Identification of a Novel KPC Variant, KPC-204, Conferring Resistance to Both Carbapenems and Ceftazidime–Avibactam in an ST11 Klebsiella pneumoniae Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. The Strains and In Vitro Susceptibility
2.2. Whole Genome Sequencing and Analysis
2.3. qRT-PCR
2.4. Cloning Experiment
2.5. Kinetic Assay and Determination of IC50 Values
2.6. Structure Prediction
2.7. Comparative Secondary and Stereoscopic Structures
2.8. Molecular Docking
2.9. Mating Experiments
3. Results
3.1. Antimicrobial Susceptibility
3.2. Genomic Analysis of Clinical K. pneumoniae Isolate 130125
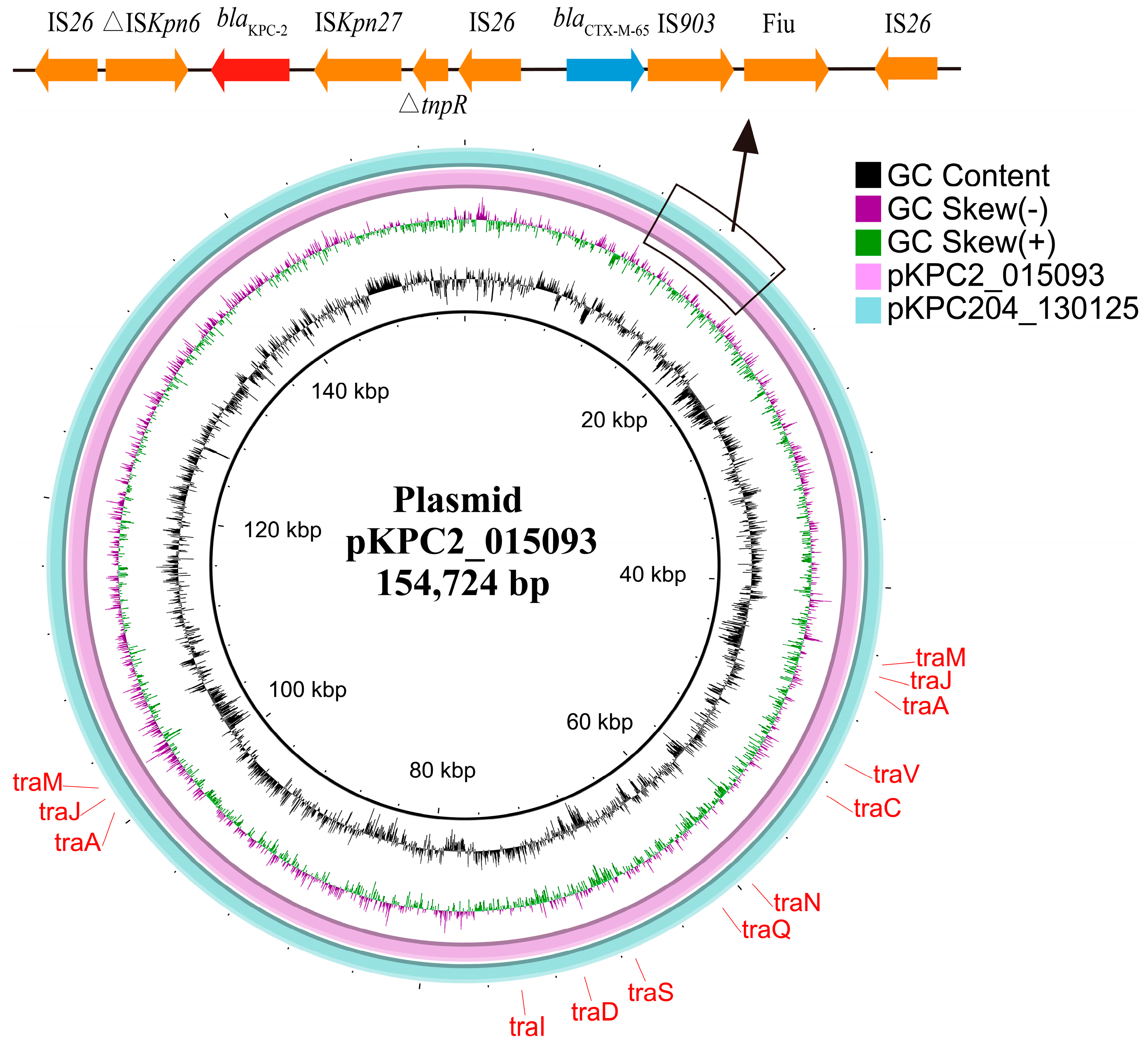
3.3. Genetic Context of blaKPC-204-Carrying Plasmid
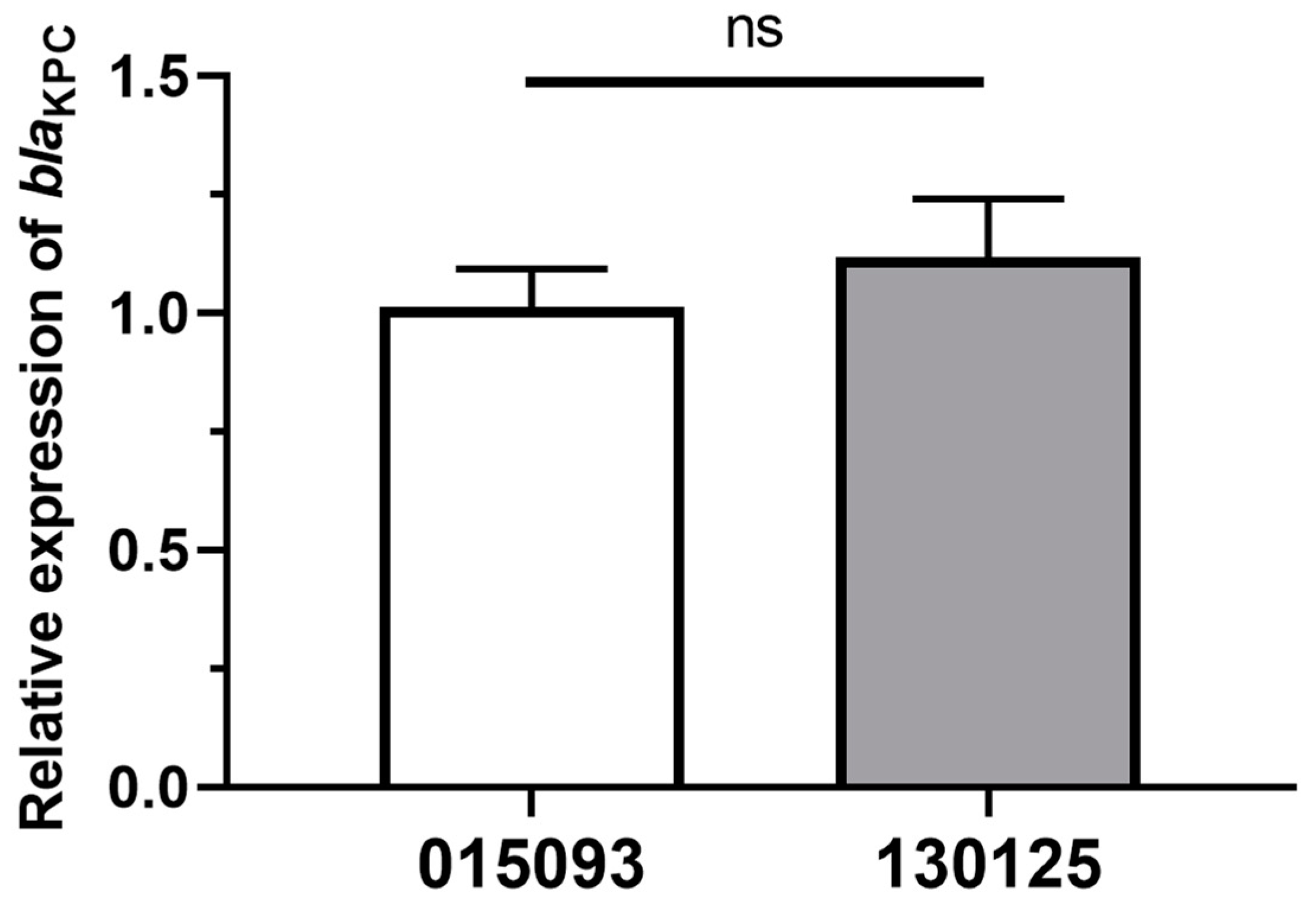
3.4. Relative blaKPC Gene’s Expression Levels
3.5. Identification of blaKPC-204 Involved in CZA Resistance
3.6. Enzyme Kinetic Parameters and IC50 Values
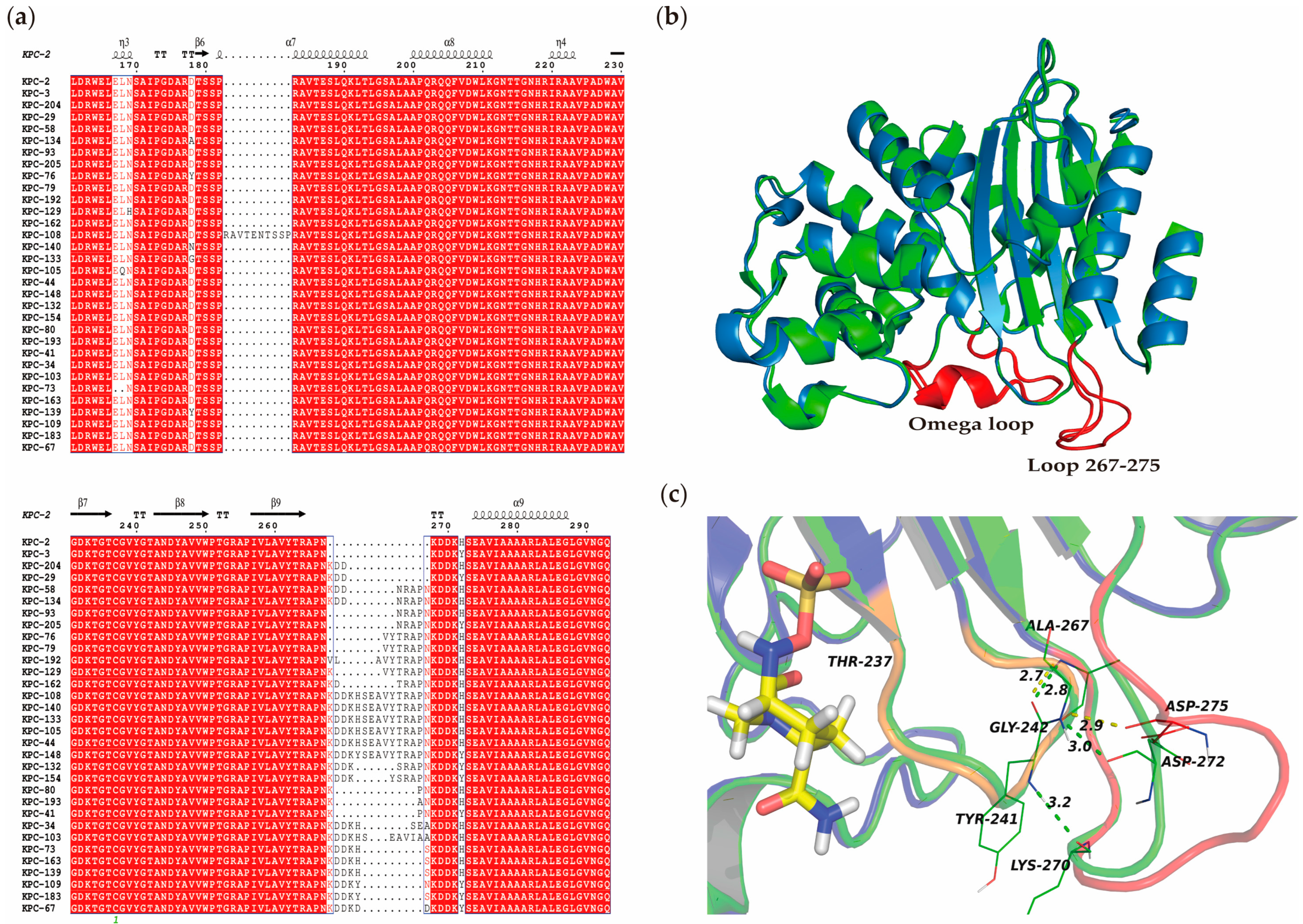
3.7. Comparative Secondary and Stereoscopic Structures of KPC-204 and Related Variants
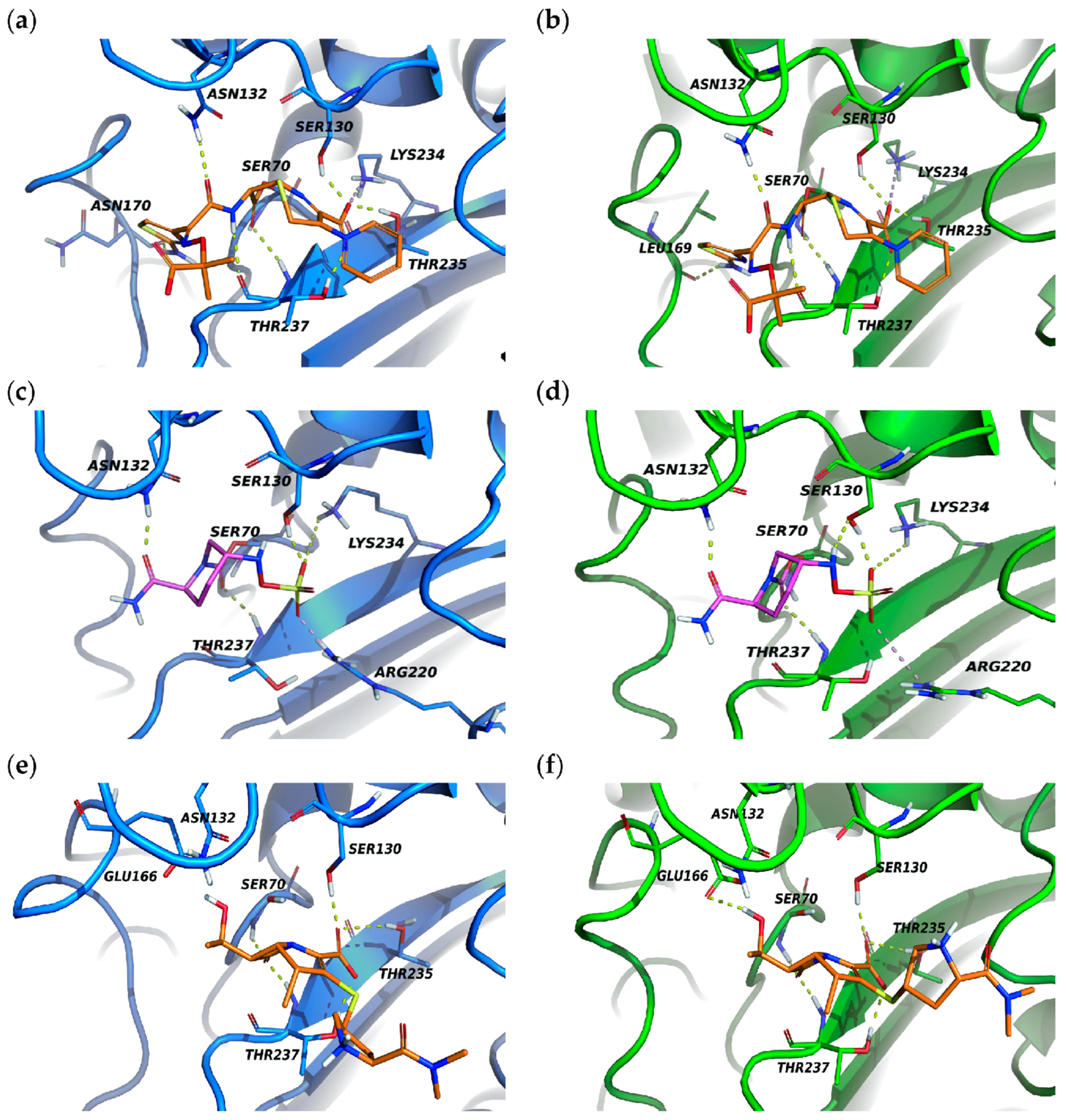
3.8. Molecular Docking of KPC-204 and KPC-2 with Ceftazidime, Avibactam and Meropenem
3.9. blaKPC-204 Was Located in a Self-Transmissible Plasmid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Song, X.; Li, M.; Yu, Z.; Cheng, W.; Yu, Z.; Zhang, W.; Zhang, Y.; Shen, A.; Sun, H.; et al. Global Spread of Carbapenem-Resistant Enterobacteriaceae: Epidemiological Features, Resistance Mechanisms, Detection and Therapy. Microbiol. Res. 2023, 266, 127249. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Klompas, M. Ceftazidime-Avibactam versus Meropenem for the Treatment of Nosocomial Pneumonia. Lancet Infect. Dis. 2018, 18, 229–231. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Interplay between β-Lactamases and New β-Lactamase Inhibitors. Nat. Rev. Microbiol. 2019, 17, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.A.; Pierrat, G.; Tenaillon, O.; Bonacorsi, S.; Bercot, B.; Jaouen, E.; Jacquier, H.; Birgy, A. Klebsiella pneumoniae Carbapenemase Variants Resistant to Ceftazidime-Avibactam: An Evolutionary Overview. Antimicrob. Agents Chemother. 2022, 66, e0044722. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shen, S.; Chen, J.; Tian, Z.; Shi, Q.; Han, R.; Guo, Y.; Hu, F. Klebsiella pneumoniae Carbapenemase Variants: The New Threat to Global Public Health. Clin. Microbiol. Rev. 2023, 36, e0000823. [Google Scholar] [CrossRef] [PubMed]
- Nicola, F.; Cejas, D.; González-Espinosa, F.; Relloso, S.; Herrera, F.; Bonvehí, P.; Smayevsky, J.; Figueroa-Espinosa, R.; Gutkind, G.; Radice, M. Outbreak of Klebsiella pneumoniae ST11 Resistant To Ceftazidime-Avibactam Producing KPC-31 and the Novel Variant KPC-115 during COVID-19 Pandemic in Argentina. Microbiol. Spectr. 2022, 10, e03733-22. [Google Scholar] [CrossRef]
- Shi, Q.; Yin, D.; Han, R.; Guo, Y.; Zheng, Y.; Wu, S.; Yang, Y.; Li, S.; Zhang, R.; Hu, F. Emergence and Recovery of Ceftazidime-Avibactam Resistance in blaKPC-33-Harboring Klebsiella pneumoniae Sequence Type 11 Isolates in China. Clin. Infect. Dis. 2020, 71, S436–S439. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Chen, L.; Kreiswirth, B.N.; Clancy, C.J. In Vitro Selection of Meropenem Resistance among Ceftazidime-Avibactam-Resistant, Meropenem-Susceptible Klebsiella pneumoniae Isolates with Variant KPC-3 Carbapenemases. Antimicrob. Agents Chemother. 2017, 61, e00079-17. [Google Scholar] [CrossRef]
- Huang, X.; Shen, S.; Chang, F.; Liu, X.; Yue, J.; Xie, N.; Yin, L.; Hu, F.; Xiao, D. Emergence of KPC-134, a KPC-2 Variant Associated with Ceftazidime-Avibactam Resistance in a ST11 Klebsiella pneumoniae Clinical Strain. Microbiol. Spectr. 2023, 11, e0072523. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Liu, C.; Zhang, Y.; Cheung, Y.C.; Wai Chi Chan, E.; Chen, S.; Zhang, R. Identification of a KPC Variant Conferring Resistance to Ceftazidime-Avibactam from ST11 Carbapenem-Resistant Klebsiella pneumoniae Strains. Microbiol. Spectr. 2022, 10, e0265521. [Google Scholar] [CrossRef]
- Li, X.; Quan, J.; Ke, H.; Wu, W.; Feng, Y.; Yu, Y.; Jiang, Y. Emergence of a KPC Variant Conferring Resistance to Ceftazidime-Avibactam in a Widespread ST11 Carbapenem-Resistant Klebsiella pneumoniae Clone in China. Front. Microbiol. 2021, 12, 724272. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ke, H.; Wu, W.; Tu, Y.; Zhou, H.; Yu, Y. Molecular Mechanisms Driving the In Vivo Development of KPC-71-Mediated Resistance to Ceftazidime-Avibactam during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. mSphere 2021, 6, e0085921. [Google Scholar] [CrossRef]
- Cano, A.; Guzman-Puche, J.; Garcia-Gutierrez, M.; Caston, J.J.; Gracia-Ahufinger, I.; Perez-Nadales, E.; Recio, M.; Natera, A.M.; Marfil-Perez, E.; Martinez-Martinez, L.; et al. Use of Carbapenems in the Combined Treatment of Emerging Ceftazidime/Avibactam-Resistant and Carbapenem-Susceptible KPC-Producing Klebsiella pneumoniae Infections: Report of a Case and Review of the Literature. J. Glob. Antimicrob. Resist. 2020, 22, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.A.; Bonacorsi, S.; Jacquier, H.; Choudhury, A.; Magnan, M.; Cointe, A.; Bercot, B.; Tenaillon, O.; Birgy, A. KPC Beta-Lactamases Are Permissive to Insertions and Deletions Conferring Substrate Spectrum Modifications and Resistance to Ceftazidime-Avibactam. Antimicrob. Agents Chemother. 2020, 64, e01175-20. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100-S32; Performance Standards for Antimicrobial Susceptibility Testing 32nd Informational Supplement. CLSI: Wayne, PA, USA, 2022.
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput ANI Analysis of 90K Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the Genomic Gold Standard for the Prokaryotic Species Definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-Access Bacterial Population Genomics: BIGSdb Software, the PubMLST.Org Website and Their Applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Wick, R.R.; Gorrie, C.; Jenney, A.; Follador, R.; Thomson, N.R.; Holt, K.E. Identification of Klebsiella Capsule Synthesis Loci from Whole Genome Data. Microb. Genom. 2016, 2, e000102. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In Silico Detection and Typing of Plasmids Using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, N.-F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple Prokaryote Genome Comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The Reference Centre for Bacterial Insertion Sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Liu, S.; Jing, L.; Yu, Z.-J.; Wu, C.; Zheng, Y.; Zhang, E.; Chen, Q.; Yu, Y.; Guo, L.; Wu, Y.; et al. ((S)-3-Mercapto-2-Methylpropanamido) Acetic Acid Derivatives as Metallo-β-Lactamase Inhibitors: Synthesis, Kinetic and Crystallographic Studies. Eur. J. Med. Chem. 2018, 145, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chao, H.; Chen, L.; Craig, P.A.; Crichlow, G.V.; Dalenberg, K.; Duarte, J.M.; et al. RCSB Protein Data Bank (RCSB.Org): Delivery of Experimentally-Determined PDB Structures alongside One Million Computed Structure Models of Proteins from Artificial Intelligence/Machine Learning. Nucleic Acids Res. 2023, 51, D488–D508. [Google Scholar] [CrossRef] [PubMed]
- Highly Accurate Protein Structure Prediction with AlphaFold|Nature. Available online: https://www.nature.com/articles/s41586-021-03819-2 (accessed on 11 May 2023).
- Robert, X.; Gouet, P. Deciphering Key Features in Protein Structures with the New ENDscript Server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Coque, T.M.; Oliver, A.; Pérez-Díaz, J.C.; Baquero, F.; Cantón, R. Genes Encoding TEM-4, SHV-2, and CTX-M-10 Extended-Spectrum Beta-Lactamases Are Carried by Multiple Klebsiella pneumoniae Clones in a Single Hospital (Madrid, 1989 to 2000). Antimicrob. Agents Chemother. 2002, 46, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Ding, B.; Ye, M.; Wang, P.; Bi, Y.; Wu, S.; Xu, X.; Guo, Q.; Wang, M. High Ceftazidime Hydrolysis Activity and Porin OmpK35 Deficiency Contribute to the Decreased Susceptibility to Ceftazidime/Avibactam in KPC-Producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2017, 72, 1930–1936. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Q.; Perlaza-Jiménez, L.; Zheng, X.; Zhao, Y.; Dhanasekaran, V.; Fang, R.; Li, J.; Wang, C.; Liu, H.; et al. First Description of Antimicrobial Resistance in Carbapenem-Susceptible Klebsiella pneumoniae after Imipenem Treatment, Driven by Outer Membrane Remodeling. BMC Microbiol. 2020, 20, 218. [Google Scholar] [CrossRef]
- Virolle, C.; Goldlust, K.; Djermoun, S.; Bigot, S.; Lesterlin, C. Plasmid Transfer by Conjugation in Gram-Negative Bacteria: From the Cellular to the Community Level. Genes 2020, 11, 1239. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Cai, L.; Zong, Z. Enhanced Survival of ST-11 Carbapenem-Resistant Klebsiella pneumoniae in the Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2020, 41, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Arcari, G.; Bibbolino, G.; Sacco, F.; Tomolillo, D.; Di Lella, F.M.; Trancassini, M.; Faino, L.; Venditti, M.; Antonelli, G.; et al. Evolutionary Trajectories toward Ceftazidime-Avibactam Resistance in Klebsiella pneumoniae Clinical Isolates. Antimicrob. Agents Chemother. 2021, 65, e0057421. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Yin, Y.; Chen, H.; Jin, L.; Gu, B.; Xie, L.; Yang, C.; Ma, X.; Li, H.; et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: Report from the China CRE Network. Antimicrob. Agents Chemother. 2018, 62, e01882-17. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, L.; Zhou, H.; Chan, E.W.; Li, J.; Fang, Y.; Li, Y.; Liao, K.; Chen, S. Nationwide Surveillance of Clinical Carbapenem-Resistant Enterobacteriaceae (CRE) Strains in China. EBioMedicine 2017, 19, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Muresu, N.; Del Rio, A.; Fox, V.; Scutari, R.; Alteri, C.; Are, B.M.; Terragni, P.; Sechi, I.; Sotgiu, G.; Piana, A. Genomic Characterization of KPC-31 and OXA-181 Klebsiella pneumoniae Resistant to New Generation of β-Lactam/β-Lactamase Inhibitor Combinations. Antibiotics 2022, 12, 10. [Google Scholar] [CrossRef]
- Antonelli, A.; Giani, T.; Di Pilato, V.; Riccobono, E.; Perriello, G.; Mencacci, A.; Rossolini, G.M. KPC-31 Expressed in a Ceftazidime/Avibactam-Resistant Klebsiella pneumoniae Is Associated with Relevant Detection Issues. J. Antimicrob. Chemother. 2019, 74, 2464–2466. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Poirel, L.; Nordmann, P. Origin of OXA-181, an Emerging Carbapenem-Hydrolyzing Oxacillinase, as a Chromosomal Gene in Shewanella xiamenensis. Antimicrob. Agents Chemother. 2011, 55, 4405–4407. [Google Scholar] [CrossRef]
- Arcari, G.; Cecilia, F.; Oliva, A.; Polani, R.; Raponi, G.; Sacco, F.; De Francesco, A.; Pugliese, F.; Carattoli, A. Genotypic Evolution of Klebsiella pneumoniae Sequence Type 512 during Ceftazidime/Avibactam, Meropenem/Vaborbactam, and Cefiderocol Treatment, Italy. Emerg. Infect. Dis. 2023, 29, 2266–2274. [Google Scholar] [CrossRef]
- Cui, X.; Shan, B.; Zhang, X.; Qu, F.; Jia, W.; Huang, B.; Yu, H.; Tang, Y.W.; Chen, L.; Du, H. Reduced Ceftazidime-Avibactam Susceptibility in KPC-Producing Klebsiella pneumoniae from Patients without Ceftazidime-Avibactam Use History—A Multicenter Study in China. Front. Microbiol. 2020, 11, 1365. [Google Scholar] [CrossRef]
- Räisänen, K.; Koivula, I.; Ilmavirta, H.; Puranen, S.; Kallonen, T.; Lyytikäinen, O.; Jalava, J. Emergence of Ceftazidime-Avibactam-Resistant Klebsiella pneumoniae during Treatment, Finland, December 2018. Eurosurveillance 2019, 24, 1900256. [Google Scholar] [CrossRef]
- Shi, Q.; Han, R.; Guo, Y.; Yang, Y.; Wu, S.; Ding, L.; Zhang, R.; Yin, D.; Hu, F. Multiple Novel Ceftazidime-Avibactam-Resistant Variants of blaKPC-2-Positive Klebsiella pneumoniae in Two Patients. Microbiol. Spectr. 2022, 10, e0171421. [Google Scholar] [CrossRef]
- Mueller, L.; Masseron, A.; Prod’Hom, G.; Galperine, T.; Greub, G.; Poirel, L.; Nordmann, P. Phenotypic, Biochemical, and Genetic Analysis of KPC-41, a KPC-3 Variant Conferring Resistance to Ceftazidime-Avibactam and Exhibiting Reduced Carbapenemase Activity. Antimicrob. Agents Chemother. 2019, 63, e01111-19. [Google Scholar] [CrossRef]
- Liu, L.; Feng, Y.; Wei, L.; Xiao, Y.; Zong, Z. KPC-2-Producing Carbapenem-Resistant Klebsiella pneumoniae of the Uncommon ST29 Type Carrying OXA-926, a Novel Narrow-Spectrum OXA β-Lactamase. Front. Microbiol. 2021, 12, 701513. [Google Scholar] [CrossRef]
- Qin, J.; Feng, Y.; Lü, X.; Zong, Z. KPC-12 with a L169M Substitution in the Ω Loop Has Reduced Carbapenemase Activity. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1761–1766. [Google Scholar] [CrossRef]
- Wang, L.; Shen, W.; Zhang, R.; Cai, J. Identification of a Novel Ceftazidime-Avibactam-Resistant KPC-2 Variant, KPC-123, in Citrobacter Koseri Following Ceftazidime-Avibactam Treatment. Front. Microbiol. 2022, 13, 930777. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, D.; Wei, Z.; Shen, P.; Zhou, Z.; Yu, Y. Complete Nucleotide Sequence of Klebsiella pneumoniae Multidrug Resistance Plasmid pKP048, Carrying blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob. Agents Chemother. 2010, 54, 3967–3969. [Google Scholar] [CrossRef]
- Cuzon, G.; Naas, T.; Nordmann, P. Functional Characterization of Tn4401, a Tn3-Based Transposon Involved in blaKPC Gene Mobilization. Antimicrob. Agents Chemother. 2011, 55, 5370–5373. [Google Scholar] [CrossRef]
- Ding, L.; Shi, Q.; Han, R.; Yin, D.; Wu, S.; Yang, Y.; Guo, Y.; Zhu, D.; Hu, F. Comparison of Four Carbapenemase Detection Methods for blaKPC-2 Variants. Microbiol. Spectr. 2021, 9, e0095421. [Google Scholar] [CrossRef] [PubMed]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.T.J.; Duran, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and Additive Effect of Oregano Essential Oil and Biological Silver Nanoparticles against Multidrug-Resistant Bacterial Strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-K.; Ma, L.; Chan, M.-C.; Lin, Y.-T.; Fung, C.-P.; Wu, T.-L.; Chuang, Y.-C.; Lu, P.-L.; Wang, J.-T.; Lin, J.-C.; et al. Carbapenem Nonsusceptible Klebsiella pneumoniae in Taiwan: Dissemination and Increasing Resistance of Carbapenemase Producers during 2012–2015. Sci. Rep. 2018, 8, 8468. [Google Scholar] [CrossRef]
| MICs (mg/L) a | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | PIP | TZP | FOX | FEP | ATM | CAZ | CZA | IPM | IMR | MEM | MEV | ETP |
| 130125 | >512 | 256 | >512 | >512 | 512 | >512 | 256 | 64 | 0.25 | 64 | 0.5 | 64 |
| 015093 | >512 | >512 | >512 | >512 | 512 | >512 | 0.5 | 128 | 0.25 | 256 | 0.06 | 64 |
| DH5α::pEKPC-2 | >512 | >512 | >512 | >512 | 512 | 128 | 0.5 | 16 | 0.125 | 8 | 0.03 | 8 |
| DH5α::pEKPC-204 | >512 | 256 | >512 | 512 | 256 | 128 | 64 | 16 | 0.125 | 16 | 0.125 | 8 |
| DH5α::pET28a | 1 | 1 | 2 | 0.06 | 0.125 | 0.25 | 0.25 | 0.25 | 0.06 | ≤0.015 | ≤0.015 | ≤0.015 |
| E. coli J53 | 1 | 1 | 1 | 0.06 | 0.125 | 0.25 | 0.125 | 0.125 | 0.06 | ≤0.015 | ≤0.015 | ≤0.015 |
| J53::pKPC2_015093 | >512 | >512 | >512 | 512 | 512 | 512 | 0.5 | 32 | 0.25 | 32 | 0.03 | 32 |
| J53::KPC204_130125 | >512 | 256 | >512 | 512 | 512 | 512 | 64 | 32 | 0.25 | 64 | 0.25 | 32 |
| Accession No. | Size, bp | Replicon Type | Resistance Genes | ||
|---|---|---|---|---|---|
| β-Lactam | Other | ||||
| 130125_chr | CP148996 | 5,462,753 | - | blaSHV-158 | aadA2, fosA6 |
| pKPC204_130125 | CP148997 | 154,728 | IncR, IncFII | blaKPC-204, blaTEM-1, blaCTX-M-65 | rmtB1 |
| p1_130125 | CP148998 | 10,060 | ColRNAI | ||
| p2_130125 | CP148999 | 5596 | - | ||
| KPC-2 | KPC-204 | |||||
|---|---|---|---|---|---|---|
| β-Lactam | Km (μM) | kcat (s−1) | kcat/Km (μM−1·s−1) | Km (μM) | kcat (s−1) | kcat/Km (μM−1·s−1) |
| Nitrocefin | 22.124 | 97.589 | 4.411 | 31.178 | 116.419 | 3.734 |
| Ceftazidime | 870.413 | 5.226 | 0.006 | 975.154 | 7.801 | 0.008 |
| Meropenem | 15.283 | 5.194 | 0.34 | 14.157 | 8.325 | 0.588 |
| IC50 (μM) | ||
|---|---|---|
| Inhibitor | KPC-2 | KPC-204 |
| Avibactam | 0.045 | 0.569 |
| Tazobactam | 1.782 | 0.083 |
| Clavulanic acid | 0.887 | 0.124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Feng, Y.; Lv, X. Identification of a Novel KPC Variant, KPC-204, Conferring Resistance to Both Carbapenems and Ceftazidime–Avibactam in an ST11 Klebsiella pneumoniae Strain. Microorganisms 2024, 12, 1193. https://doi.org/10.3390/microorganisms12061193
Gong Y, Feng Y, Lv X. Identification of a Novel KPC Variant, KPC-204, Conferring Resistance to Both Carbapenems and Ceftazidime–Avibactam in an ST11 Klebsiella pneumoniae Strain. Microorganisms. 2024; 12(6):1193. https://doi.org/10.3390/microorganisms12061193
Chicago/Turabian StyleGong, Yanqiao, Yu Feng, and Xiaoju Lv. 2024. "Identification of a Novel KPC Variant, KPC-204, Conferring Resistance to Both Carbapenems and Ceftazidime–Avibactam in an ST11 Klebsiella pneumoniae Strain" Microorganisms 12, no. 6: 1193. https://doi.org/10.3390/microorganisms12061193
APA StyleGong, Y., Feng, Y., & Lv, X. (2024). Identification of a Novel KPC Variant, KPC-204, Conferring Resistance to Both Carbapenems and Ceftazidime–Avibactam in an ST11 Klebsiella pneumoniae Strain. Microorganisms, 12(6), 1193. https://doi.org/10.3390/microorganisms12061193






