Investigating the Impact That Diagnostic Screening with Lateral Flow Devices Had on the Rabies Surveillance Program in Zanzibar, Tanzania
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Permission
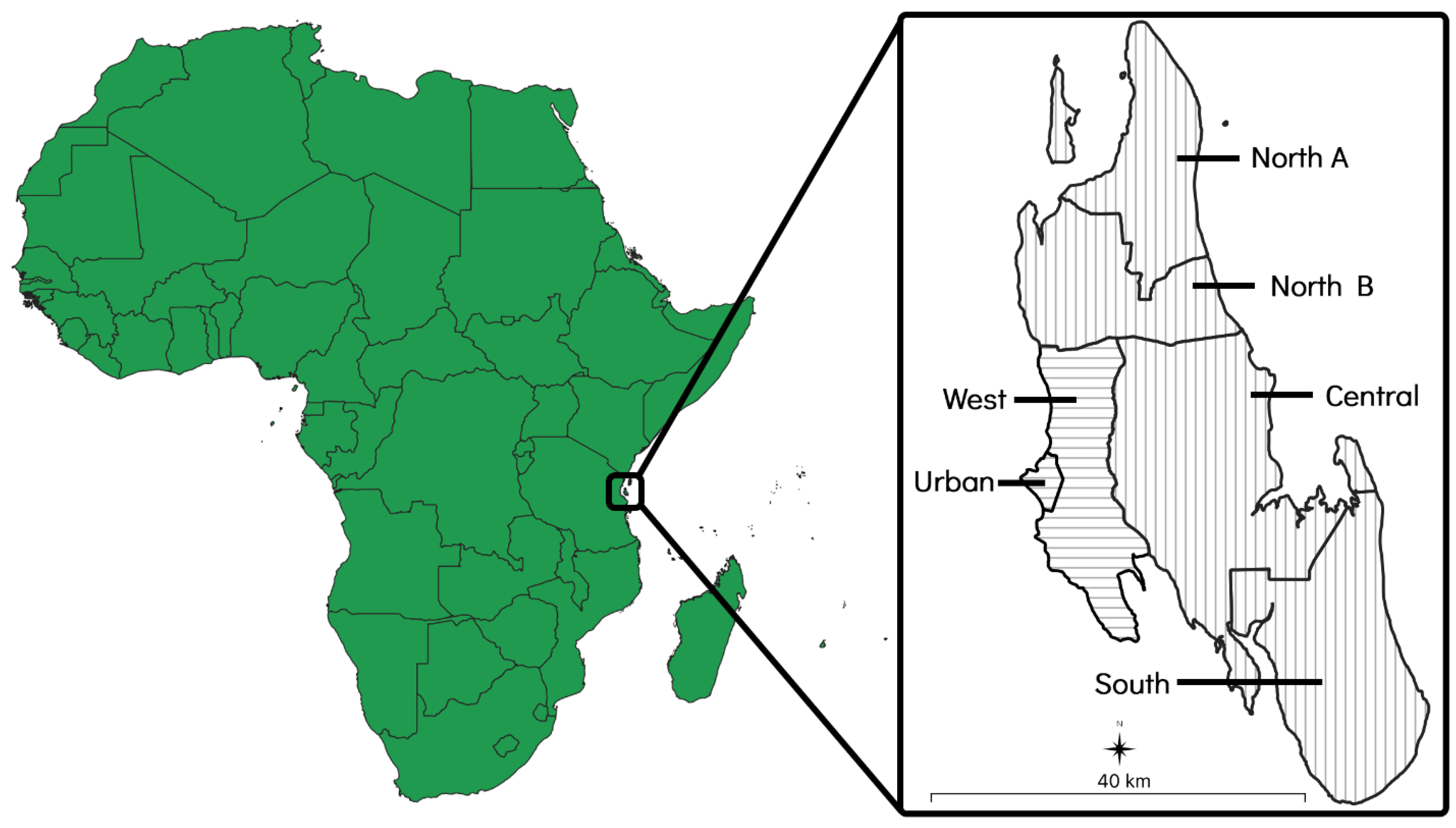
2.2. Project Site
2.3. Development of the Standard Operating Procedure
- Samples collected from animals involved in human exposures are expedited for diagnostic confirmation using a WOAH-recommended diagnostic assay, regardless of the diagnostic screening result. However, if the LFD result is rabies-positive, disease intervention efforts, such as outbreak responses and dog vaccinations, can be implemented while diagnostic confirmation is pending. Again, in all human exposure cases, diagnostic confirmation is prioritised to ensure the shortest turnaround time that can practically be achieved.
- Samples collected from animals not involved in human exposures that are negative or inconclusive during the in-field diagnostic screening process are expedited for diagnostic confirmation using a WOAH-recommended diagnostic assay before any further action is taken (if necessary).
- Samples collected from animals not involved in human exposures that test positive during the in-field screening process are treated as rabies-positive so that disease intervention efforts can be implemented in a timely manner. Such LFD rabies-positive samples are still sent for diagnostic confirmation using a WOAH-recommended diagnostic assay.
2.4. Implementation of the ‘Rapid In-Field Diagnosis and Epidemiology of Rabies’ Toolkit in Zanzibar
2.4.1. Collection of Animal Brain Samples
2.4.2. In-Field Diagnostic Screening Using a Lateral Flow Device
2.4.3. Recording the Diagnostic Screening Results
2.5. Diagnostic Confirmation
2.6. Resolving Diagnostic Incongruities
2.7. Data Analysis
3. Results
3.1. Sample Submission and Distribution
3.2. Targeted Dog Vaccinations in Response to Diagnostic Screening Results
3.3. Statistical Analysis of the Diagnostic Efficacy of the ADTEC LFD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the Global Burden of Endemic Canine Rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Tidman, R.; Thumbi, S.M.; Wallace, R.; de Balogh, K.; Iwar, V.; Dieuzy-Labaye, I.; Song, J.; Shadomy, S.; Qiu, Y.; Torres, G.; et al. United Against Rabies Forum: The One Health Concept at Work. Front. Public Health 2022, 10, 854419. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations; World Organization for Animal Health; Global Alliance for Rabies Control. Zero by 30: The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030; WHO: Geneva, Switzerland, 2018; ISBN 9789241513838. [Google Scholar]
- Scott, T.P.; Coetzer, A.; Fahrion, A.S.; Nel, L.H. Addressing the Disconnect between the Estimated, Reported and True Rabies Data: The Development of a Regional African Rabies Bulletin. Front. Vet. Sci. 2017, 4, 18. [Google Scholar] [CrossRef]
- Lembo, T.; Hampson, K.; Kaare, M.T.; Ernest, E.; Knobel, D.L.; Kazwala, R.R.; Haydon, D.T.; Cleaveland, S. The Feasibility of Canine Rabies Elimination in Africa: Dispelling Doubts with Data. PLoS Negl. Trop. Dis. 2010, 4, e626. [Google Scholar] [CrossRef]
- Banyard, A.C.; Horton, D.L.; Freuling, C.; Müller, T.; Fooks, A.R. Control and Prevention of Canine Rabies: The Need for Building Laboratory-Based Surveillance Capacity. Antivir. Res. 2013, 98, 357–364. [Google Scholar] [CrossRef]
- Nel, L.H. Discrepancies in Data Reporting for Rabies, Africa. Emerg. Infect. Dis. 2013, 19, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Coetzer, A.; Scott, T.P.; Noor, K.; Gwenhure, L.F.; Nel, L.H. A Novel Integrated and Labile EHealth System for Monitoring Dog Rabies Vaccination Campaigns. Vaccines 2019, 7, 108. [Google Scholar] [CrossRef]
- Wallace, R.M.; Undurraga, E.A.; Blanton, J.D.; Cleaton, J.; Franka, R. Elimination of Dog-Mediated Human Rabies Deaths by 2030: Needs Assessment and Alternatives for Progress Based on Dog Vaccination. Front. Vet. Sci. 2017, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Franka, R.; Wallace, R. Rabies Diagnosis and Surveillance in Animals in the Era of Rabies Elimination. Rev. Sci. Tech. 2018, 37, 359–370. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Consultation on Rabies, Third Report; World Health Organization: Geneva, Switzerland, 2018; ISBN 9789241210218.
- Eggerbauer, E.; De Benedictis, P.; Hoffmann, B.; Mettenleiter, T.C. Evaluation of Six Commercially Available Rapid Immunochromatographic Tests for the Diagnosis of Rabies in Brain Material. PLoS Negl. Trop. Dis. 2016, 10, e0004776. [Google Scholar] [CrossRef]
- Duong, V.; Tarantola, A.; Ong, S.; Mey, C.; Choeung, R.; Ly, S.; Bourhy, H.; Dussart, P.; Buchy, P. Laboratory Diagnostics in Dog-Mediated Rabies–an Overview of Performance and a Proposed Strategy for Various Settings. Int. J. Infect. Dis. 2016, 46, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Coetzer, A.; Scott, T.P.; Sabeta, C.T.; Nel, L.H. Economic and feasibility comparison of the dRIT and DFA for decentralized rabies diagnosis in resource-limited settings: The use of Nigerian dog meat markets as a case study. PLoS Negl. Trop. Dis. 2020, 14, e0008088. [Google Scholar] [CrossRef]
- Naïssengar, K.; Oussiguere, A.; Madaye, E.; Mbaipago, N.; Mindekem, R.; Moyengar, R.; Madjadinan, A.; Ngandolo, R.; Zinsstag, J.; Léchenne, M. Challenges to Improved Animal Rabies Surveillance: Experiences from Pilot Implementation of Decentralized Diagnostic Units in Chad. Acta Trop. 2021, 221, 105984. [Google Scholar] [CrossRef]
- Mfuh, K.O.; Abanda, N.N.; Titanji, B.K. Strengthening Diagnostic Capacity in Africa as a Key Pillar of Public Health and Pandemic Preparedness. PLoS Glob. Public Health 2023, 3, e0001998. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health Rabies (Infection with Rabies Virus and Other Lyssaviruses). Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.18_RABIES.pdf (accessed on 18 April 2024).
- Klein, A.; Fahrion, A.; Finke, S.; Eyngor, M.; Novak, S.; Yakobson, B.; Ngoepe, E.; Phahladira, B.; Sabeta, C.; De Benedictis, P.; et al. Further Evidence of Inadequate Quality in Lateral Flow Devices Commercially Offered for the Diagnosis of Rabies. Trop. Med. Infect. Dis. 2020, 5, 13. [Google Scholar] [CrossRef]
- Kimitsuki, K.; Saito, N.; Yamada, K.; Park, C.H.; Inoue, S.; Suzuki, M.; Saito-Obata, M.; Kamiya, Y.; Manalo, D.L.; Demetria, C.S.; et al. Evaluation of the Diagnostic Accuracy of Lateral Flow Devices as a Tool to Diagnose Rabies in Post-Mortem Animals. PLoS Negl. Trop. Dis. 2020, 14, e0008844. [Google Scholar] [CrossRef]
- Servat, A.; Robardet, E.; Cliquet, F. An Inter-Laboratory Comparison to Evaluate the Technical Performance of Rabies Diagnosis Lateral Flow Assays. J. Virol. Methods 2019, 272, 113702. [Google Scholar] [CrossRef]
- Servat, A.; Picard-Meyer, E.; Robardet, E.; Muzniece, Z.; Must, K.; Cliquet, F. Evaluation of a Rapid Immunochromatographic Diagnostic Test for the Detection of Rabies from Brain Material of European Mammals. Biologicals 2012, 40, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Audu, S.W.; Adawa, D.A.Y.; Mshelbwala, P.P.; Simon, Y.A.; Yakubu, B. Field and Laboratory Detection of Rabies Antigens in Saliva and Brains of Dogs in Nigeria: An Approach Using Rapid Immunochromatographic Test. J. Microbes Microbiol. Technol. 2017, 1, 104. [Google Scholar]
- Tenzin, T.; Lhamo, K.; Rai, P.B.; Tshering, D.; Jamtsho, P.; Namgyal, J.; Wangdi, T.; Letho, S.; Rai, T.; Jamtsho, S.; et al. Evaluation of a Rapid Immunochromatographic Test Kit to the Gold Standard Fluorescent Antibody Test for Diagnosis of Rabies in Animals in Bhutan. BMC Vet. Res. 2020, 16, 183. [Google Scholar] [CrossRef]
- Debbarma, B.; Dutta, J.B.; Isloor, S.; Baishya, B.C.H.; Boro, P.K.R.; Das, T. Incidence of Rabies in Animals of Kamrup Metro District of Assam, India Incidence of Rabies in Animals of Kamrup Metro District of Assam, India. Pharma Innov. J. 2023, 12, 169–172. [Google Scholar]
- Lechenne, M.; Naissengar, K.; Lampelletier, A.; Alfarouk, I.O.; Bourhy, H.; Zinsstag, J.; Dacheux, L. Validation of a Rapid Rabies Diagnostic Tool for Field Surveillance in Developing Countries. PLoS Neglected Trop. Dis. 2016, 10, e0005010. [Google Scholar] [CrossRef]
- Yale, G.; Gibson, A.D.; Mani, R.S.; Harsha, P.K.; Costa, N.C.; Corfmat, J.; Otter, I.; Otter, N.; Handel, I.G.; Bronsvoort, B.M.; et al. Evaluation of an Immunochromatographic Assay as a Canine Rabies Surveillance Tool in Goa, India. Viruses 2019, 11, 649. [Google Scholar] [CrossRef]
- Govindaiah Scholar, K.; Govindaiah, K.; Lakshman, D.; Shrikrishna, I.; Doddamane, R.; Ramakrishnaiah, S.; Doddappaiah, N.H.; Munivenkatappa, B.S.; Dasappa Gupta, V.M.; Gongal, G.; et al. Comparative Evaluation of Lateral Flow Assay with Direct Fluorescent Antibody Assay for Surveillance of Rabies in Animals in India. Pharma Innov. J. 2022, 11, 883–887. [Google Scholar]
- Mshelbwala, P.P.; Abdullahi, S.U.; Maikai, B.V.; Onyuche, E.T.; Ogunkoya, A.B. Evaluation of Two Rapid Diagnostic Tests for Rabies Diagnosis under Field and Laboratory Conditions in Nigeria. J. Vaccines Vaccin. 2012, 6, 6–10. [Google Scholar] [CrossRef]
- Freuling, C.M.; van der Westhuizen, J.; Khaiseb, S.; Tenzin, T.; Müller, T. From Field Tests to Molecular Tools—Evaluating Diagnostic Tests to Improve Rabies Surveillance in Namibia. Viruses 2023, 15, 371. [Google Scholar] [CrossRef]
- Certoma, A.; Lunt, R.A.; Vosloo, W.; Smith, I.; Colling, A.; Williams, D.T.; Tran, T.; Blacksell, S.D. Assessment of a Rabies Virus Rapid Diagnostic Test for the Detection of Australian Bat Lyssavirus. Trop. Med. Infect. Dis. 2018, 3, 109. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Fernández, J.C.; Salas-Rojas, C.; Estrella-Morales, J.; González, R.; Cordero-Solorzano, J.M.; Aguilar-Arguedas, O.; Soto-Araya, Y.; Perez Villalobos, D.; Leon, B. Evaluation of a Rapid Immunochromatographic Assay for the Diagnosis of Rabies in Regional Laboratories of Costa Rica. Vet. México OA 2023, 10, 1–11. [Google Scholar] [CrossRef]
- Cruz, J.L.; Garcia, A.M.; Saito, N.; Lagayan, M.G.O.; Dela Peña, R.C.; Usana, M.S.; Agustin, S.P.; Tattao, J.Z.; Mamauag, C.V.; Ducayag, O.P.; et al. Evaluation of Lateral Flow Devices for Postmortem Rabies Diagnosis in Animals in the Philippines: A Multicenter Study. J. Clin. Microbiol. 2023, 61, e0084223. [Google Scholar] [CrossRef]
- Mananggit, M.; Manalo, D.; Saito, N.; Kimitsuki, K.; Garcia, A.; LacanilaoI, P.; Ongtangco, J.; VelascoI, C.; del Rosario, M.; Lagayan, M.; et al. Lateral Flow Devices for Samples Collected by Straw Sampling Method for Postmortem Canine Rabies Diagnosis. PLoS Negl. Trop. Dis. 2021, 15, e0009891. [Google Scholar] [CrossRef]
- Voehl, K.M.; Saturday, G.A. Evaluation of a Rapid Immunodiagnostic Rabies Field Surveillance Test on Samples Collected from Military Operations in Africa, Europe, and the Middle East. Army Med. Dep. J. 2014, 27–32. [Google Scholar] [PubMed]
- Magembe, S.R. Epidemiology of Rabies in the United Republic of Tanzania. In Rabies in the Tropics; Kuwert, E., Mérieux, C., Koprowski, H., Bögel, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 392–398. [Google Scholar]
- Lushasi, K.S.; Cleaveland, S.; Changalucha, J.J.; Haydon, D.; Kazwala, R.; Lembo, T.; Masoud, M.; Maziku, M.; Mchau, G.; Mtema, Z.; et al. Progress towards Rabies Elimination from Pemba Island, Southern Tanzania. Online J. Public Health Inform. 2017, 9, e118. [Google Scholar] [CrossRef]
- Tanzania National Bureau of Statistics and President’s Office. The 2022 Population and Housing Census: Administrative Units Population Distribution Report; Tanzania National Bureau of Statistics and President’s Office: Dodoma, Tanzania, 2022.
- Barrat, J. Simple Technique for the Collection and Shipment of Brain Specimens for Rabies Diagnosis. In Laboratory Techniques in Rabies; Meslin, F.-X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organization: Geneva, Switzerland, 1996; pp. 425–432. [Google Scholar]
- Surveillance Tools. Available online: https://rabiesalliance.org/tools/surveillance-tools (accessed on 1 June 2024).
- Coetzer, A.; Nel, L.H.; Rupprecht, C. Demonstration of Lyssavirus Antigens by a Direct Rapid Immunohistochemical Test; Academic Press: Cambridge, MA, USA, 2014; Volume 1, ISBN 9780128004654. [Google Scholar]
- Real Statistics Using Excel. Available online: www.real-statistics.com (accessed on 17 January 2024).
- Minghui, R.; Stone, M.; Semedo, M.H.; Nel, L. New Global Strategic Plan to Eliminate Dog-Mediated Rabies by 2030. Lancet Glob. Health 2018, 6, e828–e829. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health. Infection with Rabies Virus. In Terrestrial Animal Health Code; WOAH: Paris, France, 2023. [Google Scholar]
- Mauti, S.; Léchenne, M.; Naïssengar, S.; Traoré, A.; Kallo, V.; Kouakou, C.; Couacy-Hymann, E.; Gourlaouen, M.; Mbilo, C.; Pyana, P.P.; et al. Field Postmortem Rabies Rapid Immunochromatographic Diagnostic Test for Resource-Limited Settings with Further Molecular Applications. J. Vis. Exp. 2020, 160, e60008. [Google Scholar] [CrossRef]



| Reason for Diagnostic Screening | Rabies Positive (%) | Rabies Negative (%) |
|---|---|---|
| Animal was showing signs of rabies and was humanely euthanised | 17 (20%) | 38 (46%) |
| Animal was showing signs of rabies and was killed by community members | 3 (4%) | 4 (5%) |
| Roadkill | 0 | 16 (19%) |
| Animal found dead | 0 | 5 (6%) |
| True Positive | False Positive | True Negative | False Negative | Diagnostic Sensitivity (95% CI) | Diagnostic Specificity (95% CI) | |
|---|---|---|---|---|---|---|
| DRIT | 20 | 0 | 63 | 0 | 100% (83.16–100%) | 100% (94.31–100%) |
| ADTEC LFD | 19 | 0 | 63 | 1 | 95% (75.13–99.87%) | 100% (94.31–100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moh’d, A.Z.; Coetzer, A.; Malan, A.J.; Scott, T.P.; Ramadhan, R.J.; Wright, N.; Nel, L.H. Investigating the Impact That Diagnostic Screening with Lateral Flow Devices Had on the Rabies Surveillance Program in Zanzibar, Tanzania. Microorganisms 2024, 12, 1314. https://doi.org/10.3390/microorganisms12071314
Moh’d AZ, Coetzer A, Malan AJ, Scott TP, Ramadhan RJ, Wright N, Nel LH. Investigating the Impact That Diagnostic Screening with Lateral Flow Devices Had on the Rabies Surveillance Program in Zanzibar, Tanzania. Microorganisms. 2024; 12(7):1314. https://doi.org/10.3390/microorganisms12071314
Chicago/Turabian StyleMoh’d, Ali Z., Andre Coetzer, Ayla J. Malan, Terence P. Scott, Ramadhan J. Ramadhan, Nicolette Wright, and Louis H. Nel. 2024. "Investigating the Impact That Diagnostic Screening with Lateral Flow Devices Had on the Rabies Surveillance Program in Zanzibar, Tanzania" Microorganisms 12, no. 7: 1314. https://doi.org/10.3390/microorganisms12071314
APA StyleMoh’d, A. Z., Coetzer, A., Malan, A. J., Scott, T. P., Ramadhan, R. J., Wright, N., & Nel, L. H. (2024). Investigating the Impact That Diagnostic Screening with Lateral Flow Devices Had on the Rabies Surveillance Program in Zanzibar, Tanzania. Microorganisms, 12(7), 1314. https://doi.org/10.3390/microorganisms12071314







