Orchid Mycorrhizal Association of Cultivated Dendrobium Hybrid and Their Role in Seed Germination and Seedling Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Sampling
2.2. Assessment of Mycorrhization
2.3. Isolation of Mycorrhizal Fungi
2.4. DNA Extraction, PCR, and Sanger Sequencing
2.5. Phylogenetic Analysis
2.6. High-Throughput Sequencing
2.7. In Vitro Symbiotic Germination
2.8. Symbiotic Culture of Seedlings
3. Results
3.1. Assessment of Mycorrhization
3.2. Molecular Identification of Mycorrhizal Fungi by Sanger Sequencing
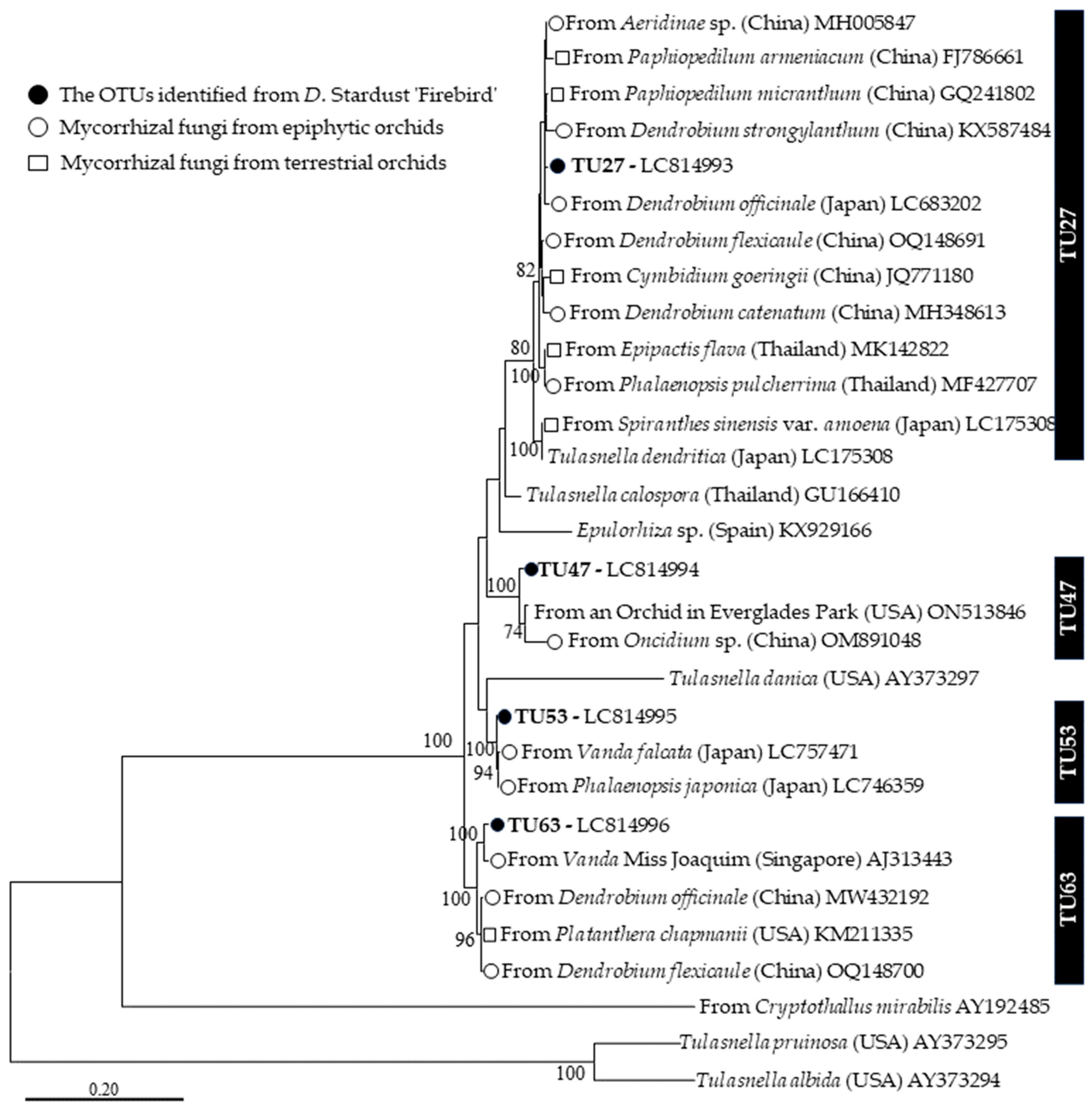
3.3. Phylogenetic Analysis
3.4. High-Throughput Sequencing
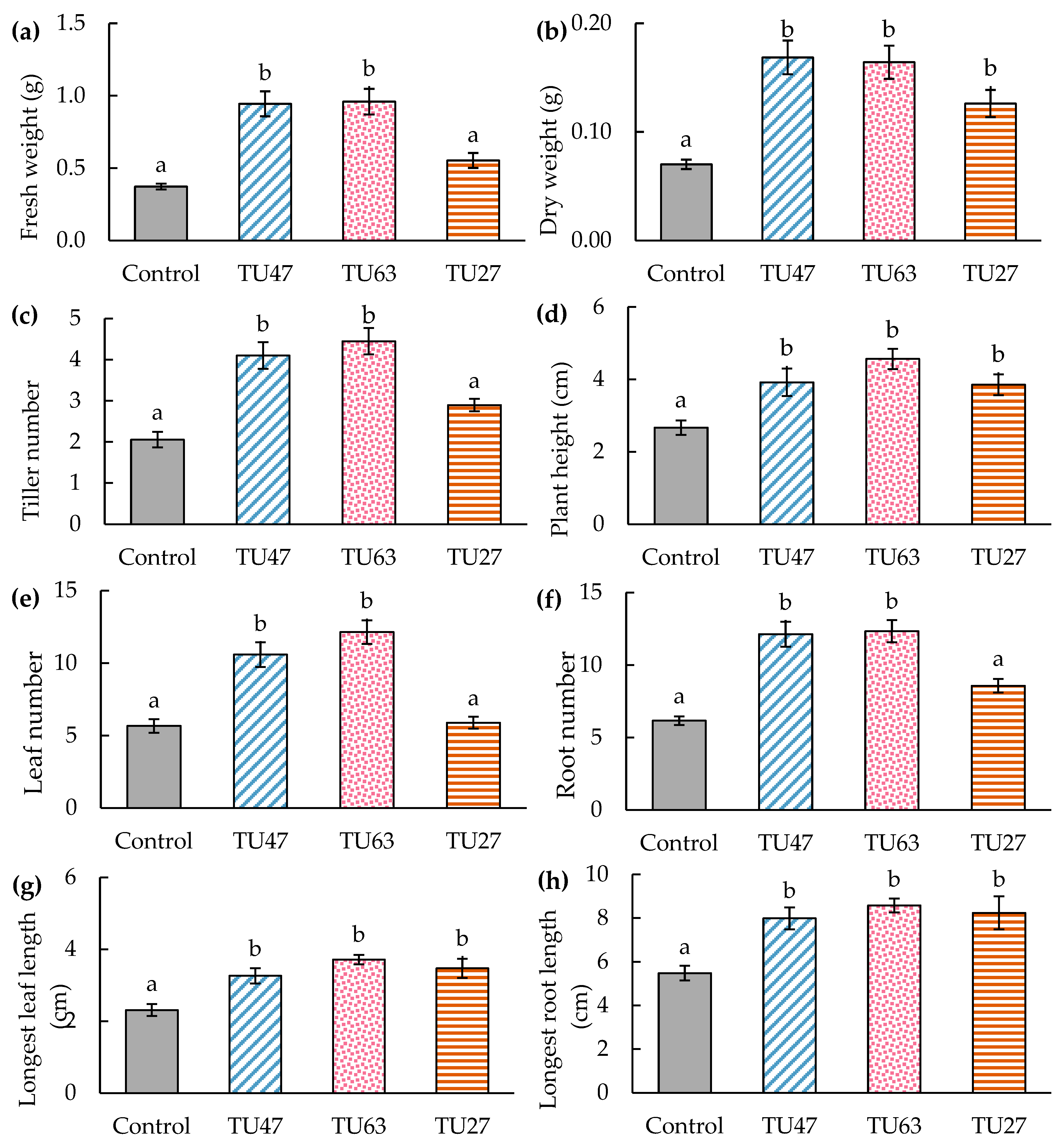
3.5. Effects of Fungal Strains on Seed Germination
3.6. Effects of Fungal Strains on Seedling Growth
4. Discussion
4.1. Assessment of Mycorrhization
4.2. Mycorrhizal Fungal Community of D. Stardust ‘Firebird’
4.3. Variation in Mycorrhizal Community Composition
4.4. Effects of Fungi on Seed Germination and Seedling Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De, L.C.; Khan, A.M.; Kumar, R.; Medhi, R.P. Orchid Farming—A remunerative approach for farmers’ livelihood. Int. J. Sci. Res. 2014, 3, 468–471. [Google Scholar]
- Yuan, S.C.; Lekawatana, S.; Amore, T.D.; Chen, F.C.; Chin, S.W.; Vega, D.M.; Wang, Y.T. The global orchid market. In The Orchid Genome; Chen, F.C., Chin, S.W., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–28. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Rasmussen, F.N. Orchid mycorrhiza: Implications of a mycophagous lifestyle. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Dearnaley, J.D.; Martos, F.; Selosse, M.A. Orchid mycorrhizas: Molecular ecology, physiology, evolution, and conservation aspects. In Fungal Associations, 2nd ed.; Hock, B., Ed.; Springer: Berlin, Germany, 2012; pp. 207–230. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Zanello, C.A.; Chen, T. An overview of orchid protocorm-like bodies: Mass propagation, biotechnology, molecular aspects, and breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Rahi, P.; Gulati, A.; Sharma, M. Improved ex vitro survival of asymbiotically raised seedlings of Cymbidium using mycorrhizal fungi isolated from distant orchid taxa. Sci. Hortic. 2013, 159, 109–116. [Google Scholar] [CrossRef]
- Chang, D.C. The screening of orchid mycorrhizal fungi (OMF) and their applications. In Orchid Biotechnology; Chen, W.H., Chen, H.H., Eds.; World Scientific: Singapore, 2007; pp. 77–98. [Google Scholar]
- Wu, P.H.; Huang, D.D.; Chang, D.C. Mycorrhizal symbiosis enhances Phalaenopsis orchid’s growth and resistance to Erwinia chrysanthemi. Afr. J. Biotechnol. 2011, 10, 10095–10100. [Google Scholar] [CrossRef]
- Bagyaraj, D.J.; Powell, C.L. Occurrence and intensity of mycorrhizal infections in cultivated orchids in some New Zealand nurseries. N. Z. J. Agr. Res. 1983, 26, 409–412. [Google Scholar] [CrossRef]
- Goh, C.J.; Sim, A.A.; Lim, G. Mycorrhizal associations in some tropical orchids. Lindleyana 1992, 7, 13–17. [Google Scholar]
- Martos, F.; Munoz, F.; Pailler, T.; Kottke, I.; Gonneau, C.; Selosse, A. The role of epiphytism in architecture and evolutionary constraint within mycorrhizal networks of tropical orchids. Mol. Ecol. 2012, 21, 5098–5109. [Google Scholar] [CrossRef]
- Rammitsu, K.; Yamamoto, N.; Chamara, R.M.S.R.; Minobe, M.; Kinoshita, A.; Kotaka, N.; Ogura-Tsujita, Y. The epiphytic orchid Vanda falcata is predominantly associated with a single Tulasnellaceae fungus in adulthood, and Ceratobasidiaceae fungi strongly induce its seed germination in vitro. Plant Species Biol. 2023, 38, 306–318. [Google Scholar] [CrossRef]
- Ming, M.A.; Tan, T.K.; Wong, S.M. Identification and molecular phylogeny of Epulorhiza isolates from tropical orchids. Mycol. Res. 2003, 107, 1041–1049. [Google Scholar] [CrossRef]
- Nontachaiyapoom, S.; Sasirat, S.; Manoch, L. Isolation and identification of Rhizoctonia-like fungi from roots of three orchid genera, Paphiopedilum, Dendrobium, and Cymbidium, collected in Chiang Rai and Chiang Mai provinces of Thailand. Mycorrhiza 2010, 20, 459–471. [Google Scholar] [CrossRef]
- Chamara, R.M.S.R.; Rammitsu, K.; Minobe, M.; Kinoshita, A.; Kotaka, N.; Yukawa, T.; OguraTsujita, Y. Mycorrhizal fungi of Phalaenopsis japonica (Orchidaceae) and their role in seed germination and seedling development. Diversity 2024, 16, 218. [Google Scholar] [CrossRef]
- Zhang, L.; Rammitsu, K.; Kinoshita, A.; Tokuhara, K.; Yukawa, T.; Ogura-Tsujita, Y. Symbiotic culture of three closely related Dendrobium species reveals a growth bottleneck and differences in mycorrhizal specificity at early developmental stages. Diversity 2022, 14, 1119. [Google Scholar] [CrossRef]
- Rammitsu, K.; Kajita, T.; Imai, R.; Ogura-Tsujita, Y. Strong primer bias for Tulasnellaceae fungi in metabarcoding: Specific primers improve the characterization of the mycorrhizal communities of epiphytic orchids. Mycoscience 2021, 62, 356–363. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Whigham, D.F. Phenology of roots and mycorrhiza in orchid species differing in phototrophic strategy. New Phytol. 2002, 154, 797–807. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Izumitsu, K.; Hatoh, K.; Sumita, T.; Kitade, Y.; Morita, A.; Tanaka, C.; Gafur, A.; Ohta, A.; Kawai, M.; Yamanaka, T.; et al. Rapid and simple preparation of mushroom DNA directly from colonies and fruiting bodies for PCR. Mycoscience 2012, 53, 396–401. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Waud, M.; Busschaert, P.; Ruyters, S.; Jacquemyn, H.; Lievens, B. Impact of primer choice on characterization of orchid mycorrhizal communities using 454 pyrosequencing. Mol. Ecol. Resour. 2014, 14, 679–699. [Google Scholar] [CrossRef] [PubMed]
- Oja, J.; Kohout, P.; Tedersoo, L.; Kull, T.; Kõljalg, U. Temporal patterns of orchid mycorrhizal fungi in meadows and forests as revealed by 454 pyrosequencing. New Phytol. 2015, 205, 1608–1618. [Google Scholar] [CrossRef]
- Rammitsu, K.; Abe, S.; Abe, T.; Kotaka, N.; Kudaka, M.; Kudaka, N.; Kinoshita, A.; Ogura-Tsujita, Y. The endangered epiphytic orchid Dendrobium okinawense has a highly specific mycorrhizal association with a single Tulasnellaceae fungus. J. For. Res. 2021, 26, 215–221. [Google Scholar] [CrossRef]
- Kuraku, S.; Zmasek, C.M.; Nishimura, O.; Katoh, K. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 2013, 41, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice, and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Turenne, C.Y.; Sanche, S.E.; Hoban, D.J.; Karlowsky, J.A.; Kabani, A.M. Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J. Clin. Microbiol. 1999, 37, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 25 December 2023).
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An analysis tool set for population genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open-source tool for metagenomics. PeerJ 2016, 4, 2584. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE QIIME Release for Fungi. 887 Version 16.10.2022. UNITE Community, 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/37953409/ (accessed on 19 May 2024). [CrossRef]
- Meng, Y.Y.; Fan, X.L.; Zhou, L.R.; Shao, S.C.; Liu, Q.; Selosse, M.A.; Gao, J.Y. Symbiotic fungi undergo a taxonomic and functional bottleneck during orchid seeds germination: A case study on Dendrobium moniliforme. Symbiosis 2019, 79, 205–212. [Google Scholar] [CrossRef]
- Ogura-Tsujita, Y.; Gebauer, G.; Xu, H.; Fukasawa, Y.; Umata, H.; Tetsuka, K.; Kubota, M.; Schweiger, I.; Yamashita, S.; Maekawa, N.; et al. The giant mycoheterotrophic orchid Erythrorchis altissima is associated mainly with a divergent set of wood-decaying fungi. Mol. Ecol. 2018, 27, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rammitsu, K.; Tetsuka, K.; Yukawa, T.; Ogura-Tsujita, Y. Dominant Dendrobium officinale mycorrhizal partners vary among habitats and strongly induce seed germination in vitro. Front. Ecol. Evol. 2022, 10, 994641. [Google Scholar] [CrossRef]
- Bertolini, V.; Cruz-Blasi, J.; Damon, A.; Mora, J.V. Seasonality and mycorrhizal colonization in three species of epiphytic orchids in southeast Mexico. Acta Bot. Bras. 2014, 28, 512–518. [Google Scholar] [CrossRef]
- Xing, X.; Ma, X.; Men, J.; Chen, Y.; Guo, S. Phylogenetic constraints on mycorrhizal specificity in eight Dendrobium (Orchidaceae) species. Sci. China Life Sci. 2017, 60, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Bayman, P.; Mosquera-Espinosa, A.T.; Saladini-Aponte, C.M.; Hurtado-Guevara, N.C.; Viera-Ruiz, N.L. Age-dependent mycorrhizal specificity in an invasive orchid, Oeceoclades maculata. Am. J. Bot. 2016, 103, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Ogura-Tsujita, Y.; Yukawa, T. High mycorrhizal specificity in a widespread mycoheterotrophic plant, Eulophia zollingeri (Orchidaceae). Am. J. Bot. 2008, 95, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Yagame, T.; Funabiki, E.; Nagasawa, E.; Fukiharu, T.; Iwase, K. Identification and symbiotic ability of Psathyrellaceae fungi isolated from a photosynthetic orchid, Cremastra appendiculata (Orchidaceae). Am. J. Bot. 2013, 100, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Padamsee, M.; Matheny, P.B.; Dentinger, B.T.; McLaughlin, D.J. The mushroom family Psathyrellaceae: Evidence for large-scale polyphyly of the genus Psathyrella. Mol. Phylogenetics Evol. 2008, 46, 415–429. [Google Scholar] [CrossRef]
- Zhao, C.; Qu, M.; Huang, R.; Karunarathna, S.C. Multi-gene phylogeny and taxonomy of the wood-rotting fungal genus Phlebia sensu lato (Polyporales, Basidiomycota). J. Fungi 2023, 9, 320. [Google Scholar] [CrossRef]
- Hu, J.J.; Li, Y.; Li, X.; Frederick, S.L.; Zhang, B. New findings of Neonothopanus (Marasmiaceae, Basidiomycota) from Ghana. Phytotaxa 2021, 512, 57–67. [Google Scholar] [CrossRef]
- Wang, B.; Dai, Y.C.; Cui, B.K.; Du, P.; Li, H.J. Wood-rotting fungi in eastern China 4. Polypores from Dagang Mountains, Jiangxi Province. Cryptogam. Mycol. 2009, 30, 233–241. [Google Scholar]
- Petrolli, R.; Selosse, M.A.; Bonillo, C.; Griveau, C.; Lalanne-Tisné, G.; Comes, B.; Kodja, H.; Martos, F. Mycorrhizal communities of Vanilla planifolia in an introduction area (La Réunion) under varying cultivation practices. Plants People Planet 2024, 6, 683–696. [Google Scholar] [CrossRef]
- Bautista, N.S.; Valentino, M.J.G. Symbiotic propagation of Dendrobium bigibbum Lindl. with selected saprophytic Basidiomycota. Biodiversitas 2023, 24, 3519–3527. [Google Scholar] [CrossRef]
- Manuel, G.; Jeannette, N.R.I. Preliminary evaluation of the potential of macromycetes as mediators in bio-hardening of orchid plantlets. Acta Sci. Agric. 2020, 4, 141–146. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Duffy, K.J.; Selosse, M.A. Biogeography of orchid mycorrhizas. In Biogeography of Mycorrhizal Symbiosis; Tedersoo, L., Ed.; Ecological Studies; Springer: Cham, Switzerland, 2017; Volume 230, pp. 159–177. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 2018, 219, 1207–1215. [Google Scholar] [CrossRef]
- Calevo, J.; Voyron, S.; Adamo, M.; Alibrandi, P.; Perotto, S.; Girlanda, M. Can orchid mycorrhizal fungi be persistently harbored by the plant host? Fungal Ecol. 2021, 53, 101071. [Google Scholar] [CrossRef]
- Bonnardeaux, Y.; Brundrett, M.; Batty, A.; Dixon, K.; Koch, J.; Sivasithamparam, K. Diversity of mycorrhizal fungi of terrestrial orchids: Compatibility webs, brief encounters, lasting relationships, and alien invasions. Mycol. Res. 2006, 111, 51–61. [Google Scholar] [CrossRef]
- Zettler, L.W.; Dvorak, C.J. Tulasnella calospora (UAMH 9824) retains its effectiveness at facilitating orchid symbiotic germination in vitro after two decades of subculturing. Bot. Stud. 2021, 62, 14. [Google Scholar] [CrossRef] [PubMed]
- Ventre Lespiaucq, A.; Jacquemyn, H.; Rasmussen, H.N.; Mendez, M. Temporal turnover in mycorrhizal interactions: A proof of concept with orchids. New Phytol. 2021, 230, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.Y.; Chen, X.M.; Guo, S.X.; Lee, Y.I. Effect of different mycobionts on symbiotic germination and seedling growth of Dendrobium officinale, an important medicinal orchid. Bot. Stud. 2020, 61, 2. [Google Scholar] [CrossRef]
- Meng, Y.; Shao, S.; Liu, S.; Gao, J. Do the fungi associated with roots of adult plants support seed germination? A case study on Dendrobium exile (Orchidaceae). Glob. Ecol. Conserv. 2019, 17, e00582. [Google Scholar] [CrossRef]
- Johnson, T.R.; Stewart, S.L.; Dutra, D.; Kane, M.E.; Richardson, L. Asymbiotic and symbiotic seed germination of Eulophia alta (Orchidaceae)—Preliminary evidence for the symbiotic culture advantage. Plant Cell Tissue Organ Cult. 2007, 90, 313–323. [Google Scholar] [CrossRef]
- Júnior, D.E.; Alves-Silva, G.; Sasamori, M.H.; da Silveira, R.M.B.; Droste, A. Successful Tulasnella amonilioides isolation from wild Cattleya intermedia and effectiveness of the mycobiont on in vitro propagation of this threatened Orchidaceae. J. Environ. Anal. Progr. 2023, 8, 009–029. [Google Scholar] [CrossRef]

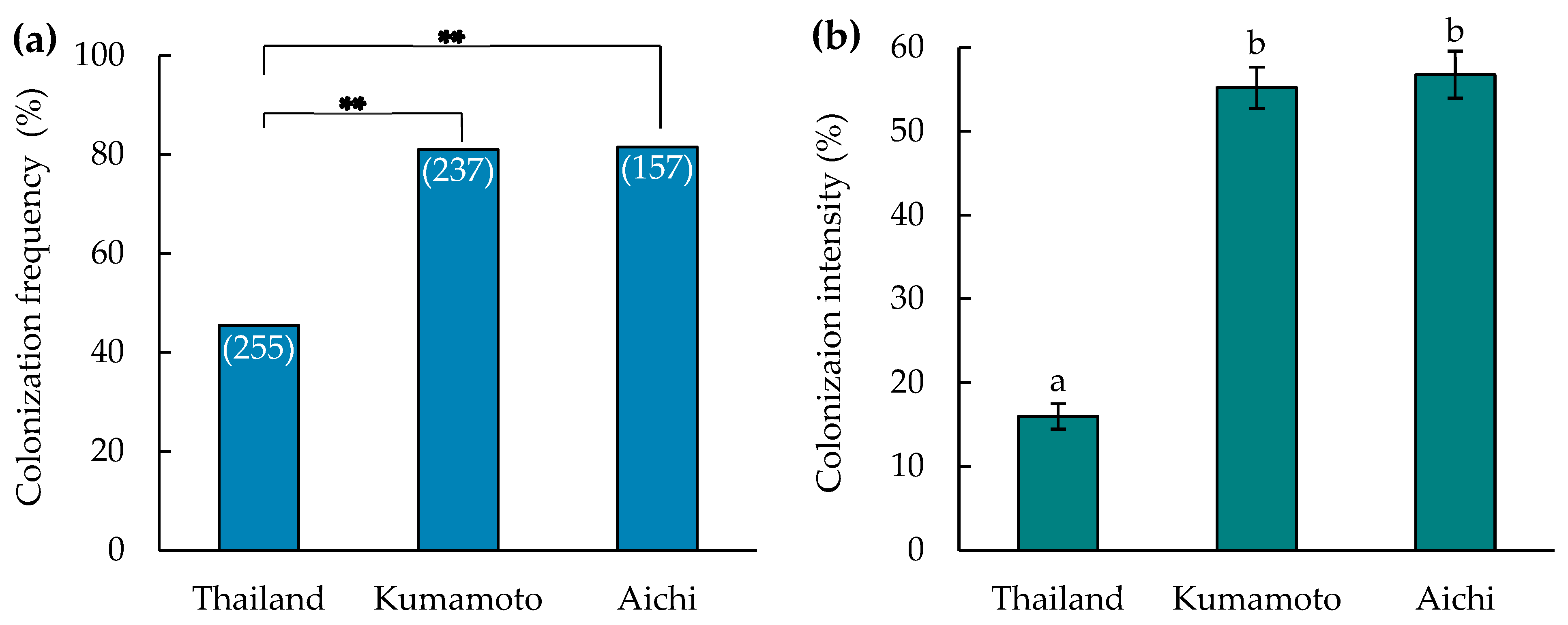




| Location | No. of Plants | No. of Roots | No. of Sections Observed | Growth Stage | Growth Medium |
|---|---|---|---|---|---|
| Chiang Mai Prov., Thailand | 5 | 64 | 255 | Juvenile | Coconut husk |
| Kumamoto Pref. Japan | 4 | 46 | 237 | Mature | Sphagnum moss |
| Aichi Pref. Japan | 4 | 28 | 157 | Mature | Coconut husk and Sphagnum moss |
| Fungal OTU | Host Orchid | DDBJ Accession No. | NBRC Accession No. |
|---|---|---|---|
| TU27 | D. officinale | LC683202 | NBRC 115262 |
| TU47 | D. Stardust ‘Firebird’ | LC814994 | NBRC 116648 |
| TU63 | D. Stardust ‘Firebird’ | LC814996 | NBRC 116574 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamara, R.M.S.R.; Miyoshi, K.; Yukawa, T.; Asai, N.; Ogura-Tsujita, Y. Orchid Mycorrhizal Association of Cultivated Dendrobium Hybrid and Their Role in Seed Germination and Seedling Growth. Microorganisms 2024, 12, 1176. https://doi.org/10.3390/microorganisms12061176
Chamara RMSR, Miyoshi K, Yukawa T, Asai N, Ogura-Tsujita Y. Orchid Mycorrhizal Association of Cultivated Dendrobium Hybrid and Their Role in Seed Germination and Seedling Growth. Microorganisms. 2024; 12(6):1176. https://doi.org/10.3390/microorganisms12061176
Chicago/Turabian StyleChamara, R. M. S. Ruwan, Kazumitsu Miyoshi, Tomohisa Yukawa, Nobuyuki Asai, and Yuki Ogura-Tsujita. 2024. "Orchid Mycorrhizal Association of Cultivated Dendrobium Hybrid and Their Role in Seed Germination and Seedling Growth" Microorganisms 12, no. 6: 1176. https://doi.org/10.3390/microorganisms12061176
APA StyleChamara, R. M. S. R., Miyoshi, K., Yukawa, T., Asai, N., & Ogura-Tsujita, Y. (2024). Orchid Mycorrhizal Association of Cultivated Dendrobium Hybrid and Their Role in Seed Germination and Seedling Growth. Microorganisms, 12(6), 1176. https://doi.org/10.3390/microorganisms12061176






