Insights into the Gut Microbiome of the South American Leaf-Toed Gecko (Phylodactylus gerropygus) Inhabiting the Core of the Atacama Desert
Abstract
1. Introduction
2. Methodology
2.1. Site Information
2.2. Animal Trapping
2.3. Microbiome Analysis
2.3.1. Stool Sample Collection and DNA Isolation
2.3.2. Targeted Library Preparation
2.3.3. Control Samples
2.3.4. Sequencing and Bioinformatics Analysis
2.4. Statistical Analysis
3. Results
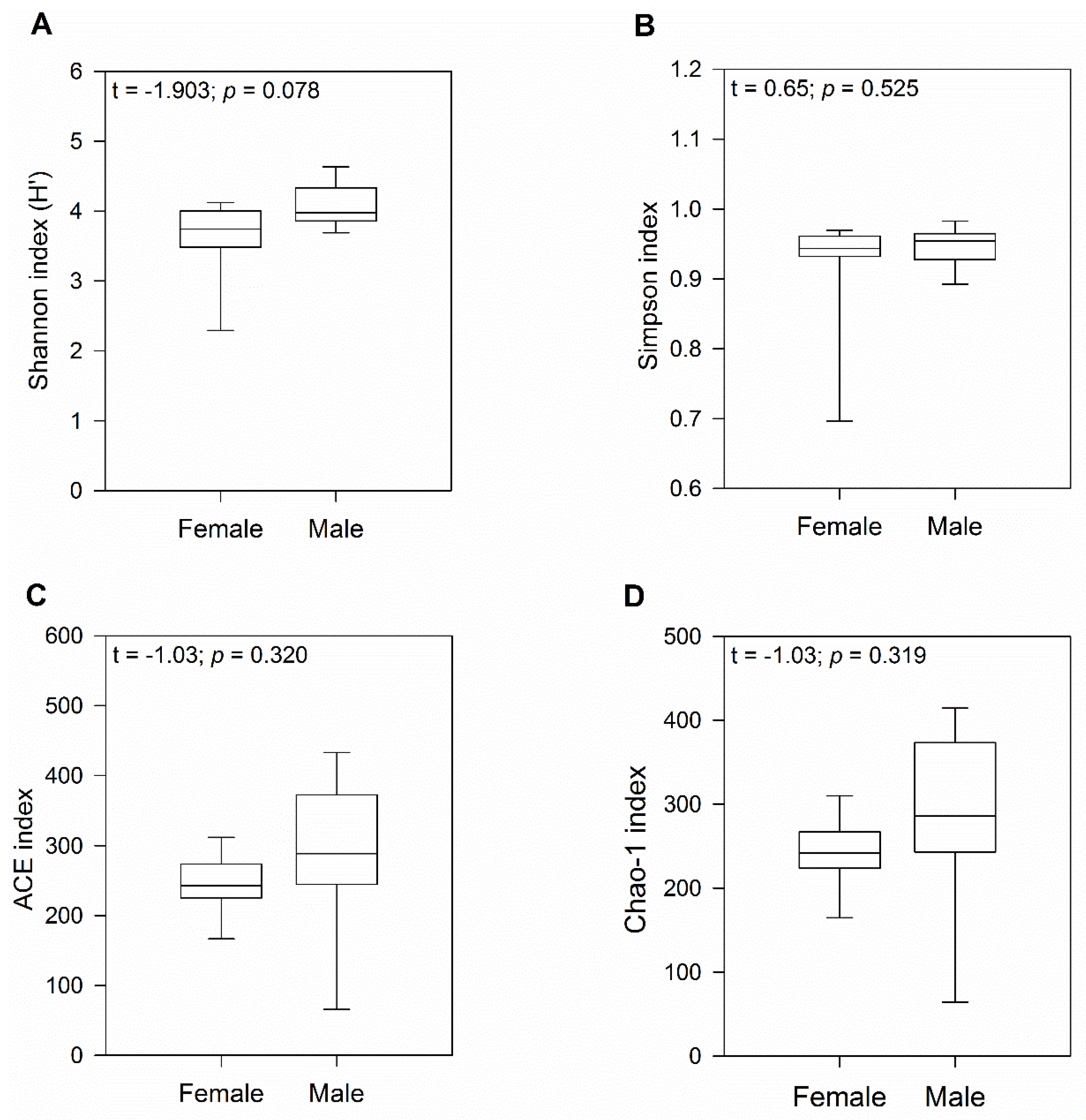
3.1. Diversity of the Gut Bacterial Communities Was Not Significantly Different between Female and Male Geckos
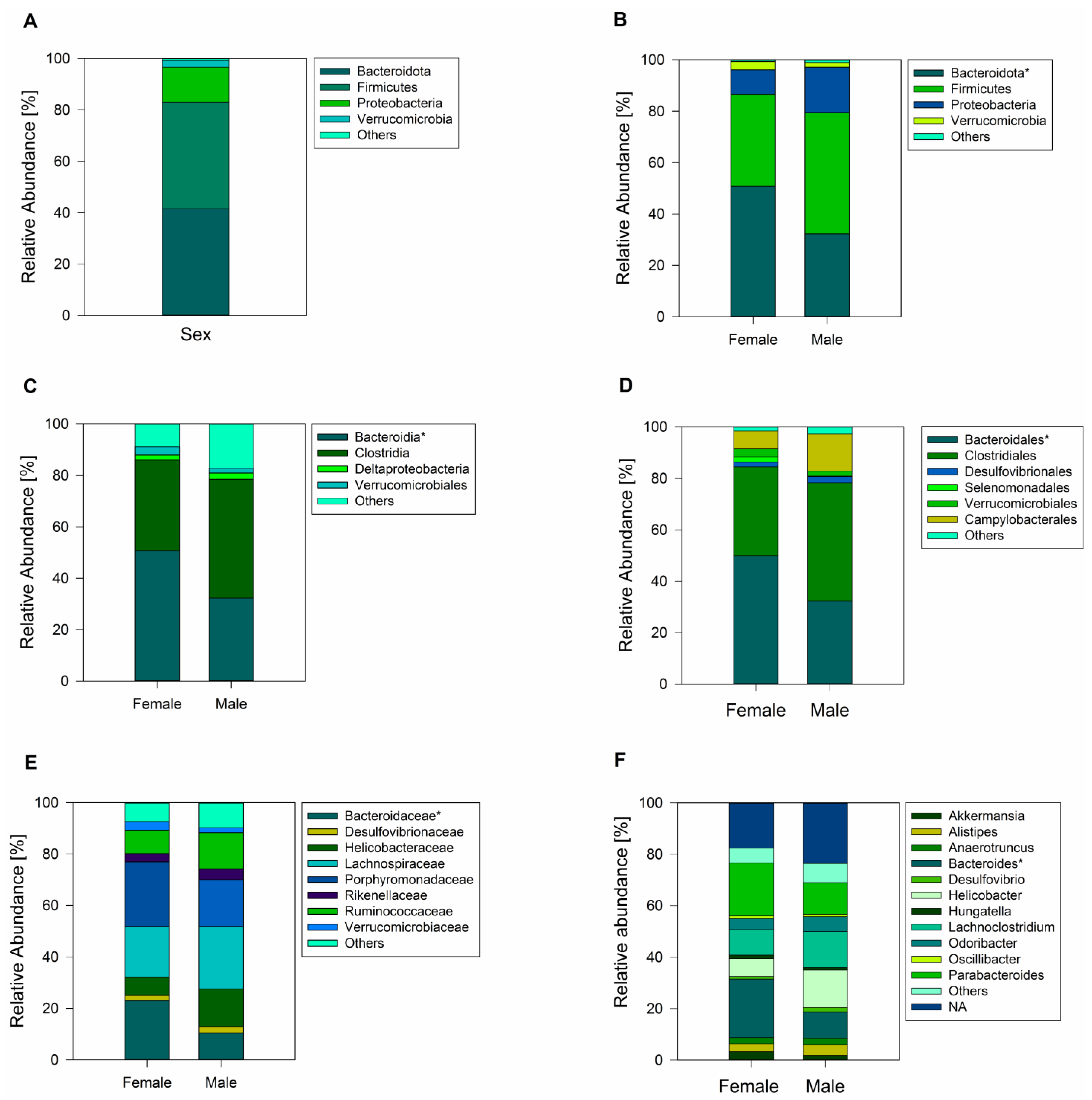
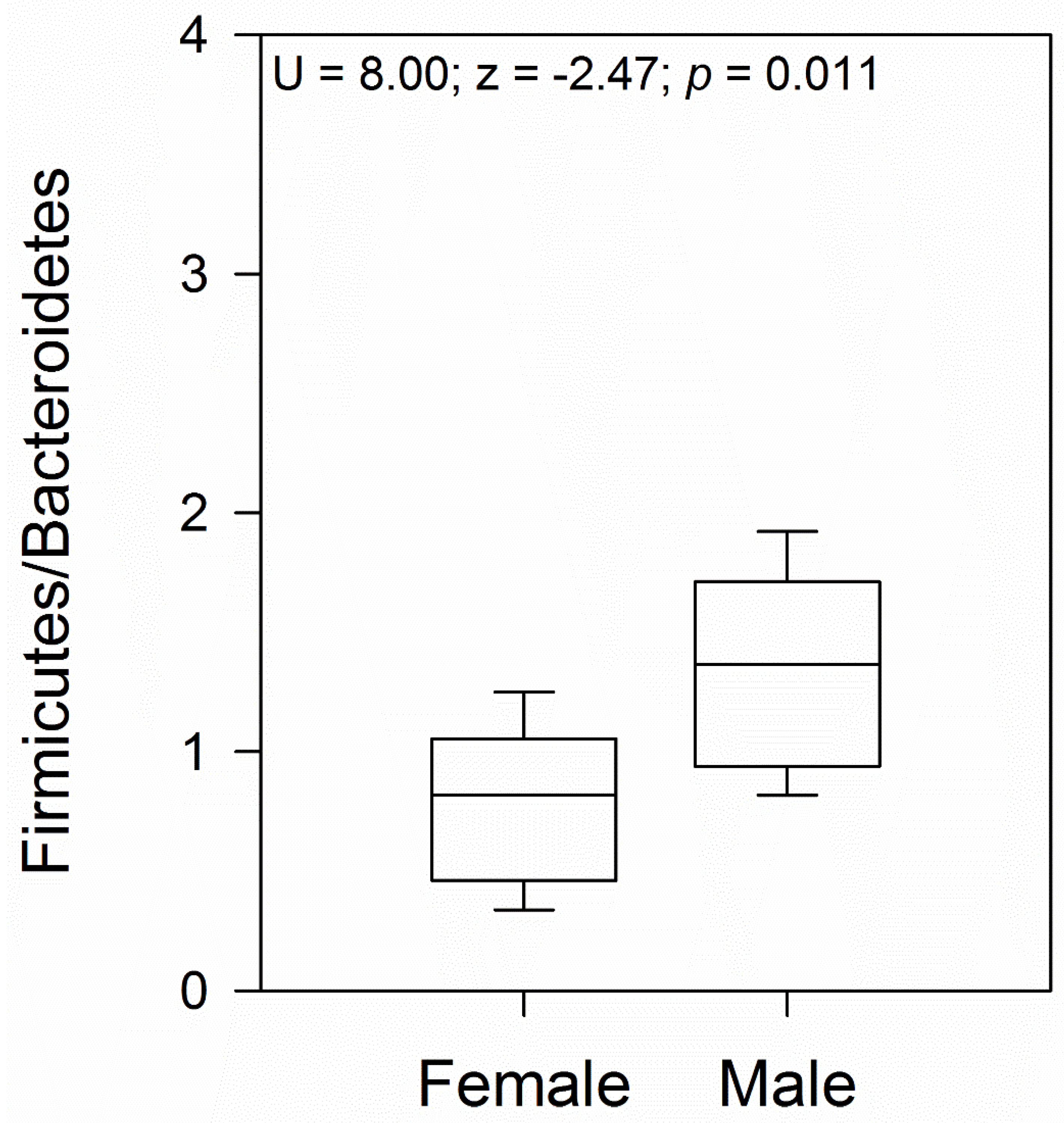
3.2. Taxonomic Characterization of the Gut Bacterial Communities in Female and Male Geckos
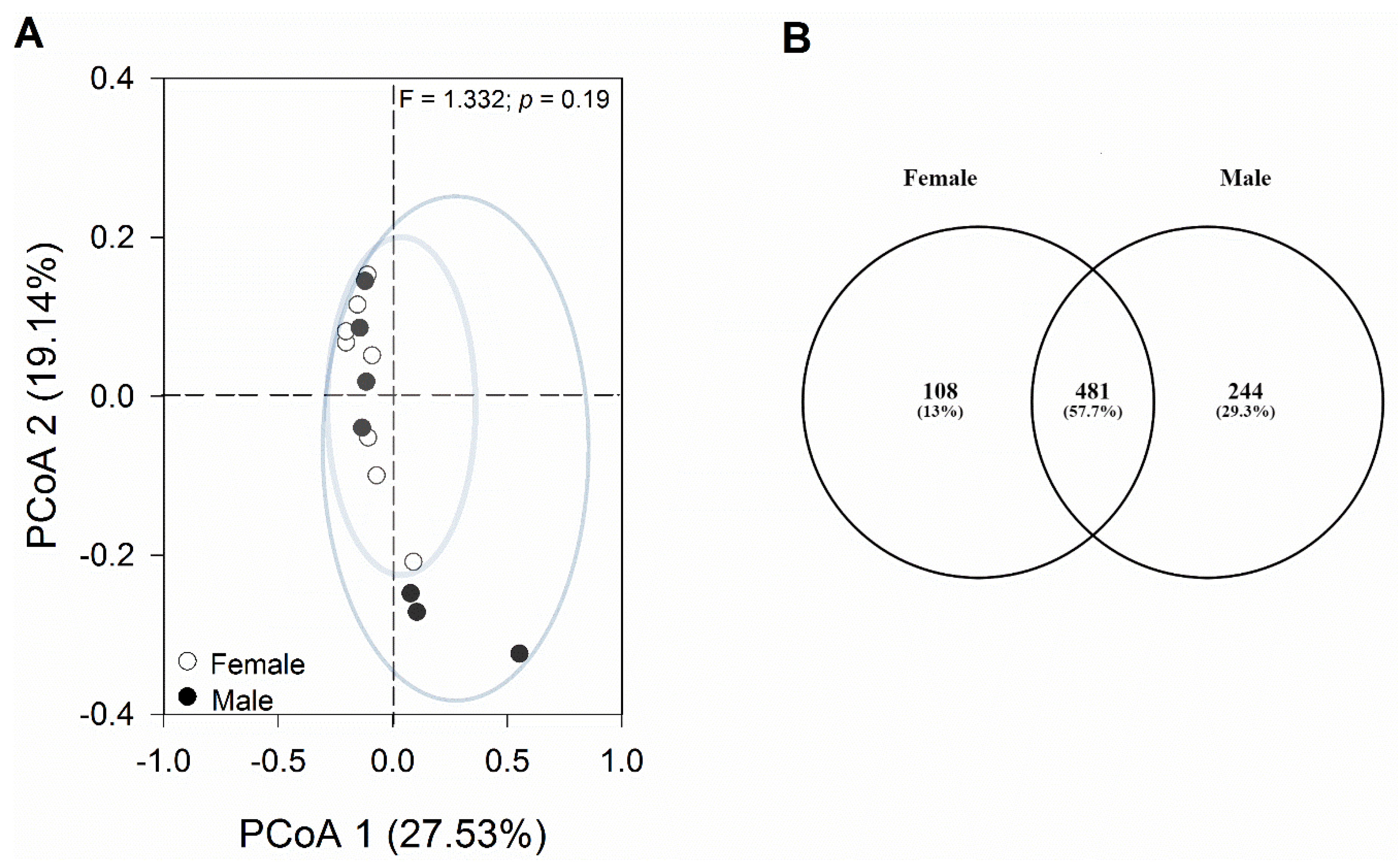
3.3. Comparable Gut Bacterial Composition in Female and Male Geckos
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waterman, T.H. Evolutionary Challenges of Extreme Environments (Part 2). J. Exp. Zool. 2001, 291, 130–168. [Google Scholar] [CrossRef] [PubMed]
- Wharton, D.A. Life at the Limits: Organisms in Extreme Environments; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Withers, P.C.; Cooper, C.E.; Maloney, S.K.; Bozinovic, F.; Cruz-Neto, A.P. Physiological Adaptations to Extreme Environments. In Ecological and Environmental Physiology of Mammals; Oxford University Press: Oxford, UK, 2016; pp. 290–392. [Google Scholar]
- Petford, M.; Alexander, G. Diel Activity Patterns of Two Syntopic Range-Restricted Geckos Suggest Idiosyncratic Responses to Climate Change. Afr. Zool. 2021, 56, 202–212. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Meng, Z.; Jia, M.; Li, R.; Yan, S.; Tian, S.; Zhou, Z.; Diao, J. Effects of L-Glufosinate-Ammonium and Temperature on Reproduction Controlled by Neuroendocrine System in Lizard (Eremias Argus). Environ. Pollut. 2020, 257, 113564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Q.; Chen, J.; Khattak, R.H.; Li, Z.; Teng, L.; Liu, Z. Insights into the Composition of Gut Microbiota in Response to Environmental Temperature: The Case of the Mongolia Racerunner (Eremias Argus). Glob. Ecol. Conserv. 2022, 36, e02125. [Google Scholar] [CrossRef]
- Akbar, N.; Khan, N.A.; Sagathevan, K.; Iqbal, M.; Tawab, A.; Siddiqui, R. Gut Bacteria of Cuora Amboinensis (Turtle) Produce Broad-Spectrum Antibacterial Molecules. Sci. Rep. 2019, 9, 17012. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Jeyamogan, S.; Ali, S.M.; Abbas, F.; Sagathevan, K.A.; Khan, N.A. Crocodiles and Alligators: Antiamoebic and Antitumor Compounds of Crocodiles. Exp. Parasitol. 2017, 183, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Cruz Soares, N.; Khan, N.A. Crocodile Gut Microbiome Is a Potential Source of Novel Bioactive Molecules. ACS Pharmacol. Transl. Sci. 2021, 4, 1260–1261. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Maciver, S.K.; Khan, N.A. Gut Microbiome–Immune System Interaction in Reptiles. J. Appl. Microbiol. 2022, 132, 2558–2571. [Google Scholar] [CrossRef]
- Kohl, K.D.; Brun, A.; Magallanes, M.; Brinkerhoff, J.; Laspiur, A.; Acosta, J.C.; Caviedes-Vidal, E.; Bordenstein, S.R. Gut Microbial Ecology of Lizards: Insights into Diversity in the Wild, Effects of Captivity, Variation across Gut Regions and Transmission. Mol. Ecol. 2017, 26, 1175–1189. [Google Scholar] [CrossRef]
- Moeller, A.H.; Ivey, K.; Cornwall, M.B.; Herr, K.; Rede, J.; Taylor, E.N.; Gunderson, A.R. The Lizard Gut Microbiome Changes with Temperature and Is Associated with Heat Tolerance. Appl. Environ. Microbiol. 2020, 86, e01181-20. [Google Scholar] [CrossRef]
- Bunker, M.E.; Arnold, A.E.; Weiss, S.L. Wild Microbiomes of Striped Plateau Lizards Vary with Reproductive Season, Sex, and Body Size. Sci. Rep. 2022, 12, 20643. [Google Scholar] [CrossRef] [PubMed]
- Escallón, C.; Belden, L.K.; Moore, I.T. The Cloacal Microbiome Changes with the Breeding Season in a Wild Bird. Integr. Org. Biol. 2019, 1, oby009. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef] [PubMed]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; Von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.O.; Gilman, F.R.; Weiss, S.L. Sex-Specific Asymmetry within the Cloacal Microbiota of the Striped Plateau Lizard, Sceloporus Virgatus. Symbiosis 2010, 51, 97–105. [Google Scholar] [CrossRef]
- Merker, G.P.; Nagy, K.A. Energy Utilization by Free-Ranging Sceloporus Virgatus Lizards. Ecology 1984, 65, 575–581. [Google Scholar] [CrossRef]
- Gamble, T.; Greenbaum, E.; Jackman, T.R.; Bauer, A.M. Into the Light: Diurnality Has Evolved Multiple Times in Geckos. Biol. J. Linn. Soc. 2015, 115, 896–910. [Google Scholar] [CrossRef]
- Tejero-Cicuéndez, H.; Simó-Riudalbas, M.; Menéndez, I.; Carranza, S. Ecological Specialization, Rather than the Island Effect, Explains Morphological Diversification in an Ancient Radiation of Geckos. Proc. R. Soc. B 2021, 288, 20211821. [Google Scholar] [CrossRef] [PubMed]
- Meiri, S. What Geckos Are–an Ecological-Biogeographic Perspective. Isr. J. Ecol. Evol. 2019, 66, 253–263. [Google Scholar] [CrossRef]
- Autumn, K.; Jindrich, D.; DeNardo, D.; Mueller, R. Locomotor Performance at Low Temperature and the Evolution of Nocturnality in Geckos. Evolution 1999, 53, 580–599. [Google Scholar] [CrossRef]
- Green, B.; Dryden, G.; Dryden, K. Field Energetics of a Large Carnivorous Lizard, Varanus Rosenbergi. Oecologia 1991, 88, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.E. Ecosystem Ecology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Nagy, K.A.; Clarke, B.C.; Seely, M.K.; Mitchell, D.; Lighton, J.R.B. Water and Energy Balance in Namibian Desert Sand-Dune Lizards Angolosaurus Skoogi (Andersson, 1916). Funct. Ecol. 1991, 5, 731–739. [Google Scholar] [CrossRef]
- De Vosjoli, P.; Klingenberg, R.; Tremper, R.; Viets, B. The Leopard Gecko Manual: Includes African Fat-Tailed Geckos; Fox Chapel Publishing: Mount Joy, PA, USA, 2011. [Google Scholar]
- Rutland, C.S.; Cigler, P.; Kubale, V. Reptilian Skin and Its Special Histological Structures. In Veterinary Anatomy and Physiology; IntechOpen: London, UK, 2019; pp. 135–156. [Google Scholar]
- Bauer, A.M.; Russell, A.P.; Shadwick, R.E. Mechanical Properties and Morphological Correlates of Fragile Skin in Gekkonid Lizards. J. Exp. Biol. 1989, 145, 79–102. [Google Scholar] [CrossRef]
- Pianka, E.R. Ecology and Natural History of Desert Lizards; Princeton University Press: Princet, NJ, USA, 1986. [Google Scholar]
- Werner, Y.L.; Bouskila, A.; Davies, S.; Werner, N. Observations and Comments on Active Foraging in Geckos. Russ. J. Herpetol. 1997, 4, 34–39. [Google Scholar] [CrossRef]
- Werner, Y.L.; Okada, S.; Ota, H.; Perry, G.; Tokunaga, S. Varied and Fluctuating Foraging Modes in Nocturnal Lizards of the Family Gekkonidae. Asiat. Herpetol. Res. 1997, 7, 153–165. [Google Scholar]
- Demangel, D. Reptiles En Chile. Fauna Nativa Ediciones. Santiago, Chile 2016. Available online: https://www.reptilesenchile.cl/index.html (accessed on 11 May 2024).
- Mella, J. Guía de Campo de Reptiles de Chile, Tomo 2: Zona Norte; Peñaloza, A.P.G., Ed.; Libreria Mackay: Santiago, Chile, 2017; 316p. [Google Scholar]
- Núñez, H.; Veloso, A. Distribución Geográfica de Las Especies de Lagartos de La Región de Antofagasta, Chile. Boletín Mus. Nac. Hist. Nat. 2001, 50, 109–120. [Google Scholar] [CrossRef]
- Zeballos, H.; López, E.; Villegas, L.; Jiménez, P.; Gutiérrez, R. Distribución de Los Reptiles de Arequipa, Sur Del Perú. Dilloniana 2002, 2, 27–34. [Google Scholar]
- Donoso-Barros, R. Reptiles de Chile; Ediciones de la Universidad de Chile: Santiago, Chile, 1966; 458p. [Google Scholar]
- Pincheira-Donoso, D. Los Geckos de Chile (Scleroglossa, Gekkonidae, Gekkoninae). Parte II: Biogeografía y Cambios Ontogenéticos En El Patrón de Coloración de Phyllodactylus Gerrhopygus. Puede La Evidencia Sostener La Presencia de Phyllodactylus Inaequalis En Chile? Multequina 2006, 15, 37–48. [Google Scholar]
- De Gamboa, M.R. Lista Actualizada de Los Reptiles de Chile. Boletín Chil. Herpetol. 2016, 3, 7–12. [Google Scholar]
- Pérez, J.; Balta, K. Ecología de Phyllodactylus Angustidigitus y P. Gerrhopygus (Squamata: Phyllodactylidae) de La Reserva Nacional de Paracas, Perú. Rev. Peru. Biol. 2011, 18, 217–223. [Google Scholar] [CrossRef]
- Reeves, J.T.; Fuhlendorf, S.D.; Davis, C.A.; Wilder, S.M. Arthropod Prey Vary among Orders in Their Nutrient and Exoskeleton Content. Ecol. Evol. 2021, 11, 17774–17785. [Google Scholar] [CrossRef] [PubMed]
- Lobos, G.; Tapia, G.; Alzamora, A.; Rojas, O. Distribución, Densidad y Nicho Isotópico En Reptiles y Mamíferos Del Desierto Absoluto de Atacama; Con Registro de Saurofagia Entre Reptiles. Gayana (Concepción) 2020, 84, 118–128. [Google Scholar] [CrossRef]
- Osborne, P.; Hall, L.J.; Kronfeld-Schor, N.; Thybert, D.; Haerty, W. A Rather Dry Subject; Investigating the Study of Arid-Associated Microbial Communities. Environ. Microbiome 2020, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- del Río, C.; Garcia, J.-L.; Osses, P.; Zanetta, N.; Lambert, F.; Rivera, D.; Siegmund, A.; Wolf, N.; Cereceda, P.; Larraín, H. ENSO Influence on Coastal Fog-Water Yield in the Atacama Desert, Chile. Aerosol Air Qual. Res. 2018, 18, 127–144. [Google Scholar] [CrossRef]
- Cereceda, P.; Larrain, H.; Osses, P.; Farias, M.; Egaña, I. The Spatial and Temporal Variability of Fog and Its Relation to Fog Oases in the Atacama Desert, Chile. Atmos. Res. 2008, 87, 312–323. [Google Scholar] [CrossRef]
- Daniels, C.B. The Role of Caudal Lipid in the Adaptive Strategy of Tail Autotomy in the Gekkonid Phyllodactylus Marmoratus (Gray, 1844). Honours Degree, University of Adelaide, Adelaide, Australia, 1981. [Google Scholar]
- Vitt, L.J.; Caldwell, J.P. Squamates Part I: Lizard. Herpetol 2014, 4, 581–582. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Zuo, S.; Zhang, J.; Chen, Z.; Chen, G.; Wang, X.; Pan, Y.; Liu, Y.; Wang, P. Protective Effect of Hydrogen Sulfide on TNF-α and IFN-γ-Induced Injury of Intestinal Epithelial Barrier Function in Caco-2 Monolayers. Inflamm. Res. 2015, 64, 789–797. [Google Scholar] [CrossRef]
- Valeri, F.; Endres, K. How Biological Sex of the Host Shapes Its Gut Microbiota. Front. Neuroendocrinol. 2021, 61, 100912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, F.; Li, T.; Dayananda, B.; Lin, L.; Lin, C. Lessons from the Diet: Captivity and Sex Shape the Gut Microbiota in an Oviparous Lizard (Calotes Versicolor). Ecol. Evol. 2022, 12, e8586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, Y.; Dai, Y.; Jiang, Y.; Lin, L.; Li, H.; Li, P.; Qu, Y.; Ji, X. Captivity Affects Diversity, Abundance, and Functional Pathways of Gut Microbiota in the Northern Grass Lizard Takydromus Septentrionalis. MicrobiologyOpen 2020, 9, e1095. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R. Prokaryotic and Eukaryotic Diversity of the Human Gut. Adv. Appl. Microbiol. 2010, 72, 43–62. [Google Scholar] [PubMed]
- Kim, Y.S.; Milner, J.A. Dietary Modulation of Colon Cancer Risk. J. Nutr. 2007, 137, 2576S–2579S. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.-H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbiol. 2011, 2, 9588. [Google Scholar] [CrossRef]
- Jiang, X.-R.; Dai, Y.-Y.; Wang, Y.-R.; Guo, K.; Du, Y.; Gao, J.-F.; Lin, L.-H.; Li, P.; Li, H.; Ji, X. Dietary and Sexual Correlates of Gut Microbiota in the Japanese Gecko, Gekko Japonicus (Schlegel, 1836). Animals 2023, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-Based Diet, a Promising Nutritional Source, Modulates Gut Microbiota Composition and SCFAs Production in Laying Hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef] [PubMed]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The First Report of the Physicochemical Structure of Chitin Isolated from Hermetia Illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’sullivan, O.; Fouhy, F.; Clarke, S.F.; O’toole, P.W.; Quigley, E.M.; Stanton, C. Composition and Energy Harvesting Capacity of the Gut Microbiota: Relationship to Diet, Obesity and Time in Mouse Models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Li, Y.; Chen, Z.; Wu, Z. Comparison of Gut Microbiota Diversity between Captive and Wild Tokay Gecko (Gekko Gecko). Front. Microbiol. 2022, 13, 897923. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- La Reau, A.J.; Meier-Kolthoff, J.P.; Suen, G. Sequence-Based Analysis of the Genus Ruminococcus Resolves Its Phylogeny and Reveals Strong Host Association. Microb. Genom. 2016, 2, e000099. [Google Scholar] [CrossRef] [PubMed]
- Teullet, S.; Tilak, M.-K.; Magdeleine, A.; Schaub, R.; Weyer, N.M.; Panaino, W.; Fuller, A.; Loughry, W.J.; Avenant, N.L.; De Thoisy, B.; et al. Metagenomics Uncovers Dietary Adaptations for Chitin Digestion in the Gut Microbiota of Convergent Myrmecophagous Mammals. mSystems 2023, 8, e00388-23. [Google Scholar] [CrossRef] [PubMed]
- Arizza, V.; Vecchioni, L.; Caracappa, S.; Sciurba, G.; Berlinghieri, F.; Gentile, A.; Persichetti, M.F.; Arculeo, M.; Alduina, R. New Insights into the Gut Microbiome in Loggerhead Sea Turtles Caretta Caretta Stranded on the Mediterranean Coast. PLoS ONE 2019, 14, e0220329. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Ma, J.-E.; Li, J.; Zhang, X.-J.; Li, L.-M.; He, N.; Liu, H.-Y.; Luo, S.-Y.; Wu, Z.-J.; Han, R.-C. Diets Alter the Gut Microbiome of Crocodile Lizards. Front. Microbiol. 2017, 8, 2073. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Kahrl, A.F.; Wu, M.; Cox, R.M. Does Adaptive Radiation of a Host Lineage Promote Ecological Diversity of Its Bacterial Communities? A Test Using Gut Microbiota of Anolis Lizards. Mol. Ecol. 2016, 25, 4793–4804. [Google Scholar] [CrossRef] [PubMed]
- Revelles, M.; Cardona, L.; Aguilar, A.; Fernández, G. The Diet of Pelagic Loggerhead Sea Turtles (Caretta Caretta) off the Balearic Archipelago (Western Mediterranean): Relevance of Long-Line Baits. J. Mar. Biol. Assoc. UK 2007, 87, 805–813. [Google Scholar] [CrossRef]
- Colston, T.J.; Jackson, C.R. Microbiome Evolution along Divergent Branches of the Vertebrate Tree of Life: What Is Known and Unknown. Mol. Ecol. 2016, 25, 3776–3800. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, R.; Zhou, X.; Ji, F.; Zhang, B. Hydrogen Sulfide Improves Colonic Barrier Integrity in DSS-Induced Inflammation in Caco-2 Cells and Mice. Int. Immunopharmacol. 2016, 39, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Nelson, T.M.; Rodriguez Lopez, C.; Sarma, R.R.; Zhou, S.J.; Rollins, L.A. A Comparison of Nonlethal Sampling Methods for Amphibian Gut Microbiome Analyses. Mol. Ecol. Resour. 2020, 20, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.F.; Wynn, M.L.; Wilson, R.S. Sex-Specific Trade-Offs and Compensatory Mechanisms: Bite Force and Sprint Speed Pose Conflicting Demands on the Design of Geckos (Hemidactylus Frenatus). J. Exp. Biol. 2013, 216, 3781–3789. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Mikami, K.; Hata, T.; Kimoto, K.; Nishino, R.; Akama, F.; Yamamoto, K.; Sudo, N.; Koga, Y.; Matsumoto, H. Effect of Gut Microbiota Early in Life on Aggressive Behavior in Mice. Neurosci. Res. 2021, 168, 95–99. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, D.S.; Beltrán, V.; Gutiérrez-Cortés, I.; Vargas, C.; Alfaro, F.D. Insights into the Gut Microbiome of the South American Leaf-Toed Gecko (Phylodactylus gerropygus) Inhabiting the Core of the Atacama Desert. Microorganisms 2024, 12, 1194. https://doi.org/10.3390/microorganisms12061194
Rivera DS, Beltrán V, Gutiérrez-Cortés I, Vargas C, Alfaro FD. Insights into the Gut Microbiome of the South American Leaf-Toed Gecko (Phylodactylus gerropygus) Inhabiting the Core of the Atacama Desert. Microorganisms. 2024; 12(6):1194. https://doi.org/10.3390/microorganisms12061194
Chicago/Turabian StyleRivera, Daniela S., Valentina Beltrán, Ignacio Gutiérrez-Cortés, Constanza Vargas, and Fernando D. Alfaro. 2024. "Insights into the Gut Microbiome of the South American Leaf-Toed Gecko (Phylodactylus gerropygus) Inhabiting the Core of the Atacama Desert" Microorganisms 12, no. 6: 1194. https://doi.org/10.3390/microorganisms12061194
APA StyleRivera, D. S., Beltrán, V., Gutiérrez-Cortés, I., Vargas, C., & Alfaro, F. D. (2024). Insights into the Gut Microbiome of the South American Leaf-Toed Gecko (Phylodactylus gerropygus) Inhabiting the Core of the Atacama Desert. Microorganisms, 12(6), 1194. https://doi.org/10.3390/microorganisms12061194






