The Value of Ursodeoxycholic Acid and Mesenchymal Stem Cells in the Treatment of Severe COVID-19
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Data Source
2.3. Statistical Analysis
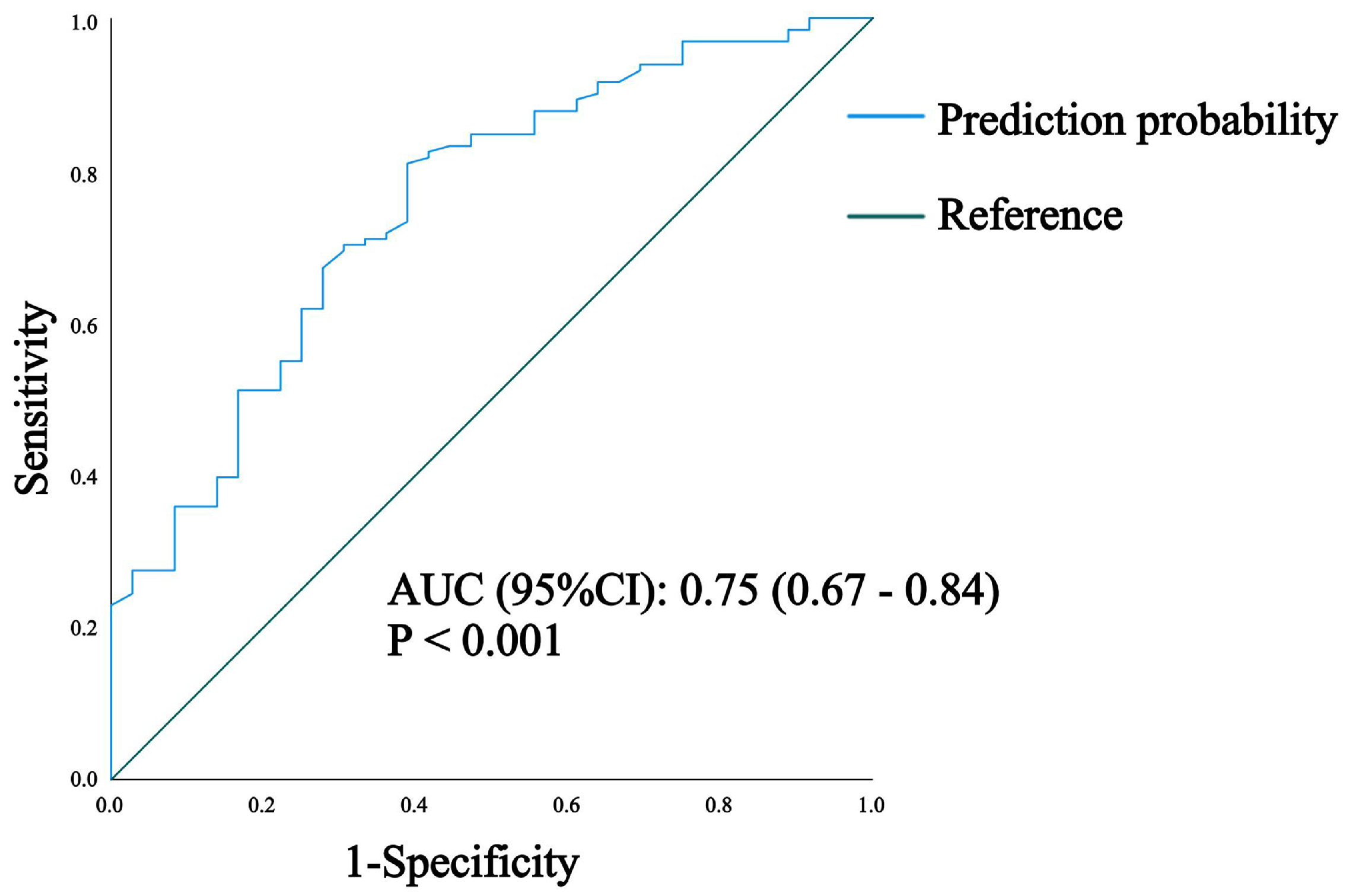
3. Results
3.1. Patients Characteristics
3.2. Results of Mortality Risk Analysis in Patients with Severe COVID-19
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, D.Y.; Abi Fadel, F.; Huang, S.; Milinovich, A.T.; Sacha, G.L.; Bartley, P.; Duggal, A.; Wang, X. Nirmatrelvir or Molnupiravir Use and Severe Outcomes From Omicron Infections. JAMA Netw. Open 2023, 6, e2335077. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-González, G.; Alamilla-Sánchez, M.; García-Macas, V.; Herrera-Acevedo, J.; Villalobos-Brito, M.; Tapia-Rangel, E.; Maldonado-Tapia, D.; López-Mendoza, M.; Cano-Cervantes, J.H.; Orozco-Vázquez, J.; et al. Impact of plasmapheresis on severe COVID-19. Sci. Rep. 2023, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Ehianeta, T.; Mzee, S.A.S.; Adebisi, M.K.; Ehianeta, O. Recent SARS-CoV-2 Outlook and Implications in a COVID-19 Vaccination Era. Infect. Microbes Dis. 2021, 3, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Montero, M.T.V.; Rowe, K.; Kirton, R.; Kunik, F., Jr. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: A review of current evidence. Expert Rev. Clin. Pharmacol. 2021, 14, 601–621. [Google Scholar] [CrossRef]
- Ngo, B.T.; Marik, P.; Kory, P.; Shapiro, L.; Thomadsen, R.; Iglesias, J.; Ditmore, S.; Rendell, M.; Varon, J.; Dubé, M.; et al. The time to offer treatments for COVID-19. Expert Opin. Investig. Drugs 2021, 30, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Vegivinti, C.T.R.; Evanson, K.W.; Lyons, H.; Akosman, I.; Barrett, A.; Hardy, N.; Kane, B.; Keesari, P.R.; Pulakurthi, Y.S.; Sheffels, E.; et al. Efficacy of antiviral therapies for COVID-19: A systematic review of randomized controlled trials. BMC Infect. Dis. 2022, 22, 107. [Google Scholar] [CrossRef]
- Montori, M.; Baroni, G.S.; Santori, P.; Di Giampaolo, C.; Ponziani, F.; Abenavoli, L.; Scarpellini, E. Liver Damage and COVID-19: At Least a “Two-Hit” Story in Systematic Review. Curr. Issues Mol. Biol. 2023, 45, 3035–3047. [Google Scholar] [CrossRef]
- Ferretti, M.T.; Martinkova, J.; Biskup, E.; Benke, T.; Gialdini, G.; Nedelska, Z.; Rauen, K.; Mantua, V.; Religa, D.; Hort, J.; et al. Sex and gender differences in Alzheimer’s disease: Current challenges and implications for clinical practice: Position paper of the Dementia and Cognitive Disorders Panel of the European Academy of Neurology. Eur. J. Neurol. 2020, 27, 928–943. [Google Scholar] [CrossRef]
- Luo, W.; He, Y.; Wei, M.G.; Lu, G.B.; Yi, Q. Paxlovid-tacrolimus drug-drug interaction caused severe diarrhea that induced combined diabetic ketoacidosis and a hyperglycemic hyperosmolar state in a kidney transplant patient: A case report. J. Med. Case Rep. 2023, 17, 406. [Google Scholar] [CrossRef]
- Cabrera, D.; Arab, J.P.; Arrese, M. UDCA, NorUDCA, and TUDCA in Liver Diseases: A Review of Their Mechanisms of Action and Clinical Applications. Handb. Exp. Pharmacol. 2019, 256, 237–264. [Google Scholar] [PubMed]
- Brevini, T.; Maes, M.; Webb, G.J.; John, B.V.; Fuchs, C.D.; Buescher, G.; Wang, L.; Griffiths, C.; Brown, M.L.; Scott, W.E., 3rd; et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature 2023, 615, 134–142. [Google Scholar] [CrossRef] [PubMed]
- John, B.V.; Bastaich, D.; Webb, G.; Brevini, T.; Moon, A.; Ferreira, R.D.; Chin, A.M.; Kaplan, D.E.; Taddei, T.H.; Serper, M.; et al. Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis. J. Intern. Med. 2023, 293, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, F.; Angelotti, G.; Masetti, C.; Shiffer, D.; Pugliese, N.; De Nicola, S.; Carella, F.; Desai, A.; Ormas, M.; Calatroni, M.; et al. Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients. Viruses 2023, 15, 1738. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Niu, S.; Guo, B.; Gao, T.; Wang, L.; Wang, Y.; Wang, L.; Tan, Y.; Wu, J.; Hao, J. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020, 53, e12939. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, C.; Chen, L.; Tang, L.; Zhu, Y.; Xu, X.; Chen, L.; Gao, H.; Lu, X.; Yu, L.; et al. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering 2020, 6, 1153–1161. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Couto, P.S.; Al-Arawe, N.; Filgueiras, I.S.; Fonseca, D.L.M.; Hinterseher, I.; Catar, R.A.; Chinnadurai, R.; Bersenev, A.; Cabral-Marques, O.; Moll, G.; et al. Systematic review and meta-analysis of cell therapy for COVID-19: Global clinical trial landscape, published safety/efficacy outcomes, cell product manufacturing and clinical delivery. Front. Immunol. 2023, 14, 1200180. [Google Scholar] [CrossRef]
- Thuy, P.X.; Bao, T.D.D.; Moon, E.Y. Ursodeoxycholic acid ameliorates cell migration retarded by the SARS-CoV-2 spike protein in BEAS-2B human bronchial epithelial cells. Biomed. Pharmacother. 2022, 150, 113021. [Google Scholar] [CrossRef]
- Vitiello, A.; Troiano, V.; La Porta, R. What will be the role of molnupiravir in the treatment of COVID-19 infection? Drugs Ther. Perspect. 2021, 37, 579–580. [Google Scholar] [CrossRef]
- Singla, S.; Goyal, S. Antiviral activity of molnupiravir against COVID-19: A schematic review of evidences. Bull. Natl. Res. Cent. 2022, 46, 62. [Google Scholar] [CrossRef] [PubMed]
- Donovan-Banfield, I.; Penrice-Randal, R.; Goldswain, H.; Rzeszutek, A.M.; Pilgrim, J.; Bullock, K.; Saunders, G.; Northey, J.; Dong, X.; Ryan, Y.; et al. Characterisation of SARS-CoV-2 genomic variation in response to molnupiravir treatment in the AGILE Phase IIa clinical trial. Nat. Commun. 2022, 13, 7284. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Neilsen, G.; Slack, R.L.; Cantara, W.A.; Castaner, A.E.; Lorson, Z.C.; Lulkin, N.; Zhang, H.; Lee, J.; Cilento, M.E.; et al. Nirmatrelvir Resistance in SARS-CoV-2 Omicron_BA.1 and WA1 Replicons and Escape Strategies. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tong, X.; Keung, W.; Arnold, L.D.; Stevens, L.J.; Pruijssers, A.J.; Kook, S.; Lopatin, U.; Denison, M.; Kwong, A.D. Evaluation of in vitro antiviral activity of SARS-CoV-2 M(pro) inhibitor pomotrelvir and cross-resistance to nirmatrelvir resistance substitutions. Antimicrob. Agents Chemother. 2023, 67, e0084023. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Iles, T.; Ikramuddin, S.; Steer, C.J. Merit of an Ursodeoxycholic Acid Clinical Trial in COVID-19 Patients. Vaccines 2020, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Abdulrab, S.; Al-Maweri, S.; Halboub, E. Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID-19-associated cytokine storm. Med. Hypotheses 2020, 143, 109897. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Wecht, S.; Rojas, M. Mesenchymal stem cells in the treatment of chronic lung disease. Respirology 2016, 21, 1366–1375. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]

| Variable | Total (n = 167) | Control Group (n = 125) | UDCA Group (n = 42) | p-Value | |
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Age (years) (Median, IQR) | 76.00 (65.00, 86.50) | 77.00 (66.00, 87.00) | 72.50 (63.00, 84.75) | 0.420 | |
| Gender (n, %) | 0.658 | ||||
| Male | 112 (67.07) | 85 (68.00) | 27 (64.29) | ||
| Female | 55 (32.93) | 40 (32.00) | 15 (35.71) | ||
| Symptoms (n, %) | |||||
| Fever | 86 (51.50) | 65 (52.00) | 21 (50.00) | 0.822 | |
| Chill | 4 (2.40) | 2 (1.60) | 2 (4.76) | 0.564 | |
| Cough | 79 (47.31) | 62 (49.60) | 17 (40.48) | 0.306 | |
| Pharyngalgia | 8 (4.79) | 7 (5.60) | 1 (2.38) | 0.669 | |
| Myalgia | 6 (3.59) | 5 (4.00) | 1 (2.38) | 0.993 | |
| Unconsciousness | 26 (15.57) | 21 (16.80) | 5 (11.90) | 0.499 | |
| Stomachache | 5 (2.99) | 2 (1.60) | 3 (7.14) | 0.193 | |
| Nausea | 5 (2.99) | 4 (3.20) | 1 (2.38) | 1.000 | |
| Vomiting | 6 (3.59) | 5 (4.00) | 1 (2.38) | 0.993 | |
| Diarrhea | 2 (1.20) | 2 (1.60) | 0 (0.00) | 1.000 | |
| Chest Tightness | 41 (24.55) | 37 (29.60) | 4 (9.52) | 0.009 | |
| Comorbidities (n, %) | |||||
| Hypertension | 108 (64.67) | 85 (68.00) | 23 (54.76) | 0.120 | |
| Diabetes | 68 (40.72) | 54 (43.20) | 14 (33.33) | 0.260 | |
| Cancer | 48 (28.74) | 30 (24.00) | 18 (42.86) | 0.019 | |
| NSD | 46 (27.54) | 33 (26.40) | 13 (30.95) | 0.568 | |
| Cardiovascular diease | 33 (19.76) | 26 (20.80) | 7 (16.67) | 0.561 | |
| Nephrosis | 39 (23.35) | 31 (24.80) | 8 (19.05) | 0.446 | |
| Hepatopathy | 28 (16.77) | 12 (9.60) | 16 (38.10) | 0.001 | |
| COPD | 16 (9.58) | 13 (10.40) | 3 (7.14) | 0.751 | |
| CBC (Median, IQR) | |||||
| Neutrophil Ratio (%) | 86.90 (77.55, 91.40) | 88.10 (79.00, 92.00) | 83.80 (76.95, 90.38) | 0.055 | |
| Lymphocyte Ratio (%) | 7.00 (4.05, 13.40) | 7.00 (3.90, 13.10) | 7.45 (4.28, 15.55) | 0.319 | |
| Monocyte Ratio (%) | 5.20 (2.55, 8.40) | 4.80 (2.40, 8.10) | 5.85 (3.50, 9.88) | 0.025 | |
| Eosinophil Ratio (%) | 0.00 (0.00, 0.20) | 0.00 (0.00, 0.10) | 0.20 (0.00, 0.95) | 0.001 | |
| Basophil Ratio (%) | 0.20 (0.10, 0.30) | 0.20 (0.10, 0.20) | 0.20 (0.10, 0.40) | 0.202 | |
| Neutrophil (10E9/L) | 6.20 (3.70, 9.05) | 6.60 (3.80, 9.30) | 5.25 (2.80, 8.05) | 0.040 | |
| Lymphocyte (10E9/L) | 0.50 (0.30, 0.80) | 0.50 (0.30, 0.80) | 0.50 (0.30, 0.88) | 0.931 | |
| Monocyte (10E9/L) | 0.40 (0.20, 0.60) | 0.40 (0.20, 0.60) | 0.40 (0.20, 0.67) | 0.463 | |
| Eosinophil (10E9/L) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) | 0.01 (0.00, 0.05) | 0.001 | |
| CRP (mg/L) (Median, IQR) | 70.60 (21.55, 127.05) | 77.40 (25.00, 141.60) | 45.30 (18.62, 93.73) | 0.070 | |
| Biochemistry (Median, IQR) | |||||
| ALT (U/L) | 26.00 (16.00, 41.50) | 25.00 (15.00, 42.00) | 27.00 (18.00, 41.00) | 0.535 | |
| AST (U/L) | 35.00 (23.00, 60.50) | 36.00 (23.00, 63.00) | 34.00 (20.50, 55.75) | 0.527 | |
| AST/ALT ratio | 1.50 (1.00, 2.30) | 1.60 (1.00, 2.30) | 1.50 (1.02, 2.18) | 0.701 | |
| Serum creatinine (μmol/L) | 100.00 (63.00, 194.00) | 119.00 (71.00, 212.00) | 66.50 (55.25, 100.00) | 0.001 | |
| Treatment (n, %) | |||||
| MSCs | 17 (10.18) | 14 (11.20) | 3 (7.14) | 0.647 | |
| Glucocorticoids | 150 (89.82) | 112 (89.60) | 38 (90.48) | 1.000 | |
| Antibiotics | 164 (98.20) | 122 (97.60) | 42 (100.00) | 0.573 | |
| Antivirals | 65 (38.92) | 48 (38.40) | 17 (40.48) | 0.811 | |
| Antifungal Drugs | 108 (64.67) | 81 (64.80) | 27 (64.29) | 0.952 | |
| Probiotics | 68 (40.72) | 50 (40.00) | 18 (42.86) | 0.744 | |
| Blood purification | 96 (57.49) | 72 (57.60) | 24 (57.14) | 0.791 | |
| Variable | Total (n = 167) | Control Group (n = 150) | MSCs Group (n = 17) | p-Value | |
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Age (years) (Median, IQR) | 76.00 (65.00, 86.50) | 76.00 (63.25, 87.00) | 77.00 (71.00, 81.00) | 0.695 | |
| Gender (n, %) | 0.744 | ||||
| Male | 112 (67.07) | 100 (66.67) | 12 (70.59) | ||
| Female | 55 (32.93) | 50 (33.33) | 5 (29.41) | ||
| Symptoms (n, %) | |||||
| Fever | 86 (51.50) | 77 (51.33) | 9 (52.94) | 0.900 | |
| Chill | 4 (2.40) | 3 (2.00) | 1 (5.88) | 0.352 | |
| Cough | 79 (47.31) | 68 (45.33) | 11 (64.71) | 0.129 | |
| Pharyngalgia | 8 (4.79) | 8 (5.33) | 0 (0.00) | 1.000 | |
| Myalgia | 6 (3.59) | 4 (2.67) | 2 (11.76) | 0.115 | |
| Unconsciousness | 26 (15.57) | 26 (17.33) | 0 (0.00) | 0.130 | |
| Stomachache | 5 (2.99) | 5 (3.33) | 0 (0.00) | 1.000 | |
| Nausea | 5 (2.99) | 4 (2.67) | 1 (5.88) | 0.419 | |
| Vomiting | 6 (3.59) | 6 (4.00) | 0 (0.00) | 1.000 | |
| Diarrhea | 2 (1.20) | 2 (1.33) | 0 (0.00) | 1.000 | |
| Chest Tightness | 41 (24.55) | 39 (26.00) | 2 (11.76) | 0.320 | |
| Comorbidities (n, %) | |||||
| Hypertension | 108 (64.67) | 97 (64.67) | 11 (64.71) | 0.997 | |
| Diabetes | 68 (40.72) | 59 (39.33) | 9 (52.94) | 0.279 | |
| Cancer | 48 (28.74) | 43 (28.67) | 5 (29.41) | 1.000 | |
| NSD | 46 (27.54) | 42 (28.00) | 4 (23.53) | 0.917 | |
| Cardiovascular diease | 33 (19.76) | 31 (20.67) | 2 (11.76) | 0.581 | |
| Nephrosis | 39 (23.35) | 36 (24.00) | 3 (17.65) | 0.776 | |
| Hepatopathy | 28 (16.77) | 26 (17.33) | 2 (11.76) | 0.810 | |
| COPD | 16 (9.58) | 14 (9.33) | 2 (11.76) | 1.000 | |
| CBC (Median, IQR) | |||||
| Neutrophil Ratio (%) | 86.90 (77.55, 91.40) | 86.85 (77.12, 91.40) | 90.00 (85.40, 92.30) | 0.155 | |
| Lymphocyte Ratio (%) | 7.00 (4.05, 13.40) | 7.10 (4.12, 14.30) | 5.40 (3.40, 7.40) | 0.170 | |
| Monocyte Ratio (%) | 5.20 (2.55, 8.40) | 5.25 (2.52, 8.47) | 5.00 (2.70, 7.80) | 0.477 | |
| Eosinophil Ratio (%) | 0.00 (0.00, 0.20) | 0.00 (0.00, 0.20) | 0.00 (0.00, 0.30) | 0.974 | |
| Basophil Ratio (%) | 0.20 (0.10, 0.30) | 0.20 (0.10, 0.30) | 0.20 (0.10, 0.20) | 0.757 | |
| Neutrophil (10E9/L) | 6.20 (3.70, 9.05) | 6.05 (3.52, 8.88) | 6.60 (5.80, 10.80) | 0.072 | |
| Lymphocyte (10E9/L) | 0.50 (0.30, 0.80) | 0.50 (0.30, 0.88) | 0.50 (0.40, 0.80) | 0.934 | |
| Monocyte (10E9/L) | 0.40 (0.20, 0.60) | 0.35 (0.20, 0.60) | 0.50 (0.30, 0.60) | 0.280 | |
| Eosinophil (10E9/L) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.03) | 0.690 | |
| CRP (mg/L)(Median, IQR) | 70.60 (21.55, 127.05) | 68.50 (22.30, 130.47) | 70.60 (19.70, 107.00) | 0.564 | |
| Biochemistry (Median, IQR) | |||||
| ALT (U/L) | 26.00 (16.00, 41.50) | 25.00 (15.25, 40.00) | 47.00 (23.00, 57.00) | 0.048 | |
| AST (U/L) | 35.00 (23.00, 60.50) | 35.00 (23.00, 60.75) | 37.00 (17.00, 54.00) | 0.721 | |
| AST/ALT ratio | 1.50 (1.00, 2.30) | 1.60 (1.10, 2.40) | 1.00 (0.70, 1.30) | 0.001 | |
| Serum creatinine (μmol/L) | 100.00 (63.00, 194.00) | 100.50 (63.00, 199.50) | 84.00 (65.00, 139.00) | 0.529 | |
| Treatment (n, %) | |||||
| UDCA | 42 (25.15) | 39 (26.00) | 3 (17.65) | 0.647 | |
| Glucocorticoids | 150 (89.82) | 133 (88.67) | 17 (100.00) | 0.298 | |
| Antibiotics | 164 (98.20) | 147 (98.00) | 17 (100.00) | 1.000 | |
| Antivirals | 65 (38.92) | 55 (36.67) | 10 (58.82) | 0.076 | |
| Antifungal Drugs | 108 (64.67) | 95 (63.33) | 13 (76.47) | 0.283 | |
| Probiotics | 68 (40.72) | 58 (38.67) | 10 (58.82) | 0.109 | |
| Blood purification | 96 (57.49) | 79 (52.67) | 17 (100) | 0.001 | |
| Variable | Beta | SE | p-Value | OR (95%CI) | RR (95%CI) |
|---|---|---|---|---|---|
| Age | 0.04 | 0.01 | 0.003 | 1.04 (1.01–1.07) | / |
| CRP | 0.00 | 0.00 | 0.250 | 1.00 (1.00–1.01) | / |
| Serum creatinine | 0.00 | 0.00 | 0.204 | 1.00 (1.00–1.00) | / |
| ALT | 0.01 | 0.01 | 0.444 | 1.01 (0.99–1.02) | |
| Sex | 1.151 (0.633–2.094) | ||||
| Female | 1.00 (Reference) | ||||
| Male | 0.18 | 0.39 | 0.647 | 1.20 (0.55–2.59) | |
| Liver disease | 1.612 (0.619–4.196) | ||||
| No | 1.00 (Reference) | ||||
| Yes | 0.58 | 0.58 | 0.310 | 1.79 (0.58–5.55) | |
| Use of MSCs | 0.397 (0.217–0.726) | ||||
| No | 1.00 (Reference) | ||||
| Yes | −1.35 | 0.53 | 0.011 | 0.26 (0.09–0.73) | |
| Use of UDCA | 0.528 (0.298–0.935) | ||||
| No | 1.00 (Reference) | ||||
| Yes | −0.85 | 0.40 | 0.035 | 0.43 (0.19–0.94) | |
| Use of antibiotics | 1.562 (0.307–7.948) | ||||
| No | 1.00 (Reference) | ||||
| Yes | 0.61 | 1.24 | 0.622 | 1.84 (0.16–20.92) | |
| Use of antivirals | 0.406 (0.224–0.734) | ||||
| No | 1.00 (Reference) | ||||
| Yes | −1.17 | 0.39 | 0.003 | 0.31 (0.15–0.67) | |
| Use of antifungal drugs | 0.805 (0.427–1.519) | ||||
| No | 1.00 (Reference) | ||||
| Yes | −0.27 | 0.40 | 0.499 | 0.76 (0.34–1.68) | |
| Use of probiotics | 0.962 (0.535–1.728) | ||||
| No | 1.00 (Reference) | ||||
| Yes | −0.05 | 0.38 | 0.896 | 0.95 (0.45–2.01) | |
| Use of blood purification | 0.890 (0.474–1.561) | ||||
| No | 1.00 (Reference) | ||||
| Yes | −0.19 | 0.38 | 0.620 | 0.83 (0.39–1.76) |
| Variable | Beta | SE | p-Value | OR (95%CI) |
|---|---|---|---|---|
| Age | 0.03 | 0.02 | 0.025 | 1.03 (1.01–1.07) |
| Use of MSCs | ||||
| No | 1.00 (Reference) | |||
| Yes | −1.57 | 0.58 | 0.007 | 0.21 (0.07–0.65) |
| Use of UDCA | ||||
| No | 1.00 (Reference) | |||
| Yes | −1.46 | 0.50 | 0.029 | 0.38 (0.16–0.91) |
| Use of antivirals | ||||
| No | 1.00 (Reference) | |||
| Yes | −0.73 | 0.44 | 0.097 | 0.48 (0.20–1.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Li, Y.; Sheng, G.; Li, L. The Value of Ursodeoxycholic Acid and Mesenchymal Stem Cells in the Treatment of Severe COVID-19. Microorganisms 2024, 12, 1269. https://doi.org/10.3390/microorganisms12071269
Zheng Q, Li Y, Sheng G, Li L. The Value of Ursodeoxycholic Acid and Mesenchymal Stem Cells in the Treatment of Severe COVID-19. Microorganisms. 2024; 12(7):1269. https://doi.org/10.3390/microorganisms12071269
Chicago/Turabian StyleZheng, Qi, Yuetong Li, Guoping Sheng, and Lanjuan Li. 2024. "The Value of Ursodeoxycholic Acid and Mesenchymal Stem Cells in the Treatment of Severe COVID-19" Microorganisms 12, no. 7: 1269. https://doi.org/10.3390/microorganisms12071269
APA StyleZheng, Q., Li, Y., Sheng, G., & Li, L. (2024). The Value of Ursodeoxycholic Acid and Mesenchymal Stem Cells in the Treatment of Severe COVID-19. Microorganisms, 12(7), 1269. https://doi.org/10.3390/microorganisms12071269






