Effect of Combining Wuyiencin and Pyrimethanil on Controlling Grape Gray Mold and Delaying Resistance Development in Botrytis cinerea
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of the Effect of Wuyiencin on Botrytis cinerea Pathogenicity
2.2.1. Colony Mycelial Growth and Conidial Germination
2.2.2. Infection Pad Formation
2.2.3. Oxalic Acid Production
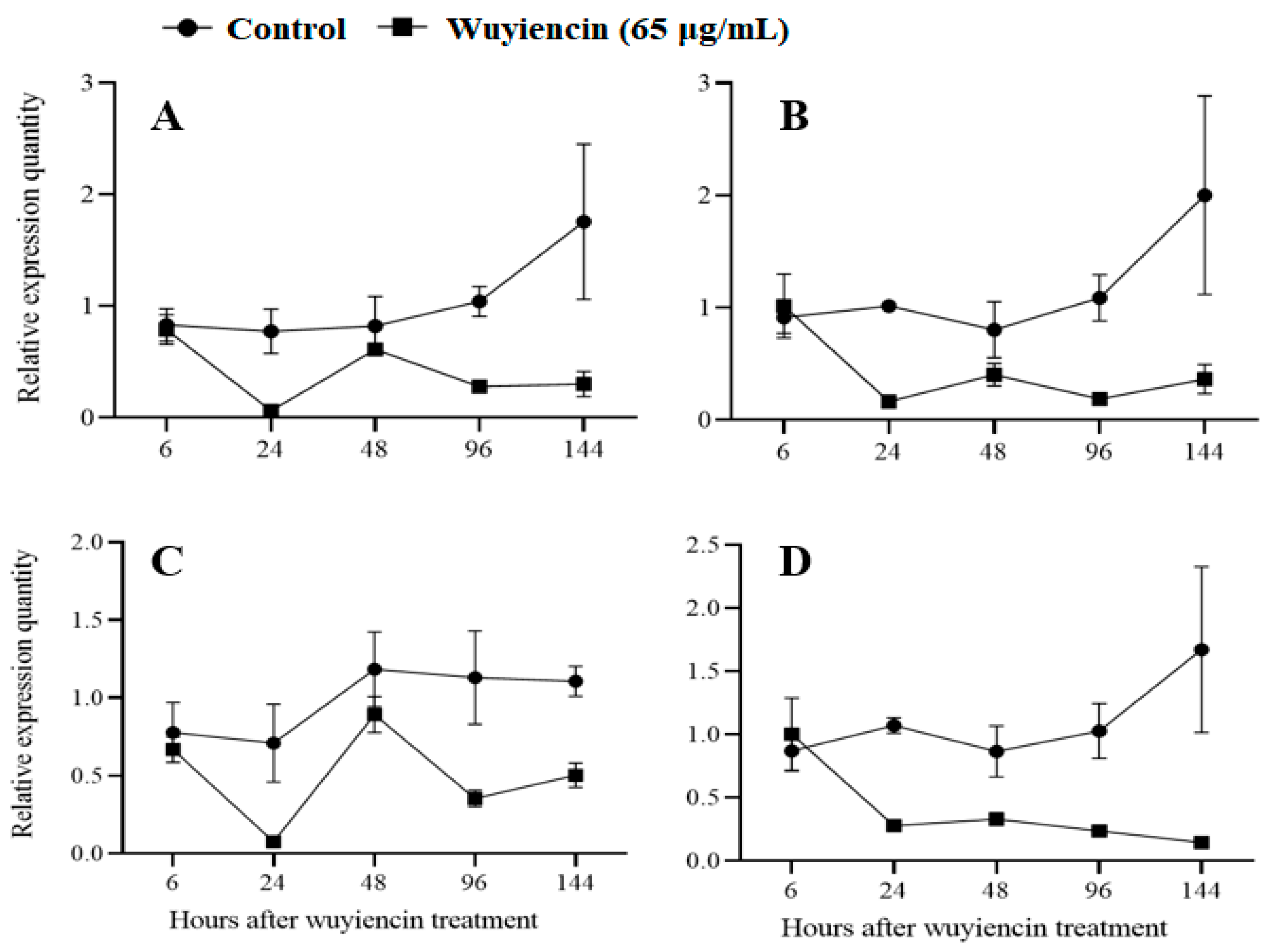
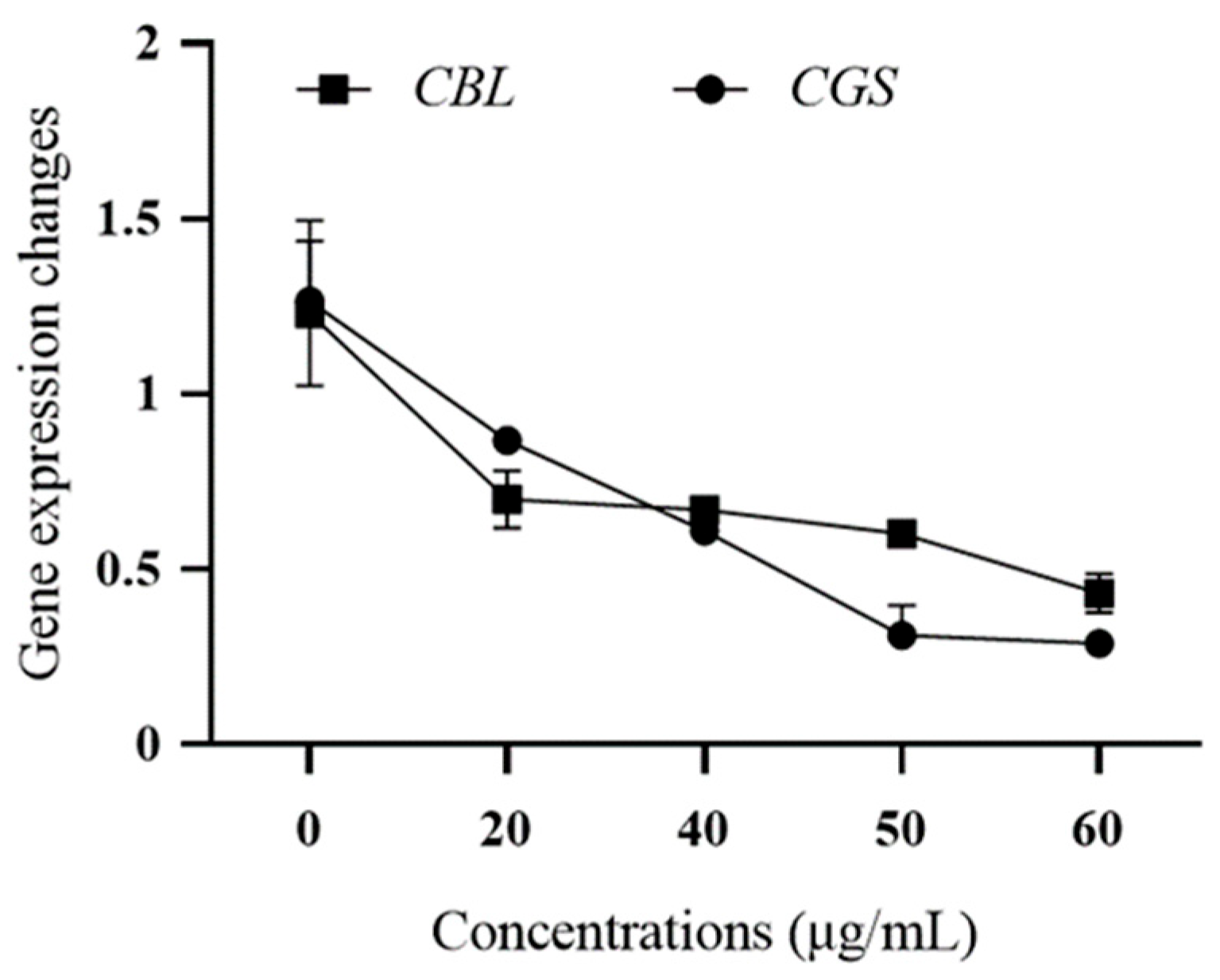
2.2.4. Pathogenicity Gene Expression
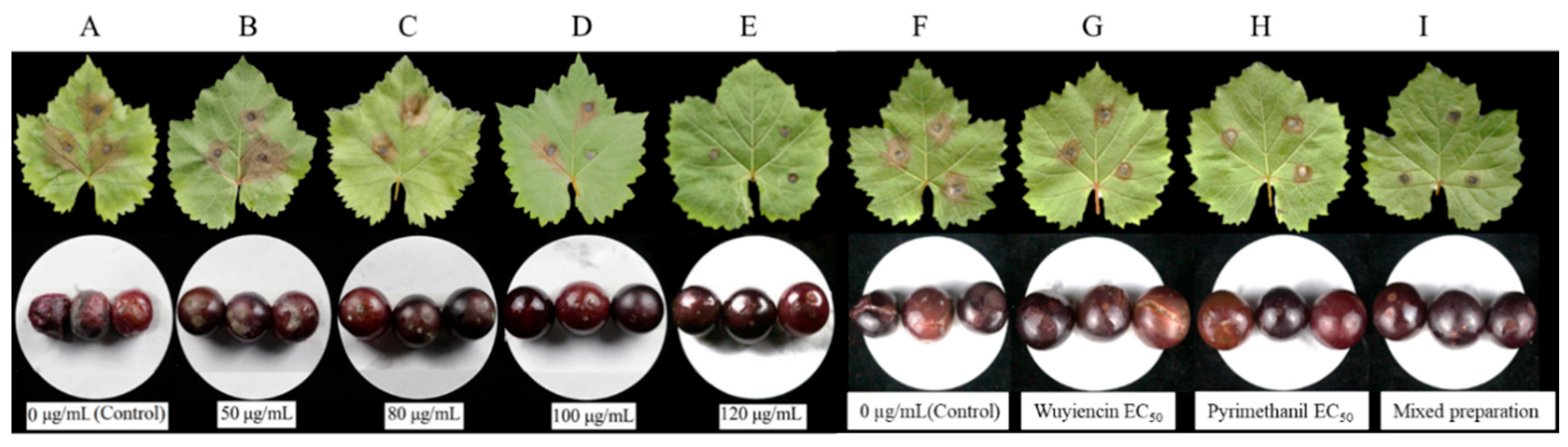
2.2.5. Leaf and Fruit Disease Suppression
2.3. Assessment of the Plant Defense Response Induced by Wuyiencin
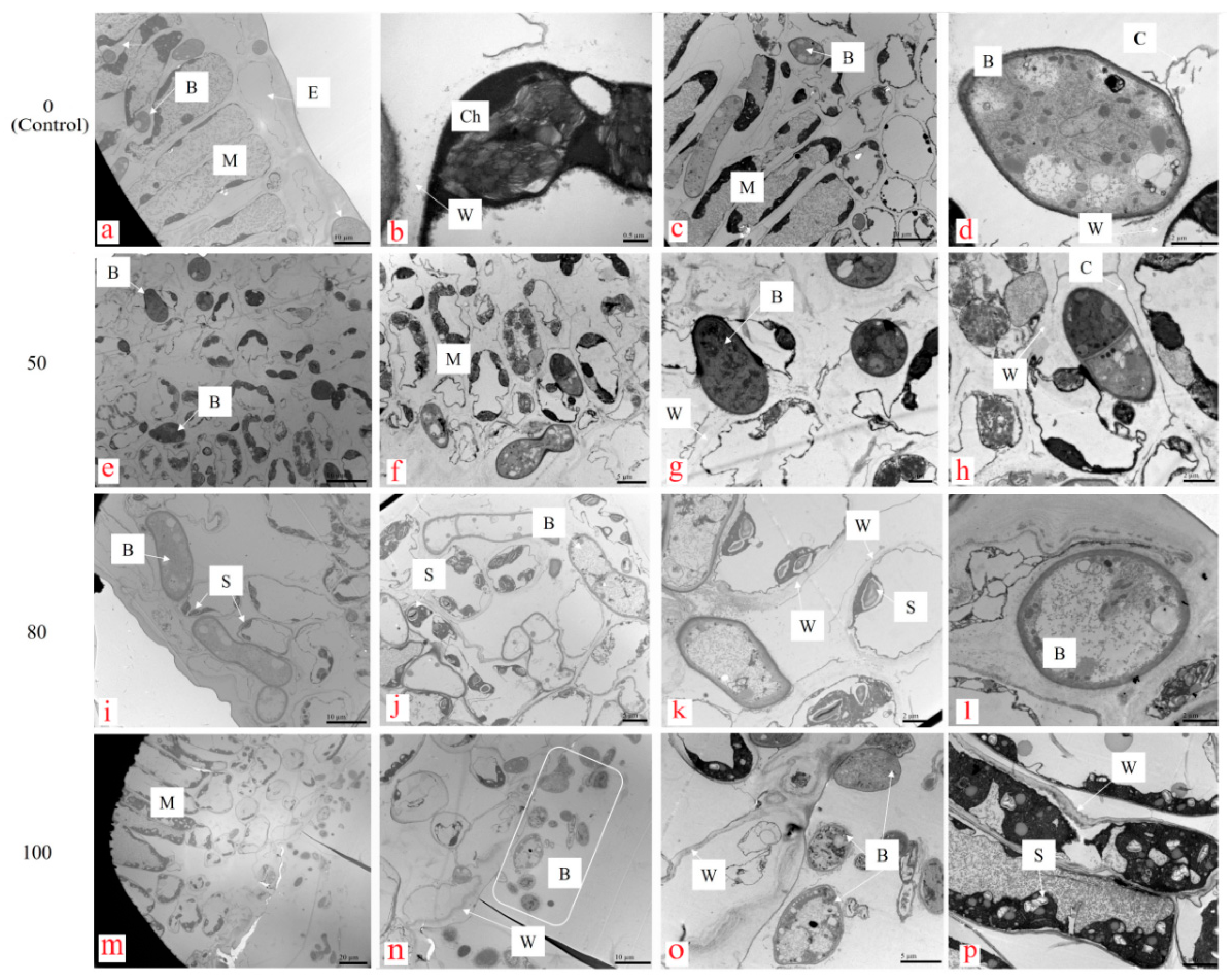
2.3.1. Changes in Leaf Cellular Organelles
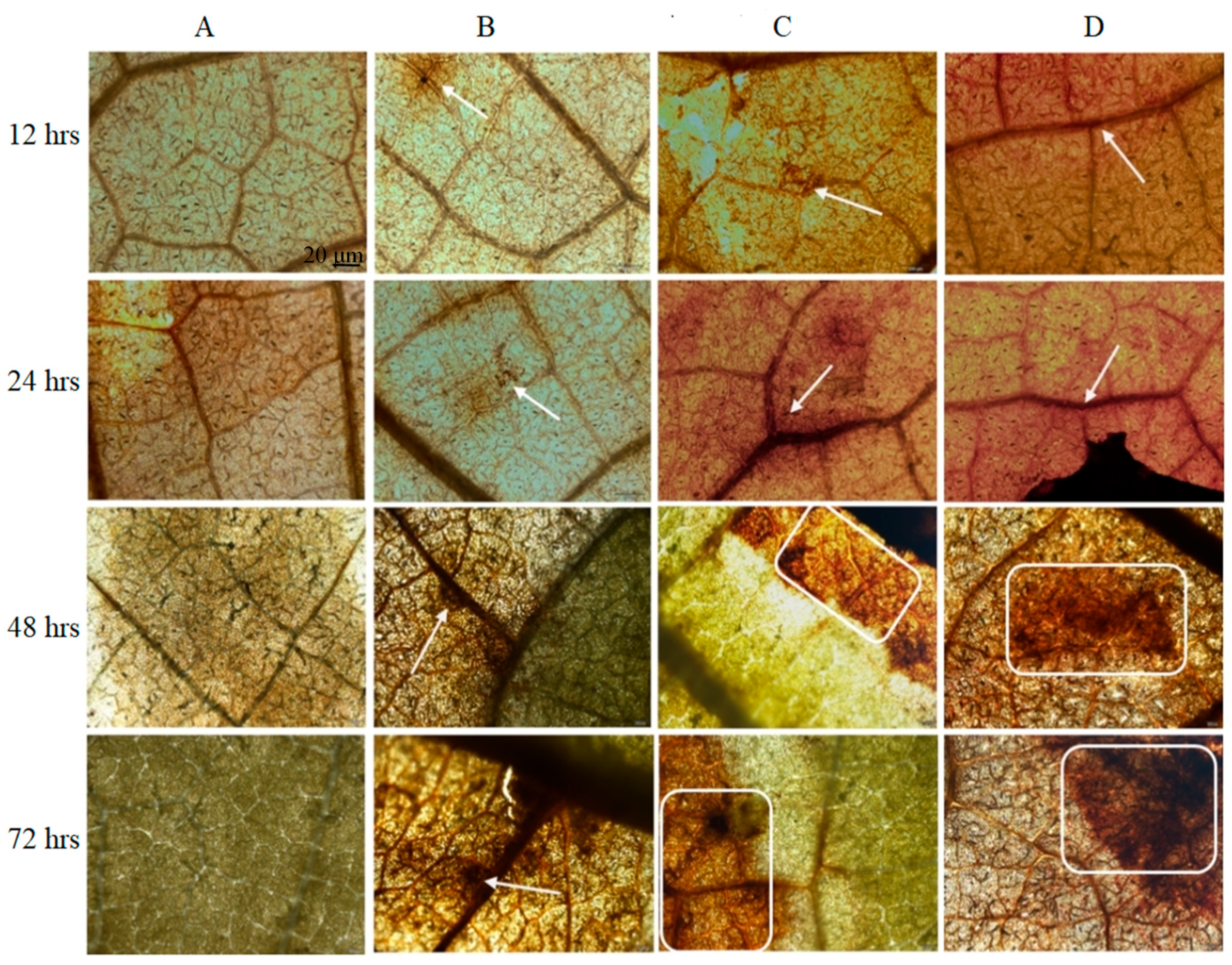
2.3.2. Callose Deposition
2.3.3. Reactive Oxygen Species Accumulation
2.4. Selection of Effective Mixed Preparations of Wuyiencin and Fungicides for Gray Mold Control
2.4.1. Screening Effective Chemical Fungicides
2.4.2. Determination of the Optimal Ratio of Candidate Fungicides in Mixed Preparations
2.4.3. Selection of the Mixed Preparation with the Highest Synergism
2.4.4. Evaluation of Botrytis cinerea Colony Growth Inhibition by the Optimal Mixed Preparation
2.4.5. Evaluation of Gray Mold Disease Suppression by the Optimal Mixed Preparation on Leaves and Fruits
2.5. Effect of Wuyiencin on Fungicide Resistance in Botrytis cinerea and Its Mechanism
2.5.1. Classification of Botrytis cinerea Strains Based on Fungicide Resistance Levels
2.5.2. Determination of the Retardation Effect of Wuyiencin on the Resistance of Botrytis cinerea Strains
2.5.3. Mechanism of the Retardation of Resistance in Botrytis cinerea by Wuyiencin
2.5.4. Determination of the Effect of Wuyiencin on Expression of the Methionine Biosynthesis Gene
2.6. Statistical Analysis of the Data
3. Results
3.1. Inhibitory Effect of Wuyiencin on Botrytis Cinerea Pathogenicity
3.1.1. Colony and Mycelial Growth
3.1.2. Conidial Germination
3.1.3. Infection Pad Formation
3.1.4. Oxalic Acid Production
3.1.5. Expression of Pathogenicity Genes
3.1.6. Disease Suppression on Leaves and Fruits
3.2. Assessment of the Plant Defense Response Induced by Wuyiencin and Its Control Effect
3.2.1. Change in Leaf Cellular Organelles
3.2.2. Callose Deposition
3.2.3. Reactive Oxygen Species Accumulation
3.3. Compound Preparations of Wuyiencin and Chemical Fungicides against Grape Gray Mold
3.3.1. Effective Chemical Fungicides
3.3.2. Synergistic Interaction of Wuyiencin with Candidate Fungicides
3.3.3. Evaluating the Synergistic Effect of Mixed Preparations
3.3.4. Inhibitory Effect of the Selected Mixed Preparation on Botrytis cinerea Colony Growth
3.3.5. Inhibitory Effect of the Optimal Mixed Preparation on Botrytis cinerea in Grape Leaves and Fruit
3.4. Wuyiencin Delayed Fungicide Resistance Development in Botrytis cinerea
3.4.1. Classification of Different Botrytis cinerea Strains Based on Fungicide Resistance
3.4.2. Retardation of Resistance Development in Botrytis cinerea Strains by Wuyiencin
3.5. Mechanism of Retardation of Resistance Development in Botrytis cinerea by Wuyiencin
3.5.1. Effect of wuyiencin on Cell Wall-Degrading Enzyme Activity in Resistant Botrytis cinerea
3.5.2. Effect of Wuyiencin on the Expression Levels of Mutant Genes of B. cinerea
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Banani, H.; Roatti, B.; Ezzahi, B.; Giovannini, O.; Gessler, G.; Pertot, I.; Perazzolli, M. Characterization of resistance mechanisms activated by Trichoderma harzianum T39 and benzothiadiazole to downy mildew in different grapevine cultivars. Plant Pathol. 2014, 63, 334–343. [Google Scholar] [CrossRef]
- Fillinger, S.; Ajouz, S.; Nicot, P.C.; Leroux, P.; Bardin, M. Functional and structural comparison of pyrrolnitrin- and iprodione-induced modifications in the class III histidine-kinase Bos1 of Botrytis cinerea. PLoS ONE 2012, 7, e42520. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef]
- Mcfeeters, H.; McFeeters, R.L. Emerging approaches to inhibit Botrytis cinerea. Int. J. Mod. Bot. 2012, 2, 127–144. [Google Scholar] [CrossRef]
- Leroux, P.; Albertini, C.; Gautier, A.; Gredt, M.; Walker, A.S. Mutations in the CYP51 gene correlated with changes in sensitivity to sterol 14 alpha-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest. Manag. Sci. 2007, 63, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Yunis, H.; Katan, T. Multiple fungicide resistance to benzimidazoles, dicarboximides and diethofencarb in field isolates of Botrytis cinerea in Israel. Plant Pathol. 1992, 41, 41–46. [Google Scholar] [CrossRef]
- Katan, T.; Elad, Y.; Yunis, H. Resistance to diethofencarb (NPC) in benomyl-resistant field isolates of Botrytis cinerea. Plant Pathol. 1989, 38, 86–92. [Google Scholar] [CrossRef]
- Myresiotis, C.; Karaoglanidis, G.; Tzavella-Klonari, K. Resistance of Botrytis cinerea isolates from vegetable crops to Anilinopyrimidine, Phenylpyrrole, Hydroxyanilide, Benzimidazole, and Dicarboximide fungicides. Plant Dis. 2007, 91, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.; Delcán, J.; Gómez, V.; Melgarejo, P. Distribution and fitness of isolates of Botrytis cinerea with multiple fungicide resistance in Spanish greenhouses. Plant Pathol. 1996, 45, 497–505. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, J.; Wei, F.; Zhu, G. Evolution of resistance to different classes of fungicides in Botrytis cinerea from greenhouse vegetables in eastern China. Phytoparasitica 2009, 37, 351–359. [Google Scholar] [CrossRef]
- Koenraadt, H.; Somerville, S.C.; Jones, A. Characterization of mutations in the beta-tubulin gene of benomyl-resistant field strains of Venturia inaequalis and other plant pathogenic fungi. Phytopathology 1992, 82, 1348–1354. [Google Scholar] [CrossRef]
- Egashira, H.; Kuwashima, A.; Ishiguro, H.; Fukushima, K.; Kaya, T.; Imanishi, S. Screening of wild accessions resistant of grey mold (Botrytis cinernea Pers.) in lycopersicon. Acta Physiol. Plant 2000, 22, 324–326. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Glättli, A.; Stammler, G.; Schlehuber, S. Mutations in the target proteins of succinate-dehydrogenase inhibitors (SDHI) and 14α-demethylase inhibitors (DMI) conferring changes in the sensitivity-structural insights from molecular modelling. In Proceedings of the Association Française de Protection des Plantes, 9ème Conférence International sur les Maladies des Plantes, Tours, France, 8–9 December 2009. [Google Scholar]
- Wang, L.; Wang, C.; Qin, L.; Liu, W.; Wang, Y. ThERF1 regulates its target genes via binding to a novel cis-acting element in response to salt stress. J. Plant Biol. 2015, 57, 838–847. [Google Scholar] [CrossRef]
- Yin, Y.N.; Kim, Y.K.; Xiao, C.L. Molecular characterization of boscalid resistance in field isolates of Botrytis cinerea from apple. Phytopathology 2011, 101, 986–995. [Google Scholar] [CrossRef]
- Banno, S.; Fukumori, F.; Ichiishi, A.; Okada, K.; Uekusa, H.; Kimura, M.; Fujimura, M. Genotyping of benzimidazole-resistant and dicarboximide-resistant mutations in Botrytis cinerea using real-time polymerase chain reaction assays. Phytopathology 2008, 98, 397–404. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Alamoudi, S.A. Using some microorganisms as biocontrol agents to manage phytopathogenic fungi: A comprehensive review. J. Plant Pathol. 2024, 106, 3–21. [Google Scholar] [CrossRef]
- Alfiky, A.E.; L’Haridon, F.; Abou-Mansour, E.; Weisskopf, L. Disease inhibiting effect of strain Bacillus subtilis EG21 and its metabolites against potato pathogens Phytophthora infestans and Rhizoctonia solani. Phytopathology 2022, 112, 2099–2109. [Google Scholar] [CrossRef]
- Chio, C.; Shrestha, S.; Carr, G.; Khatiwada, J.; Zhu, Y.; Li, O.; Chen, X.; Hu, J.; Qin, W. Optimization and purification of bioproducts from Bacillus velezensis PhCL fermentation and their potential on industrial application and bioremediation. Sci. Total Environ. 2023, 903, 166428. [Google Scholar] [CrossRef]
- Ge, X.; Wei, W.; Li, G.; Sun, M.; Li, H.; Wu, J.; Hu, F. Isolated Pseudomonas aeruginosa strain VIH2 and antagonistic properties against Ralstonia solanacearum. Microb. Pathog. 2017, 111, 519–526. [Google Scholar] [CrossRef]
- Kim, J.D.; Park, M.Y.; Jeon, B.J.; Kim, B.S. Disease control efficacy of 32,33-didehydroroflamycoin produced by Streptomyces rectiviolaceus strain DY46 against gray mold of tomato fruit. Sci. Rep. 2019, 9, 13533. [Google Scholar] [CrossRef]
- Long, C.; Wu, Z.; Deng, B. Biological control of Penicillium italicum of citrus and Botrytis cinerea of grape by strain 34-9 of Kloeckera apiculata. Eur. Food Res. Technol. 2005, 221, 197–201. [Google Scholar] [CrossRef]
- Luo, W.; Liu, L.; Qi, G.; Yang, F.; Shi, X.; Zhao, X. Embedding Bacillus velezensis NH-1 in microcapsules for biocontrol of cucumber Fusarium wilt. Appl. Environ. 2019, 85, e03128-18. [Google Scholar] [CrossRef]
- Quan, X.; Xue, B.; Yang, L.; Sun, H. Toxicity test and effectivity of the mixture of Validamycin and Bacillus subtilis to Rhizoctonia cerealis. Chin. Agric. Sci. Bull. 2011, 27, 245–248. [Google Scholar]
- Tian, Z.; Du, Y.; Yang, F.; Zhao, J.; Liu, S.; Zhang, D.; Long, C. Chromosome genome sequencing and comparative transcriptome-based analyses of Kloeckera apiculata 34-9 unveil the potential biocontrol mechanisms against citrus green mold. Front. Microbiol. 2021, 12, 752529. [Google Scholar] [CrossRef]
- Warin, I.; Chaiyawat, S.; Chiradej, C.; Montree, I.; Sorwaporn, K.; Kan, C. Bioactive compound of antifungal metabolite from Trichoderma harzianum mutant strain for the control of anthracnose of chili (Capsicum annuum L.). Philipp. Agric. Sci. 2009, 92, 392–397. [Google Scholar]
- Mikani, A.; Etebarian, H.R.; Aminian, H.; Alizadeh, A. Interaction between Pseudomonas fluorescens isolates and Thiabendazole in the control of gray mold of apple. Pak. J. Biol. Sci. 2007, 10, 2172–2177. [Google Scholar] [CrossRef]
- Zhou, T.; Northover, J.M.; Schneider, K.E.; Lu, X. Interactions between Pseudomonas syringae MA-4 and Cyprodinil in the control of blue mold and gray mold of apples. Can. J. Plant Pathol. 2002, 24, 154–161. [Google Scholar] [CrossRef]
- Bi, Q.; Han, X.; Ma, Z.; Zhao, J.; Wang, W.; Jia, H. Inhibitory effects of Bacillus subtilis HMB-20428 interacted with chemical fungicides and decrement of chemical fungicides on oomycete pathogen Plasmopara viticola. J. Plant Prot. 2018, 45, 1396–1404. [Google Scholar]
- Lv, Z.; Lu, Y.; Li, B.; Shi, L.; Zhang, K.; Ge, B. Effects of ε-Poly-L-Lysine combined with wuyiencin as a bio-fungicide against Botrytis cinerea. Microorganisms 2022, 10, 971. [Google Scholar] [CrossRef]
- Tan, W.; Li, B.; Zhang, K.; Wang, Z.; Ren, F. Survey of tea diseases in Chongqing and the efficiency of disease control with wuyiencin under field conditions. J. Southwest Univ. (Nat. Sci. Ed.) 2008, 4, 123–127. [Google Scholar]
- Yang, M.; Zhang, W.; Lv, Z.; Shi, L.; Zhang, K.; Ge, B. Evaluation of the inhibitory effects of Wuyiencin, a secondary metabolite of Streptomyces albulus CK-15, against Sclerotinia sclerotiorum in vitro. Plant. Dis. 2021, 106, 156–164. [Google Scholar] [CrossRef]
- Yang, M.; Han, X.; Xie, J.; Zhang, S.; Lv, Z.; Li, B.; Shi, L.; Zhang, K.; Zhang, K. Field application of wuyiencin against sclerotinia stem rot in soybean. Front. Sustain. Food Syst. 2022, 6, 930079. [Google Scholar] [CrossRef]
- Gong, J.L. Pathogen Identification of Coffee Anthracnose and Screening of Chemical Control Agents in China. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Cao, J.; Zhang, J.; Zhang, L.; Chen, Q.; Qin, L.; Li, G. Ultrastructural studies on the penetration process of Botrytis cinerea infection cushion on different substrate surfaces. Hubei Agric. Sci. 2014, 4, 814–817. [Google Scholar]
- Duan, L.; Wang, J.; Zhao, J. Study on conditions of colorimetric determination of oxalate content in Spinach. J. Anhui Agric. Sci. 2007, 35, 632–633. [Google Scholar]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2 Method−ΔΔCT. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, B.; Lv, Z.; Park, K.S.; Shi, L.; Zhang, K. Membrane protein Bcest is involved in hyphal growth, virulence and stress tolerance of Botrytis cinerea. Microorganisms 2023, 11, 1225. [Google Scholar] [CrossRef]
- Alvarez, M.; Pennell, R.; Meijer, P.; Ishikawa, A.; Dixon, R.; Lamb, C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 1998, 92, 773–784. [Google Scholar] [CrossRef]
- Ramel, F.; Sulmon, C.; Bogard, M.; Couée, I.; Gouesbet, G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol. 2009, 9, 28. [Google Scholar] [CrossRef]
- Horsfall, J. Fungicides and Their Action; Chronica Botanica: Waltham, MA, USA, 1945. [Google Scholar]
- Sun, Y.; Johnson, E. Analysis of joint action of insecticides against house flies. J. Econ. Entomol. 1960, 53, 887–892. [Google Scholar] [CrossRef]
- Magro, P.; Marciano, P.; Di, P. Oxalic acid production and its role in pathogenesis of Sclerotinia sclerotiorum. FEMS Microbiol. Lett. 1984, 24, 9–12. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. Regulation and function of defense-related callose deposition in plants. Int. J. Mol. Sci. 2021, 22, 2393. [Google Scholar] [CrossRef] [PubMed]
- Marček, T.; Vuletić, M.V.; Španić, V. Biochemical changes in ears of wheat genotypes subjected to Fusarium spp. attack. Acta Biol. Hung. 2018, 69, 493–504. [Google Scholar] [CrossRef]
- Blasco, C.; Picó, Y.; Manes, J.; Font, G. Determination of fungicide residues in fruits and vegetables by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. 2002, 947, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wightwick, A.; Mollah, M.; Partington, D.; Allinson, G. Copper fungicide residues in Australian vineyard soils. J. Agric. Food Chem. 2008, 56, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Hawkins, N.; Fraaije, B. The evolution of fungicide resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar] [PubMed]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Biol. Chem. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Idnurm, A.; Howlett, B. Pathogenicity genes of phytopathogenic fungi. Mol. Plant Pathol. 2001, 2, 241–255. [Google Scholar] [CrossRef]
- Nakkeeran, S.; Rajamanickam, S.; Karthikeyan, M.; Mahendra, K.; Renukadevi, P.; Johnson, I. Antimicrobial secondary metabolites from Trichoderma spp. as next generation fungicides. In Biocontrol Agents and Secondary Metabolites; Elsevier Science: Amsterdam, The Netherlands, 2021; pp. 257–282. [Google Scholar] [CrossRef]
- Zuriegat, Q.; Zheng, Y.; Liu, H.; Wang, Z.; Yun, Y. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef]
- Evidente, A.; Cimmino, A.; Masi, M. Phytotoxins produced by pathogenic fungi of agrarian plants. Phytochem. Rev. 2019, 18, 843–870. [Google Scholar] [CrossRef]
- Noyes, R.; Hancock, J. Role of oxalic acid in the Sclerotinia wilt of sunflower. Physiol. Mol. Plant Pathol. 1981, 18, 123–132. [Google Scholar] [CrossRef]
- Yang, X.; Hong, C. Biological control of Phytophthora blight by Pseudomonas protegens strain 14D5. Eur. J. Plant Pathol. 2019, 156, 591–601. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.; Wang, Q.; Suo, H.; Hamdy, A.; Song, J. Antifungal effect of metabolites from a new strain Lactiplantibacillus plantarum LPP703 isolated from naturally fermented Yak Yogurt. Foods 2023, 12, 181. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, L.; Zhang, X.; Dhanasekaran, S.; Abdelhai, M.; Yang, Q.; Jiang, Z.; Zang, H. Study on biocontrol of postharvest decay of table grapes caused by Penicillium rubens and the possible resistance mechanisms by Yarrowia lipolytica. Biol. Control 2019, 130, 110–117. [Google Scholar] [CrossRef]
- Raaijmakers, J.; Vlami, M.; De Souza, J. Antibiotic production by bacterial biocontrol agents. Antonie VanLeeuwenhoek 2004, 81, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Rahardjo, I.; Budiarto, K.; Marwoto, B. Evaluation of biological and chemical fungicides to control white rust in chrysanthemum grown under open condition. AGRIVITA J. Agric. Sci. 2019, 41, 55–63. [Google Scholar] [CrossRef]
- Li, X.; Choi, J. Development of a system for controlling ginseng Alternaria leaf blight (Alternaria panax) to reduce fungicide application and use. Res. Plant Dis. 2009, 15, 17–22. [Google Scholar] [CrossRef][Green Version]
- Gourgues, M.; Brunet-Simon, A.; Lebrun, M.H.; Levis, C. The tetraspanin BcPls1 is required for appressorium-mediated penetration of Botrytis cinerea into host plant leaves. Mol. Microbiol. 2004, 51, 619–629. [Google Scholar] [CrossRef]
- Zheng, L.; Campbell, M.; Murphy, J.; Lam, S.; Xu, J.R. The BMP1 gene is essential for pathogenicity in the gray mold fungus Botrytis cinerea. Mol. Plant-Microbe Interact. J. 2000, 13, 724–732. [Google Scholar] [CrossRef]
- Segmüller, N.; Kokkelink, L.; Giesbert, S.; Odinius, D.; van Kan, J.; Tudzynski, P. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol. Plant-Microbe Interact. J. 2008, 21, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Ten Have, A.; Mulder, W.; Visser, J.; Van Kan, J.A. The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol. Plant-Microbe Interact. J. 1998, 11, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Qin, G.; He, C.; Li, B.; Tian, S. Actin Is required for cellular development and virulence of Botrytis cinerea via the mediation of secretory proteins. mSystems 2020, 5, e00732-19. [Google Scholar] [CrossRef] [PubMed]
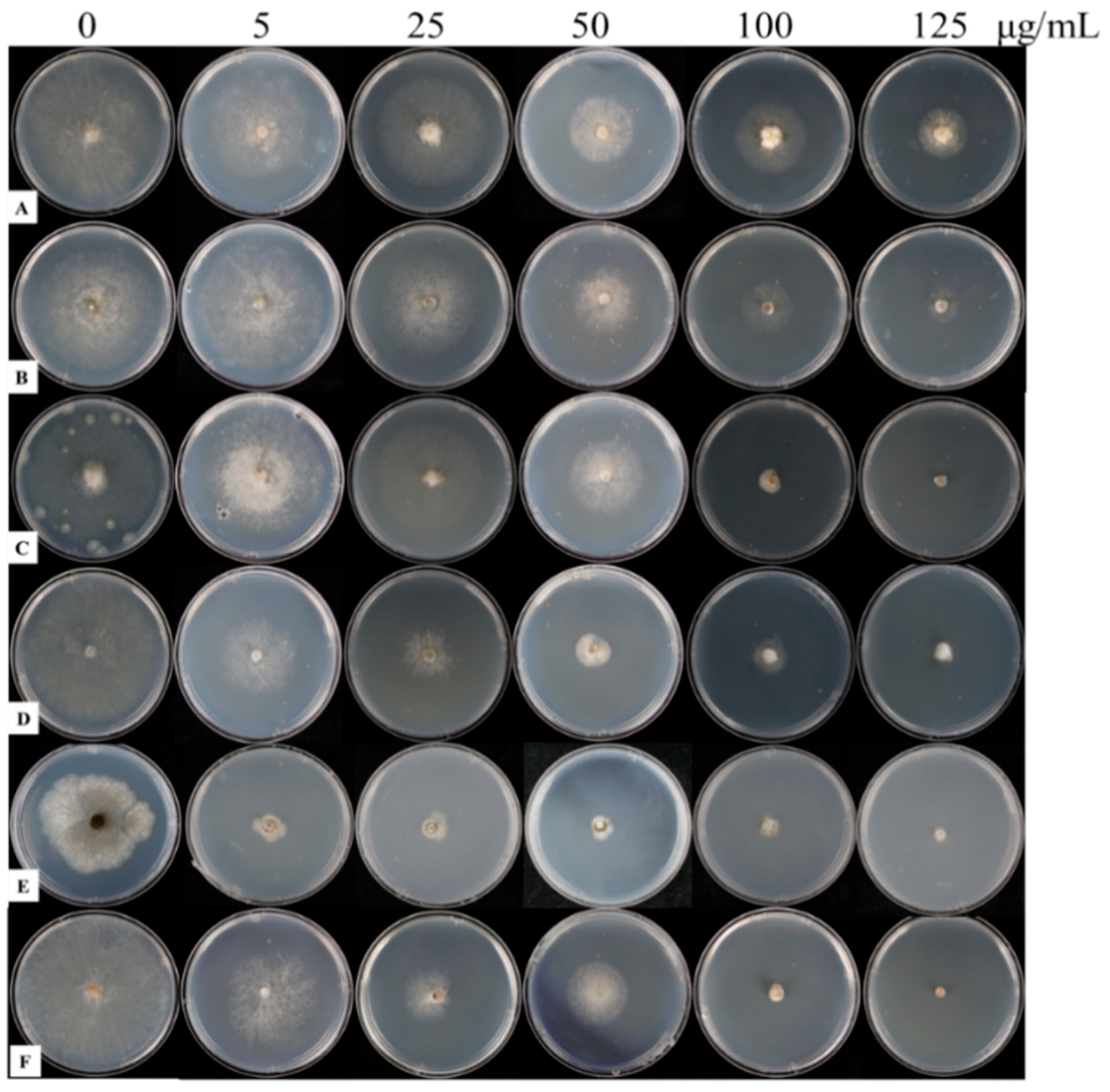
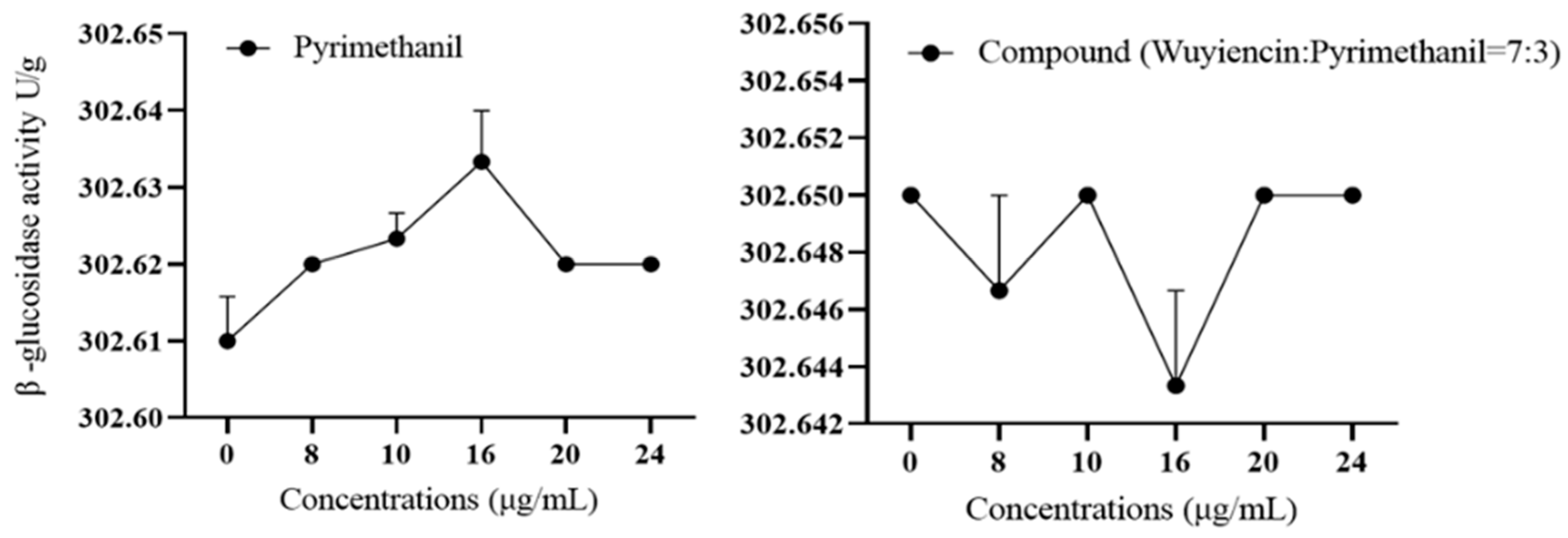
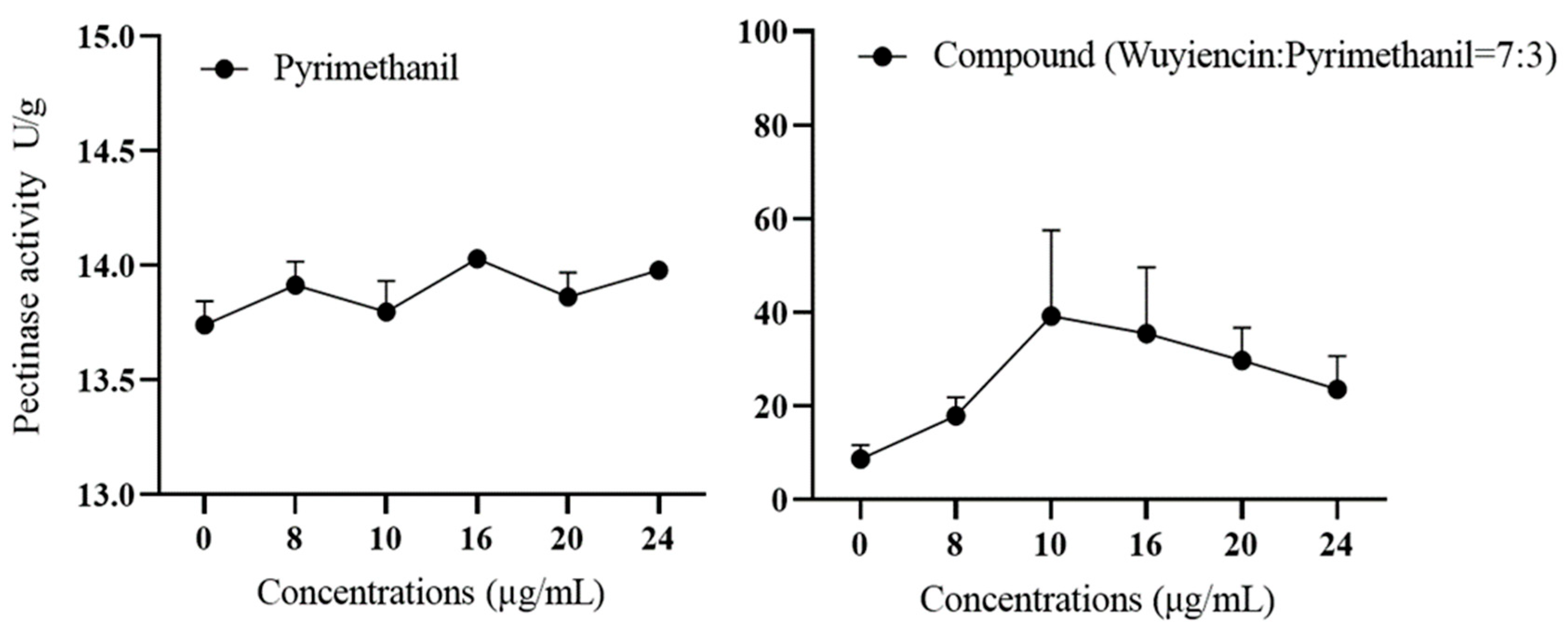
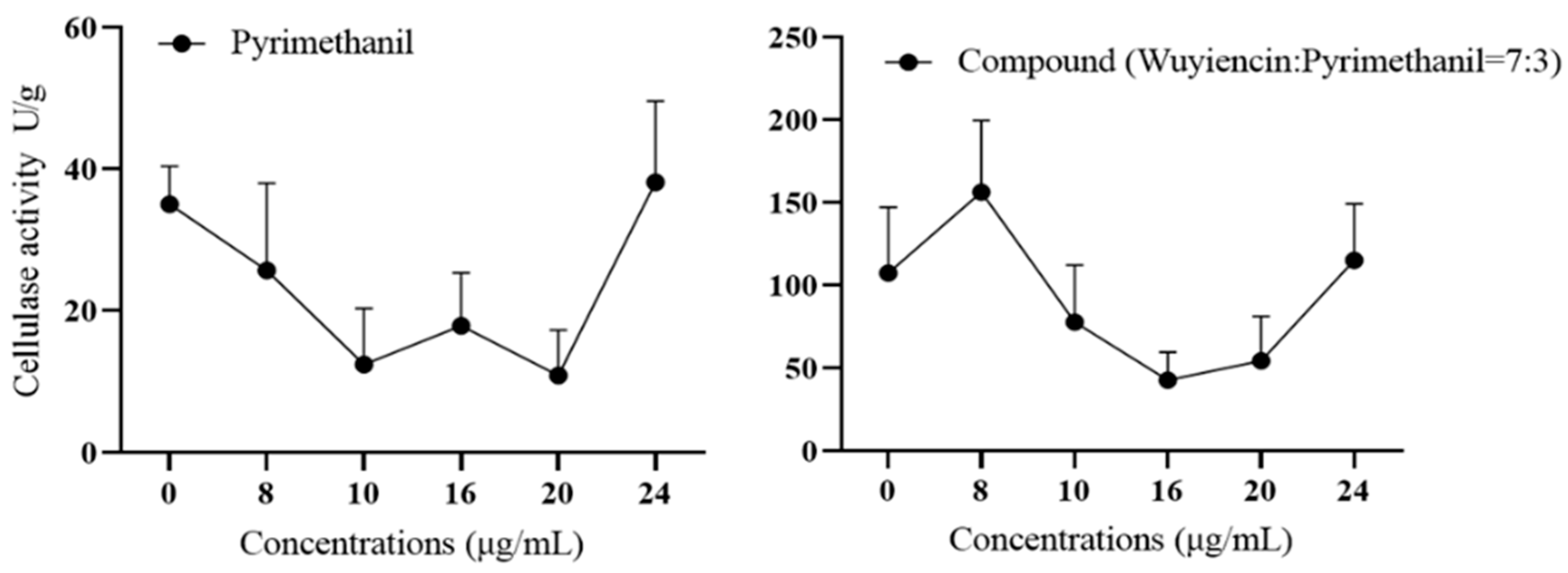
| Fungicide | Virulence Function | r2 | EC50 (mg L−1) | Range of EC50 (mg L−1) |
|---|---|---|---|---|
| Wuyiencin | y = 2.59x − 4.47 * | 0.833 | 51.213 | 31.278–71.074 |
| Pyrimethanil | y = 1.24x − 1.12 ** | 0.978 | 8.354 | 2.91–13.951 |
| Polyoxin | y = 2.8x − 6.87 ** | 0.913 | 290.059 | 230.759–462.647 |
| Iprodione | y = 1.99x − 0.97 ** | 1.000 | 4.724 | 0.216–9.728 |
| Fluopyram/trifloxystrobin | y = 0.85x − 0.08 ** | 0.956 | 1.207 | 0.002–5.317 |
| Wuyiencin + | 10:0 | 1:9 | 2:8 | 3:7 | 4:6 | 5:5 | 6:4 | 7:3 | 8:2 | 9:1 | 0:10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iprodione | TR | 1.000 | 0.781 | 1.043 | 0.901 | 0.863 | 0.904 | 0.917 | 0.888 | 0.983 | 1.006 | 1.000 |
| Type | - | Ant. | Add. | Add. | Ant. | Add. | Add. | Ant. | Add. | Add. | - | |
| Pyrimethanil | TR | 1.000 | 0.777 | 1.011 | 1.045 | 1.045 | 1.046 | 1.085 | 1.085 | 1.014 | 1.091 | 1.000 |
| Type | - | Ant. | Add. | Add. | Add. | Add. | Syn. | Syn. | Add. | Syn. | - | |
| Fluopyram· trifloxystrobin | TR | 1.000 | 1.050 | 0.934 | 1.050 | 1.046 | 1.118 | 1.163 | 1.142 | 1.156 | 1.157 | 1.000 |
| Type | - | Add. | Add. | Add. | Add. | Syn. | Syn. | Syn. | Syn. | Syn. | - |
| Preparation of Wuyiencin (A) + | Ratio (A:B) | Virulence Function | r2 | EC50 (μg mL−1) | CTC |
|---|---|---|---|---|---|
| Pyrimethanil (B) | 10:0 | y = 2.59x − 4.47 * | 0.833 | 51.213 | — |
| 6:4 | y = 1.46x − 1.57 ** | 0.978 | 11.553 | 145.2 | |
| 7:3 | y = 1.26x − 0.85 ** | 0.922 | 5.153 | 391.4 | |
| 9:1 | y = 1.48x − 1.38 ** | 0.947 | 8.642 | 391.7 | |
| 0:10 | y = 1.24x − 1.12 ** | 0.978 | 8.354 | — | |
| Fluopyram·trifloxystrobin (B) | 10:0 | y = 2.59x − 4.47 ** | 0.833 | 51.213 | — |
| 6:4 | y = 1.00x − 0.84 ** | 0.990 | 6.926 | 42.1 | |
| 8:2 | y = 1.41x − 1.07 ** | 0.915 | 6.030 | 91.4 | |
| 9:1 | y = 0.33x − 0.23 ** | 0.968 | 4.845 | 205.5 | |
| 0:10 | y = 0.85x − 0.08 ** | 0.956 | 1.207 | — |
| Fungicide | Effect of Leaves Control | Effect of Fruits Control | ||
|---|---|---|---|---|
| Lesion Diameter (cm) | Suppression Rate (%) | Disease Incidence (%) | Disease Suppression Rate (%) | |
| Control | 1.43 ± 0.34 a | 0.0 | 76.40 ± 1.40 a | 0.0 |
| Wuyiencin EC50 | 0.75 ± 0.26 b | 47.56 | 48.61 ± 1.39 c | 36.37 |
| PyrimethanilEC50 | 1.01 ± 0.28 ab | 29.37 | 51.47 ± 1.40 b | 32.63 |
| Wuyiencin EC50 + Pyrimethanil EC50 (7:3) | 0.70 ± 0.24 b | 51.05 | 25.93 ± 1.61 d | 66.06 |
| Strain | Virulence Regression Curve (y=) | EC50 (μg mL−1) | R2 | 95% Confidence Interval | Resistance Coefficient RR | Resistance Level |
|---|---|---|---|---|---|---|
| 57 | 0.54x−0.87 | 40.973 | 0.805 | 4.646–603.053 | 450.25 | High resistance |
| 59 | 1.95x−3.67 | 77.030 | 0.988 | 66.152–91.451 | 846.48 | High resistance |
| 61 | 1.47x−2.65 | 63.446 | 0.945 | 39.814–44.648 | 697.21 | High resistance |
| 65 | 0.92x−0.92 | 9.892 | 0.921 | 1.445–20.506 | 108.70 | High resistance |
| 503 | 0.42x + 0.07 | 0.565 | 0.753 | 0.001–2.871 | 6.208 | Sensitive resistance |
| 514 | 1.54x−1.92 | 17.329 | 0.928 | 3.021–37.097 | 190.43 | High resistance |
| Strain | Fungicides Concentration (μg mL−1) | Disease Spot Diameter (cm) | Control Efficiency (%) |
|---|---|---|---|
| 57 | Control | 7.18 ± 0.60 a | \ |
| 5 | 4.68 ± 0.70 b | 34.82 | |
| 25 | 4.61 ± 0.79 b | 35.79 | |
| 50 | 3.23 ± 0.21 c | 55.01 | |
| 100 | 3.09 ± 0.33 cd | 56.96 | |
| 125 | 2.57 ± 0.14 b | 64.21 | |
| 59 | Control | 5.90 ± 0.35 b | \ |
| 5 | 6.23 ± 0.82 a | −5.59 | |
| 25 | 4.81 ± 0.37 c | 18.47 | |
| 50 | 3.97 ± 0.31 d | 32.71 | |
| 100 | 2.48 ± 0.14 e | 57.97 | |
| 125 | 1.89 ± 0.22 f | 67.97 | |
| 61 | Control | 6.53 ± 0.44 a | \ |
| 5 | 6.05 ± 0.24 b | 7.35 | |
| 25 | 5.33 ± 0.15 c | 18.38 | |
| 50 | 3.83 ± 0.26 d | 41.35 | |
| 100 | 2.42 ± 0.43 e | 62.94 | |
| 125 | 1.32 ± 0.15 f | 79.79 | |
| 65 | Control | 6.81 ± 0.21 a | \ |
| 5 | 3.83 ± 0.38 b | 43.76 | |
| 25 | 3.10 ± 0.40 c | 54.48 | |
| 50 | 1.66 ± 0.31 d | 75.62 | |
| 100 | 1.20 ± 0.10 e | 82.38 | |
| 125 | 0.90 ± 0.00 e | 86.78 | |
| 503 | Control | 5.90 ± 0.30 a | \ |
| 5 | 1.95 ± 0.24 b | 66.95 | |
| 25 | 1.60 ± 0.27 c | 72.88 | |
| 50 | 1.58 ± 0.17 c | 73.22 | |
| 100 | 1.15 ± 0.23 d | 80.51 | |
| 125 | 0.75 ± 1.64 e | 87.29 | |
| 514 | Control | 7.47 ± 0.35 a | \ |
| 5 | 5.78 ± 0.62 b | 22.62 | |
| 25 | 3.14 ± 0.58 c | 57.97 | |
| 50 | 2.60 ± 0.20 d | 65.19 | |
| 100 | 0.58 ± 0.46 d | 92.24 | |
| 125 | 0.00 ± 0.00 d | 100.00 |
| Algebra | Index | Pyrimethanil EC50 | Wuyiencin EC50 + Pyrimethanil EC50 |
|---|---|---|---|
| 1 | Toxicity regression curve | y = 1.24x − 1.12 | y = 1.26x − 0.85 |
| EC50 (µg mL−1) | 8.354 | 5.153 | |
| Resistance multiplicity | 1 | 1 | |
| 8 | Toxicity regression curve | y = 2.29x − 1.76 | y = 2.06x − 1.82 |
| EC50 | 6.086 | 7.610 | |
| Resistance multiplicity | 1.37 | 0.68 | |
| 10 | Toxicity regression curve | y = 0.77x + 0.13 | y = 2.03x − 1.59 |
| EC50 | 0.663 | 6.008 | |
| Resistance multiplicity | 9.18 | 1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Li, B.; Li, J.; Zhang, K.; Ran, L.; Ge, B. Effect of Combining Wuyiencin and Pyrimethanil on Controlling Grape Gray Mold and Delaying Resistance Development in Botrytis cinerea. Microorganisms 2024, 12, 1383. https://doi.org/10.3390/microorganisms12071383
Xie J, Li B, Li J, Zhang K, Ran L, Ge B. Effect of Combining Wuyiencin and Pyrimethanil on Controlling Grape Gray Mold and Delaying Resistance Development in Botrytis cinerea. Microorganisms. 2024; 12(7):1383. https://doi.org/10.3390/microorganisms12071383
Chicago/Turabian StyleXie, Jiabei, Boya Li, Jia Li, Kecheng Zhang, Longxian Ran, and Beibei Ge. 2024. "Effect of Combining Wuyiencin and Pyrimethanil on Controlling Grape Gray Mold and Delaying Resistance Development in Botrytis cinerea" Microorganisms 12, no. 7: 1383. https://doi.org/10.3390/microorganisms12071383
APA StyleXie, J., Li, B., Li, J., Zhang, K., Ran, L., & Ge, B. (2024). Effect of Combining Wuyiencin and Pyrimethanil on Controlling Grape Gray Mold and Delaying Resistance Development in Botrytis cinerea. Microorganisms, 12(7), 1383. https://doi.org/10.3390/microorganisms12071383






