 30.0 ± 7.5 | −25.5 | |
| B + R | 29.5 ± 12.5 | 101.3 ± 34.2 | −4.7 | 7.1 ± 0.7 | 28.8 ± 6.5 | −28.4 | |
| F100 | WI | 36.0 ± 11.1 n.s. | 115.6 ± 32.6 n.s. | 5.9 ± 1.7 b | 36.1 ± 8.0 n.s. | ||
| B | 54.5 ± 8.8 | 99.3 ± 47.5 | −14 | 12.9 ± 1.9 a | 33.8 ± 3.1 | −6.4 | |
| R | 31.2 ± 12.7 | 129.6 ± 43.7 | 12.1 | 7.7 ± 3.1 b | 34.7 ± 5.5 | −3.9 | |
| B + R | 32.2 ± 14.1 | 77.8 ± 10.2 | −32.6 | 6.6 ± 0.7 b | 33.6 ± 12.0 | −7 | |
| Fert. | Inoc. | pH | Acidity | Sugars | Ratio | Firmness |
|---|---|---|---|---|---|---|
| g. citric acid/100 mL | Brix° | Brix: Acidity | kg | |||
| F0 | WI | 3.32 ± 0.11 n.s. | 0.77 ± 0.00 a | 11.2 ± 0.3 c | 14.6 c | 0.100 ± 0.01 c |
| B | 3.28 ± 0.05 | 0.62 ± 0.04 b | 14.4 ± 0.5 a | 23.4 a | 0.169 ± 0.01 a | |
| R | 3.36 ± 0.07 | 0.64 ± 0.06 b | 12.6 ± 1.3 bc | 19.7 b | 0.119 ± 0.01 b | |
| B + R | 3.32 ± 0.11 | 0.69 ± 0.02 ab | 14.1 ± 0.8 ab | 20.4 ab | 0.166 ± 0.01 a | |
| F50 | WI | 3.36 ± 0.06 n.s. | 0.72 ± 0.02 a | 10.2 ± 0.4 c | 14.3 c | 0.104 ± 0.00 c |
| B | 3.09 ± 0.03 | 0.58 ± 0.00 b | 15.5 ± 0.4 a | 27.0 a | 0.181 ± 0.02 a | |
| R | 3.42 ± 0.01 | 0.78 ± 0.08 a | 11.5 ± 0.6 b | 15.3 c | 0.104 ± 0.01 c | |
| B + R | 3.31 ± 0.09 | 0.69 ± 0.10 ab | 15.1 ± 0.3 a | 22.2 b | 0.156 ± 0.01 b | |
| F100 | WI | 3.37 ± 0.12 n.s. | 0.70 ± 0.03 n.s. | 11.5 ± 0.2 c | 16.3 b | 0.113 ± 0.01 c |
| B | 3.29 ± 0.08 | 0.66 ± 0.04 | 15.5 ± 0.5 a | 23.5 a | 0.143 ± 0.02 b | |
| R | 3.38 ± 0.08 | 0.69 ± 0.07 | 12.8 ± 0.2 bc | 18.5 b | 0.114 ± 0.01 c | |
| B + R | 3.22 ± 0.01 | 0.62 ± 0.04 | 14.2 ± 1.6 ab | 23.0 a | 0.184 ± 0.00 a |
| Fert. | Inoc. | Yield | Yield:LA | Yield:DW | |
|---|---|---|---|---|---|
| Fruits per m2 | t·ha−1 | ||||
| F0 | WI | 21.8 ± 6.0 n.s. | 5.9 ± 0.9 n.s. | 6.1 ± 1.1 n.s. | 151 ± 28 n.s. |
| B | 25.3 ± 3.2 | 5.4 ± 2.2 | 9.3 ± 4.8 | 233 ± 117 | |
| R | 24.3 ± 9.3 | 5.8 ± 1.9 | 9.7 ± 5.4 | 143 ± 64 | |
| B + R | 18.6 ± 5.8 | 4.9 ± 1.5 | 11.8 ± 3.7 | 136 ± 28 | |
| F50 | WI | 20.7 ± 4.4 n.s. | 4.8 ± 1.0 n.s. | 7.1 ± 1.6 b | 120 ± 18 b |
| B | 15.9 ± 1.8 | 3.8 ± 0.6 | 4.2 ± 0.7 c | 116 ± 21 b | |
| R | 23.2 ± 5.2 | 5.7 ± 1.0 | 9.2 ± 3.0 ab | 192 ± 12 a | |
| B + R | 21.9 ± 4.1 | 5.4 ± 1.0 | 9.5 ± 1.8 a | 191 ± 42 a | |
| F100 | WI | 19.2 ± 1.3 b | 4.9 ± 0.6 b | 7.5 ± 0.3 n.s. | 137 ± 17 b |
| B | 28.2 ± 4.5 a | 7.2 ± 1.4 a | 7.9 ± 1.0 | 215 ± 60 a | |
| R | 16.8 ± 2.7 b | 4.4 ± 0.8 b | 9.9 ± 5.9 | 125 ± 10 b | |
| B + R | 19.7 ± 4.9 b | 4.5 ± 0.6 b | 9.8 ± 3.4 | 142 ± 32 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huasasquiche, L.; Alejandro, L.; Ccori, T.; Cántaro-Segura, H.; Samaniego, T.; Quispe, K.; Solórzano, R. Bacillus subtilis and Rhizophagus intraradices Improve Vegetative Growth, Yield, and Fruit Quality of Fragaria × ananassa var. San Andreas. Microorganisms 2024, 12, 1816. https://doi.org/10.3390/microorganisms12091816
Huasasquiche L, Alejandro L, Ccori T, Cántaro-Segura H, Samaniego T, Quispe K, Solórzano R. Bacillus subtilis and Rhizophagus intraradices Improve Vegetative Growth, Yield, and Fruit Quality of Fragaria × ananassa var. San Andreas. Microorganisms. 2024; 12(9):1816. https://doi.org/10.3390/microorganisms12091816
Chicago/Turabian StyleHuasasquiche, Lucero, Leonela Alejandro, Thania Ccori, Héctor Cántaro-Segura, Tomás Samaniego, Kenyi Quispe, and Richard Solórzano. 2024. "Bacillus subtilis and Rhizophagus intraradices Improve Vegetative Growth, Yield, and Fruit Quality of Fragaria × ananassa var. San Andreas" Microorganisms 12, no. 9: 1816. https://doi.org/10.3390/microorganisms12091816
APA StyleHuasasquiche, L., Alejandro, L., Ccori, T., Cántaro-Segura, H., Samaniego, T., Quispe, K., & Solórzano, R. (2024). Bacillus subtilis and Rhizophagus intraradices Improve Vegetative Growth, Yield, and Fruit Quality of Fragaria × ananassa var. San Andreas. Microorganisms, 12(9), 1816. https://doi.org/10.3390/microorganisms12091816






