Filling the Knowledge Gap Regarding Microbial Occupational Exposure Assessment in Waste Water Treatment Plants: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy, Inclusion and Exclusion Criteria
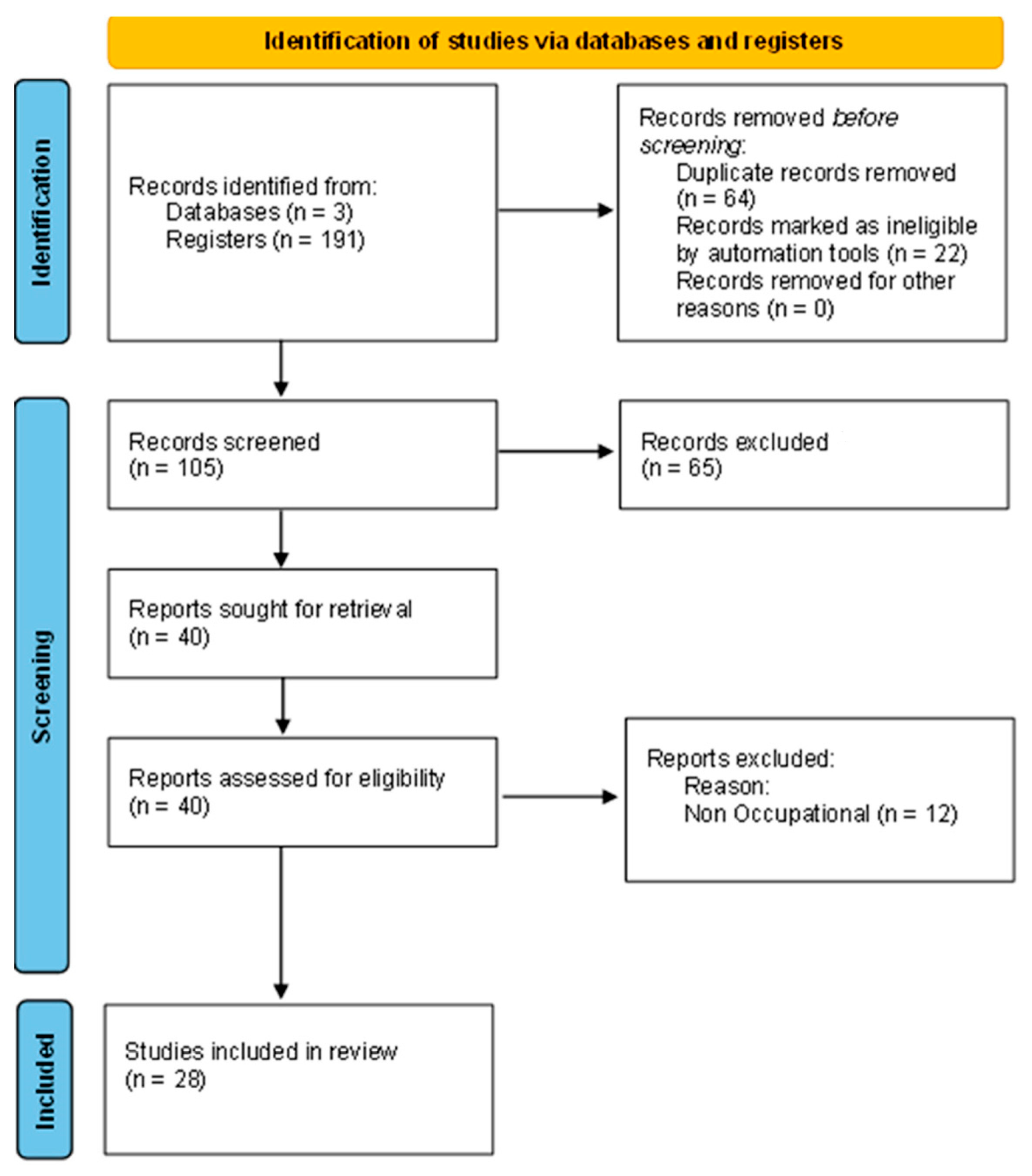
2.2. Studies Selection and Information to Be Retrieved from the Papers
2.3. Quality Assessment
3. Results
Extracted Data
| Title | Type of WWTP | Microbe Assessed | Sampling Methods | Season of Sampling | Sampling Sites and Number of Samples | Assays | Main Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Exposure to Airborne Noroviruses and Other Bioaerosol Components at a Wastewater Treatment Plant in Denmark | no data | Noroviruses (NoVs), Adenoviruses, Endotoxin, Bacteria and Molds | Air samples: Active methods—Filtration (Personal Dust Sampling-Inhable GSP samplers with teflon filters or polycarbonate filters, average sampling period 242 min), Stationary measurements of “total dust” | no data | Personal dust sampling was carried out on 16 workers, on different wastewater processes; stationary sampling was carried out in the aeration basin at 1.5 m above the ground level | Culture-based methods (DG18 agar for cultivable moulds, nutrient agar for cultivable bacteria) | NoVs and endotoxin were detected at concentrations that could pose an occupational health risk. Positive correlations between exposure to endotoxin, bacteria, moulds and NoVs were found and indicate that the exposure to bioaerosols may be related to work tasks. | [18] |
| ADMS simulation and influencing factors of bioaerosol diffusion from BRT under different aeration modes in six wastewater treatment plants | Municipal WWTP | Bacteria and Intestinal Bacteria | Air samples: Active methods—Impaction (Andersen six-stage cascade impactor, flow rate = 28.3 L/min; TH-150 medium flow sampler) | Seasonal (spring) | 1.5 m above aeration tanks of 6 Municipal Wastewater Treatment Plant (MWWT), 6 samples were taken at each sampling site, (n = 36) | Culture-based methods (LB medium for bacteria, and for intestinal bacteria, MAC); Ion chromatography and Illumina MiSeq high-throughtput sequencing | The concentrations of bacteria and, specifically, intestinal bacteria in the bioaerosols ranged from 389 CFU/m3 to 1536 CFU/m3 and 30 CFU/m3 to 152 CFU/m3, respectively, and the proportion of the intestinal bacteria was 8.85%. The proportion of intestinal bacteria (75.79%) produced via surface aeration by Biological Reaction Tanks (BRT) attached to large-sized bioaerosol particles was higher than that of a BRT undergoing the bottom aeration process (37.28%). The main microorganisms found in the bioaerosols included Moraxellaceae, Escherichia–Shigella, Psychrobacter, and Cyanobacteria. | [22] |
| Spatio-temporal variations in airborne bacteria from the municipal wastewater treatment plant: a case study in Ahvaz, Iran | Municipal WWTP | Airborne Bacteria | Air Samples: Passive methods (microbiological sampling index of microbial air contamination (1/1/1 standard)) | Seasonal (warm and cold) | Grit chamber (GCh), primary sludge dewatering basin (PSDB) and at the aeration tank (AT); (n = 180) | Culture-based methods (Trwith nystatin (250 mg/L) to inhibit fungal growth); PCR-RFLP | The dominant bacterial genus included Bacillus pumilus (26.7%), Staphylococcus arlettae (23.2%), Kocuria turfanensis (13.6%), and Alicycliphilus (9.2%), and they increased with high temperatures and wind speed, and decreased with high humidity. | [6] |
| Emission level, particle size and exposure risks of airborne bacteria from theoxidation ditch for seven months observation | WWTP with orbal oxidation ditch process | Airbone Bacteria | Air samples: Active—Impaction (Andersen six-stage cascade impactor, flow rate = 28.3 L/min); Material collection (raw water in the oxidation ditch) | Seasonal (spring and summer) | ConS: Control site was set 300 m upwind from the oxidation ditch; AWS (above water surface): above water surface; AWS-0.5: above water surface 1 m; AWS-3: above water surface 3 m; ARB (above rotating brushes)-25: after the rotating brushes 25 m; ARB-55: after the rotating brushes 55 m; ARB-210: after the rotating brushes 210 m; (n = 168) | Culture-based methods (with nutrient agar for mesophilic bacteria) for air samples; Gradient gel electrophoresis for 16S rDNA; PCR | Spatial and seasonal variations in the concentrations of airborne bacteria emissions were detected. The highest concentration was observed near the rotor disc aerators (RDAs) (835 ± 91 CFU/m3 to 8916 ± 155 CFU/m3) during each sampling process, with the concentration decreasing by 76.70% and 79.91% as sampling distance and height increased, respectively. Most of the airborne bacteria were coarse particles that exceeded 4.7 μm in size. The dominant bacteria were Bacillus sp., Lysinibacillus sp., and Sphingomonas sp. | [23] |
| Aerosol partitioning potential of bacteria presenting antimicrobial resistance from different stages of a small decentralized septic treatment system | On-site/decentralized WWTP | Antibiotic-Resistant Bacteria (ARB) | Air samples: Active method—Impinger (Wetted wall cyclone collectors (WWC)); Material collection (stainless steel porTable 600 mL water dipper (Grainger Industrial Supply)) | Seasonal (summer and winter) | Aerosol and water samples were collected at the four tanks; 600 mL of water and 1500 L of air at each tank | Kirby–Bauer testing for antibiotic resistance, quantitative Polymerase Chain Reaction (qPCR); 16SrRNA-based Illumina sequencing | As expected, the higher concentration of bacteria was found when the lids were open; in the summer, Legionella was found in the water tanks 1 and 3, and in the water tank 1 Pseudomonas was present; in the winter, Legionella was also present in the water tank 1; bioaerossol samples showed a higher antimicrobial resistance of 50% (at four of the eight antbiotics tested), and the higher antimicrobial resistance of the water samples was 87.5% (resistance in the 7 of the 8 antibiotics). | [36] |
| Identification of airborne fungi’s concentrations in indoor and outdoor air of municipal wastewater treatment plant | Municipal WWTP with conventional activated sludge treatment process | Fungi | Air Samples: Passive methods (microbiological sampling index of microbial air contamination (1/1/1 standard)) | Seasonal (warm and cold) | Grit chamber tank, primary sludge dewatering basin, aeration tank, upstream and downstream of dominant wind blowing at the site and at the administrative building (n = 240) | Culture-based methods (SDA with chloramphenicol antibiotic (100 mg/L) to inhibit bacterial growth) | The greatest release of fungal aerosols occurred in the cold season while the minimum release occurred in the warm season; the highest concentrations of fungi were observed in the grit chamber unit; Cladosporium (39.23%) and Alternaria (19.87%) were the airborne fungal genera most common. | [29] |
| Aspergillus spp. prevalence in different Portuguese occupational environments: What is the real scenario in high load settings? | no data | Aspergillus spp. | Air samples: Active methods—Impaction (Millipore air Tester, flow rate = 140 L/min) and Impinger (Coriolis μ air sampler, flow rate = 300 L/min); Passive methods: surface samples (swabs) | 1 year longitudinal study | Sampling occured at 2 Watewater Treatment Plant (WWTP); 26 air sample and 15 surface samples | Culture-based methods (MEA); Real Time PCR (RT-PCR) | At both WWTPs were found 33 different species of Aspergillus spp. (18 at WWTP1 and 15 at WWTP2), 7 species were only isolated in surfaces (5 in the WWTP1 and 2 at WWTP2), and 12 different Aspergillus sections were identified (6 in both WWTP). | [14] |
| Wastewater treatment plant workers’ exposure and methods for risk evaluation of their exposure | WWTP with anaerobic–anoxic–oxic process | Airborne Bacteria, Enteric Bacteria, Endotoxins | Air Samples: Active methods—Filtration (personal and stationary GSP samplers with polycarbonate filters or with Teflon filter, flow rate = 3.5 L/min) and Impaction (Andersen six-stage cascade impactor, flow-rate 28.3 L/min) | 1 year longitudinal study | Stationary samples were taken in the grid chamber house and in the aeration tank (106 personal GSP samples, 12 stationary GSP samples), and 141.5 L to 843 L of air by ASCI were taken over the year | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) | A total of 22.36% of the bacteria potentially inhaled by WWTP workers seem to be from the air around the aeration tank and 22.40% from the grid house; Staphylococcus (13.2%) and Aeromonas (11.7%) were the dominant genera at the aeration tank, while Acinetobacter (25.6%) was the dominant in grid house. | [19] |
| Anaerobic bacteria in wastewater treatment plant | Mechanical–biological WWTP | Anaerobic Bacteria | Air samples: Active method—Impaction (Andersen six-stage cascade impactor, flow-rate = 28.3 L/min); Material collection (sewage and sludge samples were taken directly into 50 mL sterile screwed-off Falcon tubes) | Seasonal (summer and winter) | Bar screens, containers with solids in the screens’ hall, primary settling tank, sewage sludge pumping station, aeration basins incineration plant, sludge-thickening building, and at the background of WWTP (n = 22) | Culture-based methods (Schaedler agar with 5% additive of sheep blood for bacterial growth); PCR (for confirmation of Clostridium isolates); Biochemical API 20A test (bioMérieux) | Some of the anaerobic bacteria identified belongs to the risk group 2 (according to the EU Directive 2000/54/EC); Actinomyces, Bifidobacterium, Clostridium and Propionibacterium genera were identified in wastewater and in the air. | [16] |
| Bioaerosols emission characteristics from wastewater treatment aeration tanks and associated health risk exposure assessment during autumn and winter | Municipal WWTP with rotating disc aeration tank, adopted with DE oxidation ditch treatment process, and microporous aeration tank and adopted with Anaerobic–anoxic–oxic process | Escherichia coli and Staphylococcus aureus | Air samples: Active—Impaction (Andersen six-stage cascade impactor, flow-rate = 28.3 L/min); Material collection (500 mL wastewater samples were taken by a sterility water sampling bottle) | Seasonal (autumn and winter) | The sampling was carried out at 3 WWTPs, and they were located in the middle of the center corridor of the second microporous aeration tank and the first rotating disc aeration tank from north to south | Culture-based methods (for S. aureus MYP was used, and MAC for E. coli) | Staphylococcus aureus was about 2 times higher in winter than in autumn, while Escherichia coli in autumn was about 9 times higher than in winter. | [24] |
| Influence of seasons and sites on bioaerosols in indoor wastewater treatment plants and proposal for air quality indicators | Municipal WWTP with pre-, primary and secondary treatments | Bacteria and Endotoxins | Air samples: Active—Impaction (Andersen six-stage cascade impactor, flow rate = 28.3 L/min) and Filtration (37 mm cassettes (SKC) loaded with binder-free glass fiber filters, flow-rate = 2 L/min) | Seasonal (warm and cold) | Screening, degreasing/grit removal, settling tanks and biofiltration | Culture-based methods (TSA to collect total culturable aerobic bacteria and Gram-negative selective agar (GNSA) for culturable Gram-negative bacteria). In addition to total bacteria (bacteria 16S rDNA), specific qPCR was used to monitor bacteria from human flora: E. coli, Klebsiella pneumonia, Pseudomonas aeruginosa, and fresh water environment: Aeromonas hydrophila. | The average concentration of culturable Gram-negative bacteria was approximately 100 CFU/m3 for both seasons. Only two WWTPs showed concentrations of culturable Gram-negative bacteria higher than the recommended exposure limit (1000 CFU/m3 according to Institut Robert Sauvé en Santé et en Sécurité du Travail (IRSST). Several values were close to the limit. | [37] |
| Assessment of bioaerosol contamination (bacteria and fungi) in the largest urban wastewater treatment plant in the Middle East | Municipal WWTP with air diffusion by fine bubble diffusers | Airborne Bacteria and Fungi | Air samples: Active method—Impaction (QuickTake 30 sample pump equipped with the Bio Stage single-stage cascade impactor, flow rate = 28.3 L/min) | 1 year longitudinal study | Area adjacent to the aeration tank and secondary sedimentation units, near the tricking filter, near the sludge storage tank and sludge dewatering unit, adjacent the screening, grit chamber, and primary sedimentation unit and outside of the WWTP were the locations of the sampling; (n = 240) | Culture-based methods (TSA for airborne bacteria growth, and SDA for fungal growth) | Maximum bacterial concentration was found in the aeration tank in the summer, and the minimum was in the sludge dewatering unit during the winter; maximum and minimum fungal concentrations were in primary treatment and sludge dewatering unit in winter and summer, respectively. Micrococcus spp. and Staphylococcus spp. had the highest emission of bacteria in the winter and summer, respectively. Cladosporium spp., Penicillium spp., Aspergillus spp. and Alternaria spp. were the dominant fungi. | [30] |
| Characterization of the airborne bacteria community at different distances from the rotating brushes in a wastewater treatment plant by 16S rRNA gene clone libraries | Municipal WWTP with orbal oxidation ditch treatment process | Airborne Bacteria | Air samples: Active methods—Impaction (six-stage Impacting Airborne Microorganism Sampler—FA-1, 28.3 L/min) and Impinger (SKC BioSampler, flow rate = 12.5 L/min) | no data | Aerosol samples were collected at different distances from the rotating brushes in the oxidation ditch; 1.5 L for each sample | Culture-based methods; PCR; Sequencing | The majority of bacteria in the bioaerosols were Proteobacteria and Bacteroidetes around the oxidation ditch. The study concluded that the rotating brush aeration was the main source of bioaerosols in the oxidation ditch. | [25] |
| Genomic insight into transmission mechanisms of carbapenem-producing Citrobacter spp. isolates between the WWTP and connecting rivers | WWTP with anaerobic–anoxic–oxic treatment process | Carbapenem-Resistant Citrobacter spp. (CRCS) | Material collection (wastewater and sludge mixtures samples with a total volume of 1 L) | Seasonal (spring, summer, autumn and winter) | Water inlet, anaerobic tank, aerobic tank, sludge thickening tank, activated sludge tank, mud cake storage area, and water outlet; In total, 136 river water and 51 river sediment samples were collected and 189 samples were gathered from the WWTP. | Culture-based methods; PCR; 16s RNA sequencing; MALDI-TOF MS | In total, 14 CRCS were detected in 376 environmental samples, including those from the inlet (n = 7), anaerobic tank (n = 2), and rivers (n = 5). Citrobacter braakii (n = 6) was the dominant subtype among 14 CRCS isolates, followed by Citrobacter freundii (n = 5), Citrobacter sedlakii (n = 2), and Citrobacter werkmanii (n = 1). All CRCS showed resistance to the studied antibiotics. | [26] |
| Aspergillus flavus contamination in two Portuguese wastewater treatment plants | WWTP with primary, secondary and tertiary treatment processes | Aspergillus spp. | Air samples: Active methods—Impaction (Millipore, flow rate = 140 L/min) and Impinger (Coriolis µ air sampler, flow rate = 300 L/min); Passive methods: surface samples (swabs) | Seasonal (winter) | Ten sampling locations were established at the two WWTP for assessing indoor air contamination: lift station, flotation sludge, sludge dewatering, screening, co-generation, aerobic digestion (secondary treatment), canteen, operation room, grit removal, and administration room. An outdoor reference sampling was also included; air samples: 26 indoor and 2 outdoor; surface samples: 17 indoor | Culture-based methods (MEA); RT-PCR | In both WWTPs, Aspergillus versicolor (38%), Aspergillus candidus (29.1%), and Aspergillus sydowii (12.7%) were the most common. In the surfaces were Aspergillus flavus (47.3%), Aspergillus fumigatus (34.4%), and Aspergillus sydowii (10.8%). The isolates of Aspergillus flavus that were inoculated in coconut agar medium were not identified as toxigenic, and were not detected by RT-PCR. | [13] |
| Bioaerosol emissions and detection of airbone antibiotic resistance genes from a wastewater treatment plant | Municipal WWTP with activated sludge treatment process | Culturable Bacteria and Fungi; Fluorescent Bioaerosols | Air Samples: Active method—Impaction (Reuter Centrifugal Sampler High Flow, flow-rate = 100 L/min) and Impinger (SKC Biosampler, flow-rate = 12.5 L/min; Particulate matter (Ultraviolet aerodynamic 190 particle sizer (UV-APS) | Seasonal (spring, summer, autumn, and winter) | Sludge thickening basin, biological reaction basin, screen room | Culture-based methods (with TSA and MEA for bacterial and fungal growth, respectively); PCR | Highest concentrations in sludge thickening basin (bacteria: 1697 CFU/m3, fungi: 930 CFU/m3). Bacterial concentrations met Chinese standards, but fungal levels exceeded World Health Organization (WHO) recommendations in some areas. | [4] |
| Occupational Exposure to Staphylococcus aureus in the Wastewater Treatment Plants Environment | Municipal WWTPs with mechanical, chemical and biological treatments processes | Staphylococcus aureus | Air samples: Active methods—Impaction (1-step portable air sampler made by Burkard, flow rate = 20 L/min) and Filtration (GilAir-5 pump and an open-faced aerosol sampler with a gelatin filter of a 37 mm in diameter and 3 µm pores at a flow rate of 3 L/min); Material collection (raw wastewater discharged into the wwtp and treated wastewater) | Seasonal (spring and summer) | The study was conducted in 16 WWTPs in Poland, representing different treatment technologies; a total of 286 samples were collected, including 253 air samples and 33 wastewater samples | Culture-based methods (chromogenic substrate CHROMID® S. aureus Elite agar); MALDI-TOF, and an automatic method for antibiotic resistence na alysis (WalkAway system) | The study identified Staphylococcus aureus, including antibiotic-resistant strains, in wastewater and air samples from WWTP. Workers engaged in mechanical treatment faced the highest health risk. | [17] |
| COVID-19 infection risk from exposure to aerosols of wastewater treatment plants | Municipal with activated sludge treatment process | SARS-CoV-2 | Air samples: Active method—Impinger (Portable pumps; flow rate = 7.5–8.5 L/min); Material collection (Grab samples—raw wastewater was colected in 250 mL in sterile glasses) | 1 year longitudinal study | Pumping station and activated sludge plants; a total of 24 raw wastewater samples were collected, with 12 samples from each of the two wastewater treatment plants (WWTPs) and 15 air samples. | RT-qPCR | SARS-CoV-2 RNA was found in 37.5% of wastewater samples. Detected in 5 of 12 samples from WWTP A and 4 of 12 samples from WWTP B. The highest concentration was observed at the pumping station. | [31] |
| Dispersion and Risk Assessment of Bacterial Aerosols Emitted from Rotating- Brush Aerator during Summer in a Wastewater Treatment Plant of Xi’an, China | WWTP with oxidation ditch process | Bacteria | Air samples: Active method—Impaction (Andersen six-stage cascade impactor, flow rate = 28.3 L/min) | Seasonal (summer) | Directly Downwind Sites: 2 m downwind 5 m downwind 10 m downwind 30 m downwind 50 m downwind 100 m downwind Lateral Sites: G1 (5 m laterally from the aerator) G2 (5 m laterally from the aerator) | Culture-based methods | Higher airborne bacteria concentrations were observed closer to the aerator. | [5] |
| Airborne bacteria in a wastewater treatment plant: Emission characterization, source analysis and health risk assessment | WWTP with anaerobic–anoxic–oxic process | Bacteria | Air samples: Active method—Impaction (Quartz membranes (90 mm, Whatman QM-A), flow-rate = 100 L/min and TH-150) | Seasonal (spring, summer and winter) | The WWTP has various treatment stages, including CS (possibly activated sludge), AGC (grit chamber), PST (primary settling tank), AnT (possibly anoxic tank), AeT (aeration tank), and SST (secondary settling tank). Indoor facilities like CS and SDH (sludge dewatering with decanter centrifuges) were compared with outdoor facilities like AGC, PST, and AeT. | High-throughput sequencing techniques | Concentrations varied by site and season. Treatment stages were significant emission sources. | [27] |
| Quantifying the Relationship between SARS-CoV-2 Wastewater Concentrations and Building-Level COVID-19 Prevalence at an Isolation Residence: A Passive Sampling Approach | no data | SARS-CoV-2 | Passive method (tampons made from rayon with a polyester string) | Seasonal (spring) | Approximately 190 feet from the isolation residence, and the wastewater influent at this location was restricted to the isolation building | RT-qPCR | The virus was detected over 16 weeks, demonstrating its feasibility for identifying residential halls with infected individuals. The daily viral wastewater load showed a positive association with the building’s COVID-19 prevalence. | [35] |
| Assessment of airborne virus contamination in wastewater treatment plants | no data | Adenovirus (AdV); Norovirus (NoV); Hepatitis E Virus (HEV) | Air samples: Active method—Impaction (3 μm pore size, 25 mm gelatine filters embedded in standard cassettes using MSA Escort Elf or SKC pocket pump 210–1002, flow rate = 4 L/min) | Seasonal (summer and winter) | Inside (Enclosed Area): One sample was collected in the enclosed area, specifically near the water inlet. The sampling point inside was close to the rake that removes large particles from incoming water. Outside (Unenclosed Area): Another sample was collected in the unenclosed area, specifically above the bubbling aeration basin; 123 air samples from 31 WWTPs. | qPCR | AdV-F was present in all WWTPs during summer and 97% during winter. Concentrations were higher in summer, reaching a maximum of 2.27 × 106 genome equivalent/m3. AdV-E/D were detected in winter, only in a few samples. NoV was detected in only 3 out of 123 air samples, with concentrations below quantification limits. HEV was not detected in any of the samples. | [20] |
| Airborne bacteria and fungi in a wastewater treatment plant: type and characterization of bio-aerosols, emission characterization and mapping | no data | Bacteria and Fungi | Air samples: Active method—Impaction (One-Stage Andersen cascade impactor, flow rate = 28.3 L/min) | Seasonal (spring, summer and winter) | ETP (Entrance of Treatment Plant), Gch (Grit Chamber), SDB (Sludge Drying Bed), Aea tank (Aeration tank), and Lab (Laboratory Building). Within the mentioned areas, specific points were chosen for sampling, such as the pumping station, additional points in SDB, Gch, Aea tank, and the laboratory. | PCR; biochemical tests: urease, oxidative fermentative (OF), oxidase, catalase, triple sugar iron (TSI), eosin methylene blue (EMB), and Indole-Methyl red-Voges-Proskauer-Citrate (IMViC) test | Various bacteria were identified (some with pathogenic potential), and fungi were present in the air of the WWTP. Bacterial concentrations exceeded the standards, as is the case of Staphylococcus and Enterobacteriaceae. Fungal concentrations varied seasonally and by location. The relationship between meteorological parameters and bio-aerosols was explored, with temperature showing significance. Particulate matter, especially PM10, correlated significantly with fungal concentrations. | [33] |
| Exposure to Bioaerosol from Sewage Systems | no data | Mesophilic Bacteria; Coliform Bacteria; Aspergillus fumigatus | Air samples: Active methods—Impaction (MAS-100, flow rate = 100 L/min) and Impinger (SKC Biosampler, flow rate = 12.5 ± 0.1 L/min) | Seasonal (summer and winter) | At hospital sewage (K1), relief chamber of a combined sewage overflow (K2) and in the area of a city treatment plant (K3); 30 air samples | Culture-based methods (Blood agar was used for mesophilic bacteria and Aspergillus fumigatus, Endoagar for coliform bacteria, Coli-ID agar for Escheriachia coli, Hektoenagar for Salmonella sp., and Camplylobacter agar with selective supplement for Camplylobacter sp.) | Mesophilic Bacteria Concentrations: Location K1 had concentrations ten times higher than ambient air, attributed to the small chamber size. Location K2 exhibited concentrations comparable to ambient air, possibly due to the large size and good ventilation of the relief chamber. In the encased grit chamber (K3), mesophilic bacteria concentrations were significantly higher than in K1, K2, and ambient air. Coliform bacteria concentrations were generally low, with the highest load found in the encased grit chamber (K3). Coliform bacteria were infrequently found in aerosols of wastewater plants. Aspergillus fumigatus was detected at all sampling sites both indoors and outdoors. | [21] |
| Methicillin-Resistant Staphylococcus aureus (MRSA) Detected at Four U.S. Wastewater Treatment Plants | WWTP with primary, secondary and tertiary treatment processes | Methicillin-resistant Staphylococcus aureus (MRSA) | Material collection (Grab Samples—Samples were collected in 1-L sterile polyethylene Nalgene® Wide Mouth Environmental Sample Bottles) | 1 year longitudinal study | Mid-Atlantic WWTP1 Mid-Atlantic WWTP2 Midwest WWTP1 Midwest WWTP2; 44 grab samples were collected | Gram stain; coagulase and catalase tests; PCR | MRSA was detected in 50% of wastewater samples, at all WWTPs studied. MSSA (Methicillin-Susceptible Staphylococcus aureus) was also detected in 55% of wastewater samples, at all WWTPs. The occurrence of MRSA and MSSA varied across WWTPs, sampling dates, and sampling locations. MRSA isolates showed resistance to multiple antibiotics, including those approved for treating MRSA infections. MSSA isolates also exhibited antibiotic resistance patterns that varied by WWTP. In total, 93% of MRSA isolates were multidrug-resistant (MDR), while 29% of MSSA isolates were MDR. | [34] |
| Characterization and source analysis of indoor/outdoor culturable airborne bacteria in a municipal wastewater treatment plant | Municipal WWTP with anaerobic–anoxic–oxic treatment process | Airborne Bacteria, Enterobacteriaceae and Opportunistic Pathogens | Air Sample: Active method—Impaction (Andersen six-stage cascade impactor, flow rate = 28.3 L/min) | Seasonal (spring, summer, autumn and winter) | Four specific sampling sites were selected within the plant: fine screens room (FS), aeration tank (AT), sludge dewatering house (SDH), and an external upwind control site; 48 air samples | Culture-based methods; Illumina MiSeq sequencing | FS had over ten times higher concentrations of culturable airborne bacteria compared to the outdoor aeration tank. Particle size distribution of culturable airborne bacteria varied between sampling sites. Enterobacteriaceae and opportunistic pathogens were detected indoors, primarily sourced from wastewater and sludge (were not detected outdoors). | [28] |
| Assessment of indoor airborne contamination in a wastewater treatment plant | Municipal WWTP with preliminary, primary, secondary, tertiary and sludge treatments, and deodorization processes | Bacteria and Fungi | Air Sample: Active method—Impaction (MAS 100, flow rate = 100 L/min) | Seasonal (summer, autumn and winter) | Bar Rack Chamber SEDIPAC 3D (Degritting/Degreasing/Primary Sedimentation Facility) Secondary Sedimentation Tanks (Two Locations) Sludge Thickener Sludge Dehydration Chamber Sludge Disposal Area Outdoor Control Sampling Point | Culture-based methods (TSA for total bacteria, Mannitol salt agar and MAC for Gram-positive and Gram-negative bacteria, respectively, and DG18 for total fungi) | Out of 3 sampling campaigns, in the first one (with the highest ambient temperature) the total airborne bacteria and fungi concentrations were the highest. Gram-positive bacteria were the most dominant, and Aspergillus, Penicillium, Cladosporium, and Alternaria were the most common fungi. | [15] |
| Estimation of health risks caused by exposure to enteroviruses from agricultural application of wastewater effluents | WWTPs with conventional activated sludge processes | Fecal Coliforms and Enteric Viruses | Material collection (effluent samples were collected in 1-L sterile glasses) | Seasonal (spring, summer, autumn and winter) | 30 effluent samples (15 from each WWTP) | Culture-based methods | A high fecal coliform concentration was observed in the WWTPs. Enteric viruses were also detected, peaking in summer/autumn. There was a high risk for farmers (EV infection and disease burden) and risk for lettuce consumers, exceeding WHO guidelines. | [32] |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koseoglu-Imer, D.Y.; Oral, H.V.; Coutinho Calheiros, C.S.; Krzeminski, P.; Güçlü, S.; Pereira, S.A.; Surmacz-Górska, J.; Plaza, E.; Samaras, P.; Binder, P.M.; et al. Current challenges and future perspectives for the full circular economy of water in European countries. J. Environ. Manag. 2023, 345, 118627. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, U.; Sarpong, G.; Gude, V.G. Transitioning Wastewater Treatment Plants toward Circular Economy and Energy Sustainability. ACS Omega 2021, 6, 11794–11803. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewska, E. Emission of bacteria and fungi in the air from wastewater treatment plants—A review. Front. Biosci. Sch. Ed. 2011, 3, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, L.; Zhang, X.; Xu, C.; Dong, L.; Yao, M. Bioaerosol emissions and detection of airborne antibiotic resistance genes from a wastewater treatment plant. Atmos. Environ. 2016, 124, 404–412. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Qiu, X.; Zhang, Y.; Wang, H. Dispersion and Risk Assessment of Bacterial Aerosols Emitted from Rotating-Brush Aerator during Summer in a Wastewater Treatment Plant of Xi’an, China. Aerosol Air Qual. Res. 2013, 13, 1807–1814. [Google Scholar] [CrossRef]
- Talepour, N.; Hassanvand, M.S.; Abbasi-Montazeri, E.; Latifi, S.M.; Jaafarzadeh Haghighi Fard, N. Spatio-temporal variations of airborne bacteria from the municipal wastewater treatment plant: A case study in Ahvaz, Iran. J. Environ. Health Sci. Eng. 2020, 18, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; Gomes, A.Q.; Sabino, R.; Seco, A.; Viegas, S. Fungal Contamination in Two Portuguese Wastewater Treatment Plants. J. Toxicol. Environ. Health A 2014, 77, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhao, R.; Su, G.; Liu, B.; Liu, W.; Xu, J.; Li, Q.; Meng, J. Metagenomic surveillance of antibiotic resistome in influent and effluent of wastewater treatment plants located on the Qinghai-Tibetan Plateau. Sci. Total Environ. 2023, 870, 162031. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.; Dias, M.; Cervantes, R.; Pena, P.; Santos, J.; Vasconcelos Pinto, M.; Viegas, C. One Health Approach to Tackle Microbial Contamination on Poultries—A Systematic Review. Toxics 2023, 11, 374. [Google Scholar] [CrossRef]
- Dias, M.; Gomes, B.; Cervantes, R.; Pena, P.; Viegas, S.; Viegas, C. Microbial Occupational Exposure Assessments in Sawmills—A Review. Atmosphere 2022, 13, 266. [Google Scholar] [CrossRef]
- Daae, H.L.; Heldal, K.K.; Madsen, A.M.; Olsen, R.; Skaugset, N.P.; Graff, P. Occupational exposure during treatment of offshore drilling waste and characterization of microbiological diversity. Sci. Total Environ. 2019, 681, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Viegas, C.; Dias, R.; Gomes, A.Q.; Meneses, M.; Sabino, R.; Viegas, S. Aspergillus flavus contamination in two Portuguese wastewater treatment plants. J. Toxicol. Environ. Health A 2014, 77, 796–805. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Aspergillus spp. prevalence in different Portuguese occupational environments: What is the real scenario in high load settings? J. Occup. Environ. Hyg. 2017, 14, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.V.; Miranda, S.; Monteiro, R.A.R.; Lopes, F.V.S.; Madureira, J.; Silva, G.V.; Pestana, N.; Pinto, E.; Vilar, V.J.P.; Boaventura, R.A.R. Assessment of indoor airborne contamination in a wastewater treatment plant. Environ. Monit. Assess. 2013, 185, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Cyprowski, M.; Stobnicka-Kupiec, A.; Ławniczek-Wałczyk, A.; Bakal-Kijek, A.; Gołofit-Szymczak, M.; Górny, R.L. Anaerobic bacteria in wastewater treatment plant. Int. Arch. Occup. Environ. Health 2018, 91, 571–579. [Google Scholar] [CrossRef]
- Kozajda, A.; Jeżak, K. Occupational exposure to Staphylococcus aureus in the wastewater treatment plants environment. Med. Pr. 2020, 71, 265–278. [Google Scholar] [CrossRef]
- Uhrbrand, K.; Schultz, A.; Madsen, A. Exposure to Airborne Noroviruses and Other Bioaerosol Components at a Wastewater Treatment Plant in Denmark. Food Environ. Virol. 2011, 3, 130–137. [Google Scholar] [CrossRef]
- Lu, R.; Frederiksen, M.W.; Uhrbrand, K.; Li, Y.; Østergaard, C.; Madsen, A.M. Wastewater treatment plant workers’ exposure and methods for risk evaluation of their exposure. Ecotoxicol. Environ. Saf. 2020, 205, 111365. [Google Scholar] [CrossRef]
- Masclaux, F.G.; Hotz, P.; Gashi, D.; Savova-Bianchi, D.; Oppliger, A. Assessment of airborne virus contamination in wastewater treatment plants. Environ. Res. 2014, 133, 260–265. [Google Scholar] [CrossRef]
- Haas, D.; Unteregger, M.; Habib, J.; Galler, H.; Marth, E.; Reinthaler, F.F. Exposure to Bioaerosol from Sewage Systems. Water. Air. Soil Pollut. 2010, 207, 49–56. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Wild, O.; Zhang, S.; Yang, K.; Wang, W.; Li, L. ADMS simulation and influencing factors of bioaerosol diffusion from BRT under different aeration modes in six wastewater treatment plants. Water Res. 2023, 231, 119624. [Google Scholar] [CrossRef]
- Yang, K.; Li, L.; Wang, Y.; Xue, S.; Han, Y.; Liu, J. Emission level, particle size and exposure risks of airborne bacteria from the oxidation ditch for seven months observation. Atmos. Pollut. Res. 2019, 10, 1803–1811. [Google Scholar] [CrossRef]
- Zhao, X.; An, D.; Liu, M.; Ma, J.; Ali, W.; Zhu, H.; Li, M.; Ai, X.; Nasir, Z.A.; Alcega, S.G.; et al. Bioaerosols emission characteristics from wastewater treatment aeration tanks and associated health risk exposure assessment during autumn and winter. Sci. Total Environ. 2022, 851, 158106. [Google Scholar] [CrossRef]
- Han, Y.; Li, L.; Liu, J. Characterization of the airborne bacteria community at different distances from the rotating brushes in a wastewater treatment plant by 16S rRNA gene clone libraries. J. Environ. Sci. 2013, 25, 5–15. [Google Scholar] [CrossRef]
- Wu, T.; Zou, H.; Xia, H.; Zhou, Z.; Zhao, L.; Meng, M.; Li, Q.; Guan, Y.; Li, X. Genomic insight into transmission mechanisms of carbapenem-producing Citrobacter spp. isolates between the WWTP and connecting rivers. Ecotoxicol. Environ. Saf. 2023, 262, 115150. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, L.; Wang, Y.; Xue, S.; Han, Y.; Liu, J. Airborne bacteria in a wastewater treatment plant: Emission characterization, source analysis and health risk assessment. Water Res. 2019, 149, 596–606. [Google Scholar] [CrossRef]
- Xu, G.; Han, Y.; Li, L.; Liu, J. Characterization and source analysis of indoor/outdoor culturable airborne bacteria in a municipal wastewater treatment plant. J. Environ. Sci. 2018, 74, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Talepour, N.; Hassanvand, M.S.; Abbasi-Montazeri, E.; Latifi, S.M.; Jaafarzadeh Haghighi Fard, N.; Shenavar, B. Identification of airborne fungi’s concentrations in indoor and outdoor air of municipal wastewater treatment plant. Environ. Health Eng. Manag. 2020, 7, 143–150. [Google Scholar] [CrossRef]
- Niazi, S.; Hassanvand, M.S.; Mahvi, A.H.; Nabizadeh, R.; Alimohammadi, M.; Nabavi, S.; Faridi, S.; Dehghani, A.; Hoseini, M.; Moradi-Joo, M.; et al. Assessment of bioaerosol contamination (bacteria and fungi) in the largest urban wastewater treatment plant in the Middle East. Environ. Sci. Pollut. Res. 2015, 22, 16014–16021. [Google Scholar] [CrossRef]
- Gholipour, S.; Mohammadi, F.; Nikaeen, M.; Shamsizadeh, Z.; Khazeni, A.; Sahbaei, Z.; Mousavi, S.M.; Ghobadian, M.; Mirhendi, H. COVID-19 infection risk from exposure to aerosols of wastewater treatment plants. Chemosphere 2021, 273, 129701. [Google Scholar] [CrossRef] [PubMed]
- Moazeni, M.; Nikaeen, M.; Hadi, M.; Moghim, S.; Mouhebat, L.; Hatamzadeh, M.; Hassanzadeh, A. Estimation of health risks caused by exposure to enteroviruses from agricultural application of wastewater effluents. Water Res. 2017, 125, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Jari, H.; Maleki, A.; Dehestaniathar, S.; Mohammadi, E.; Darvishi, E.; Hedayati, M.; Marzban, N.; Van Tai, T.; Nouri, B. Airborne bacteria and fungi in a wastewater treatment plant: Type and characterization of bio-aerosols, emission characterization and mapping. Aerobiologia 2022, 38, 163–176. [Google Scholar] [CrossRef]
- Rosenberg Goldstein, R.E.; Micallef, S.A.; Gibbs, S.G.; Davis, J.A.; He, X.; George, A.; Kleinfelter, L.M.; Schreiber, N.A.; Mukherjee, S.; Sapkota, A.; et al. Methicillin-resistant Staphylococcus aureus (MRSA) detected at four U.S. wastewater treatment plants. Environ. Health Perspect. 2012, 120, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Acer, P.T.; Kelly, L.M.; Lover, A.A.; Butler, C.S. Quantifying the Relationship between SARS-CoV-2 Wastewater Concentrations and Building-Level COVID-19 Prevalence at an Isolation Residence: A Passive Sampling Approach. Int. J. Environ. Res. Public Health 2022, 19, 11245. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.E.; Pak, H.; Gerlich, R.; Jantrania, A.; Smith, B.L.; King, M.D. Aerosol partitioning potential of bacteria presenting antimicrobial resistance from different stages of a small decentralized septic treatment system. Aerosol Sci. Technol. 2023, 57, 517–531. [Google Scholar] [CrossRef]
- Mbareche, H.; Dion-Dupont, V.; Veillette, M.; Brisebois, E.; Lavoie, J.; Duchaine, C. Influence of seasons and sites on bioaerosols in indoor wastewater treatment plants and proposal for air quality indicators. J. Air Waste Manag. Assoc. 2022, 72, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mbareche, H.; Lindsley, W.G.; Duchaine, C. Field sampling of indoor bioaerosols. Aerosol Sci. Technol. 2020, 54, 572–584. [Google Scholar] [CrossRef]
- Oliveira, K.; Viegas, C.; Ribeiro, E. MRSA Colonization in Workers from Different Occupational Environments—A One Health Approach Perspective. Atmosphere 2022, 13, 658. [Google Scholar] [CrossRef]
- Viegas, C.; Dias, M.; Pacífico, C.; Faria, T.; Clérigo, A.; Dias, H.; Caetano, L.; Carolino, E.; Gomes, A.; Viegas, S. Portuguese cork industry: Filling the knowledge gap regarding occupational exposure to fungi and related health effects. Front. Public Health 2024, 12, 1355094. [Google Scholar]
- Khan, M.M.; Siddiqi, S.A.; Farooque, A.A.; Iqbal, Q.; Shahid, S.A.; Akram, M.T.; Rahman, S.; Al-Busaidi, W.; Khan, I. Towards Sustainable Application of Wastewater in Agriculture: A Review on Reusability and Risk Assessment. Agronomy 2022, 12, 1397. [Google Scholar] [CrossRef]
- Chen, C.; He, R.; Cheng, Z.; Han, M.; Zha, Y.; Yang, P.; Yao, Q.; Zhou, H.; Zhong, C.; Ning, K. The Seasonal Dynamics and the Influence of Human Activities on Campus Outdoor Microbial Communities. Front. Microbiol. 2019, 10, 1579. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Wurster, S.; Jenks, J.D.; Sati, H.; Gangneux, J.-P.; Egger, M.; Alastruey-Izquierdo, A.; Ford, N.P.; Chowdhary, A.; Sprute, R.; et al. Impact of climate change and natural disasters on fungal infections. Lancet Microbe 2024. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, A.; Garrido-Chamorro, S.; Barreiro, C. Microorganisms and Climate Change: A Not So Invisible Effect. Microbiol. Res. 2023, 14, 918–947. [Google Scholar] [CrossRef]
- Mao, J.; Tang, Y.; Wang, Y.; Huang, J.; Dong, X.; Chen, Z.; Lai, Y. Particulate Matter Capturing via Naturally Dried ZIF-8/Graphene Aerogels under Harsh Conditions. iScience 2019, 16, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Anon International Labour Organization. Encyclopaedia of Occupational Health and Safety; International Labour Organization: Geneve, Switzerland, 1998. [Google Scholar]
- Dias, M.; Viegas, C. Fungal Prevalence on Waste Industry—Literature Review Encyclopedia of Mycology ed Ó Zaragoza and A Casadevall; Elsevier: Oxford, UK, 2021; pp. 99–106. [Google Scholar]
- Meadow, J.F.; Altrichter, A.E.; Kembel, S.W.; Kline, J.; Mhuireach, G.; Moriyama, M.; Northcutt, D.; O’Connor, T.K.; Womack, A.M.; Brown, G.Z.; et al. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air source. Indoor Air 2014, 24, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.B.; Keady, P.B.; Brewer, T.E.; Clements, N.; Morgan, E.E.; Awerbuch, J.; Miller, S.L.; Fierer, N. Impacts of flood damage on airborne bacteria and fungi in homes after the 2013 Colorado Front Range flood. Environ. Sci. Technol. 2015, 49, 2675–2684. [Google Scholar] [CrossRef]
- Afanou, K.A.; Eduard, W.; Laier Johnsen, H.B.; Straumfors, A. Fungal Fragments and Fungal Aerosol Composition in Sawmills. Ann. Work Expo. Health 2018, 62, 559–570. [Google Scholar] [CrossRef]
- Duchaine, C.; Mériaux, A.; Thorne, P.S.; Cormier, Y. Assessment of particulates and bioaerosols in eastern Canadian sawmills. AIHAJ J. Sci. Occup. Environ. Health Saf. 2000, 61, 727–732. [Google Scholar]
- Gomes, B.; Pena, P.; Cervantes, R.; Dias, M.; Viegas, C. Microbial Contamination of Bedding Material: One Health in Poultry Production. Int. J. Environ. Res. Public Health 2022, 19, 16508. [Google Scholar] [CrossRef]
- Foddai, A.C.G.; Grant, I.R. Methods for detection of viable foodborne pathogens: Current state-of-art and future prospects. Appl. Microbiol. Biotechnol. 2020, 104, 4281–4288. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Frederiksen, M.W.; Jacobsen, M.H.; Tendal, K. Towards a risk evaluation of workers’ exposure to handborne and airborne microbial species as exemplified with waste collection workers. Environ. Res. 2020, 183, 109177. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Viegas, S.; Gomes, A.; Täubel, M.; Sabino, R. Exposure to Microbiological Agents in Indoor and Occupational Environments; Springer: Cham, Swizerland, 2017. [Google Scholar]
- Black, W.D. A comparison of several media types and basic techniques used to assess outdoor airborne fungi in Melbourne, Australia. PLoS ONE 2020, 15, e0238901. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Dias, M.; Carolino, E.; Sabino, R. Culture media and sampling collection method for aspergillus spp. Assessment: Tackling the gap between recommendations and the scientific evidence. Atmosphere 2021, 12, 23. [Google Scholar] [CrossRef]
- Thorne, P.S.; Kiekhaefer, M.S.; Whitten, P.; Donham, K.J. Comparison of bioaerosol sampling methods in barns housing swine. Appl. Environ. Microbiol. 1992, 58, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.S.; Hsieh, Y.-H.; Simpson, S.; Kerdahi, K.; Sulaiman, I.M. Evaluation of Two Standard and Two Chromogenic Selective Media for Optimal Growth and Enumeration of Isolates of 16 Unique Bacillus Species. J. Food Prot. 2017, 80, 952–962. [Google Scholar] [CrossRef]
- Amrane, S.; Raoult, D.; Lagier, J.-C. Metagenomics, culturomics, and the human gut microbiota. Expert Rev. Anti Infect. Ther. 2018, 16, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Lagier, J.C.; Raoult, D.; Khelaifia, S. Bacterial culture through selective and non-selective conditions: The evolution of culture media in clinical microbiology. New Microbes New Infect. 2019, 34, 100622. [Google Scholar] [CrossRef] [PubMed]
- Franchitti, E.; Pascale, E.; Fea, E.; Anedda, E.; Traversi, D. Methods for Bioaerosol Characterization: Limits and Perspectives for Human Health Risk Assessment in Organic Waste Treatment. Atmosphere 2020, 11, 452. [Google Scholar] [CrossRef]
- WHO. WHO Updates List of Drug-Resistant Bacteria Most Threatening to Human Health; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. 2021, 19, e06651. [Google Scholar]
- Yalin, D.; Craddock, H.A.; Assouline, S.; Ben Mordechay, E.; Ben-Gal, A.; Bernstein, N.; Chaudhry, R.M.; Chefetz, B.; Fatta-Kassinos, D.; Gawlik, B.M.; et al. Mitigating risks and maximizing sustainability of treated wastewater reuse for irrigation. Water Res. X 2023, 21, 100203. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, X.; Jiang, X.-T.; Yang, Y.; Li, B.; Shum, M.H.-H.; Lam, T.T.Y.; Leung, G.M.; Rose, J.; Sanchez-Cid, C.; et al. Toward a Universal Unit for Quantification of Antibiotic Resistance Genes in Environmental Samples. Environ. Sci. Technol. 2023, 57, 9713–9721. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Manaia, C.; Berendonk, T.U.; Cytryn, E.; Bayona, J.; Chefetz, B.; Slobodnik, J.; Kreuzinger, N.; Rizzo, L.; Malato, S.; et al. COST Action ES1403 New and emerging challenges and opportunities in wastewater reuse (NEREUS). Environ. Sci. Pollut. Res. Int. 2015, 22, 7183–7186. [Google Scholar] [CrossRef]
- Ariyadasa, S.; Taylor, W.; Weaver, L.; McGill, E.; Billington, C.; Pattis, I. Nonbacterial Microflora in Wastewater Treatment Plants: An Underappreciated Potential Source of Pathogens. Microbiol. Spectr. 2023, 11, e0048123. [Google Scholar] [CrossRef]
- Corbu, V.M.; Gheorghe-Barbu, I.; Dumbravă, A.Ș.; Vrâncianu, C.O.; Șesan, T.E. Current Insights in Fungal Importance—A Comprehensive Review. Microorganisms 2023, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Warnasuriya, S.D.; Udayanga, D.; Manamgoda, D.S.; Biles, C. Fungi as environmental bioindicators. Sci. Total Environ. 2023, 892, 164583. [Google Scholar] [CrossRef]
- Viegas, C.; Eriksen, E.; Gomes, B.; Dias, M.; Cervantes, R.; Pena, P.; Carolino, E.; Twarużek, M.; Caetano, L.A.; Viegas, S.; et al. Comprehensive assessment of occupational exposure to microbial contamination in waste sorting facilities from Norway. Front. Public Health 2023, 11, 1297725. [Google Scholar] [CrossRef]
- Marchand, G.; Wingert, L.; Viegas, C.; Caetano, L.; Viegas, S.; Twaruzek, M.; Lacombe, N.; Lanoie, D.; Valois, I.; Gouin, F.; et al. Assessment of waste workers occupational risk to microbial agents and cytotoxic effects of mixed contaminants present in the air of waste truck cabin and ventilation filters. J. Air Waste Manag. Assoc. 2024, 74, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Salambanga, F.R.D.; Wingert, L.; Valois, I.; Lacombe, N.; Gouin, F.; Trépanier, J.; Debia, M.; Soszczyńska, E.; Twarużek, M.; Kosicki, R.; et al. Microbial contamination and metabolite exposure assessment during waste and recyclable material collection. Environ. Res. 2022, 212, 113597. [Google Scholar] [CrossRef]
- Viegas, C.; Pena, P.; Dias, M.; Gomes, B.; Cervantes, R.; Carolino, E.; Twarużek, M.; Soszczyńska, E.; Kosicki, R.; Caetano, L.A.; et al. Microbial contamination in waste collection: Unveiling this Portuguese occupational exposure scenario. J. Environ. Manag. 2022, 314, 115086. [Google Scholar] [CrossRef]
- Viegas, S.; Viegas, C.; Martins, C.; Assunção, R. Occupational Exposure to Mycotoxins—Different Sampling Strategies Telling a Common Story Regarding Occupational Studies Performed in Portugal (2012–2020). Toxins 2020, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Menendez, O.; Alastruey-Izquierdo, A.; Mellado, E.; Cuenca-Estrella, M. Triazole Resistance in Aspergillus spp.: A Worldwide Problem? J. Fungi 2016, 2, 21. [Google Scholar] [CrossRef]
- Macedo, D.; Brito Devoto, T.; Pola, S.; Finquelievich, J.L.; Cuestas, M.L.; Garcia-Effron, G. A Novel Combination of CYP51A Mutations Confers Pan-Azole Resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Camps, S.M.T.; van der Linden, J.W.M.; Li, Y.; Kuijper, E.J.; van Dissel, J.T.; Verweij, P.E.; Melchers, W.J.G. Rapid Induction of Multiple Resistance Mechanisms in Aspergillus fumigatus during Azole Therapy: A Case Study and Review of the Literature. Antimicrob. Agents Chemother. 2012, 56, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J.F. Emergence of Azole-Resistant Aspergillus fumigatus Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health. PLoS Pathog. 2013, 9, e1003633. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; Cuenca-Estrella, M.; Mellado, E. Triazole Resistance in Aspergillus Species: An Emerging Problem. Drugs 2017, 77, 599–613. [Google Scholar] [CrossRef]
- Berger, S.; El Chazli, Y.; Babu, A.F.; Coste, A.T. Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? Front. Microbiol. 2017, 8, 1024. [Google Scholar] [CrossRef]
- Verweij, P.E.; Snelders, E.; Kema, G.H.; Mellado, E.; Melchers, W.J. Azole resistance in Aspergillus fumigatus: A side-effect of environmental fungicide use? Lancet Infect. Dis. 2009, 9, 789–795. [Google Scholar] [CrossRef]
- Van Der Torre, M.H.; Novak-Frazer, L.; Rautemaa-Richardson, R. Detecting Azole-Antifungal Resistance in Aspergillus fumigatus by Pyrosequencing. J. Fungi 2020, 6, 12. [Google Scholar] [CrossRef]
- Gonçalves, P.; Melo, A.; Dias, M.; Almeida, B.; Caetano, L.A.; Veríssimo, C.; Viegas, C.; Sabino, R. Azole-Resistant Aspergillus fumigatus Harboring the TR34/L98H Mutation: First Report in Portugal in Environmental Samples. Microorganisms 2020, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; WHO: Geneva, Switzerland, 2024. [Google Scholar]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Articles published from 1 January 2010 to 8 September 2023 Articles published in English Articles summarising research results from any country Original scientific articles on the subject Articles focused to microbial occupational exposure | Articles published prior to 2010 Articles published in other language Abstracts of congress, reports, reviews/state-of-the-art articles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riesenberger, B.; Rodriguez, M.; Marques, L.; Cervantes, R.; Gomes, B.; Dias, M.; Pena, P.; Ribeiro, E.; Viegas, C. Filling the Knowledge Gap Regarding Microbial Occupational Exposure Assessment in Waste Water Treatment Plants: A Scoping Review. Microorganisms 2024, 12, 1144. https://doi.org/10.3390/microorganisms12061144
Riesenberger B, Rodriguez M, Marques L, Cervantes R, Gomes B, Dias M, Pena P, Ribeiro E, Viegas C. Filling the Knowledge Gap Regarding Microbial Occupational Exposure Assessment in Waste Water Treatment Plants: A Scoping Review. Microorganisms. 2024; 12(6):1144. https://doi.org/10.3390/microorganisms12061144
Chicago/Turabian StyleRiesenberger, Bruna, Margarida Rodriguez, Liliana Marques, Renata Cervantes, Bianca Gomes, Marta Dias, Pedro Pena, Edna Ribeiro, and Carla Viegas. 2024. "Filling the Knowledge Gap Regarding Microbial Occupational Exposure Assessment in Waste Water Treatment Plants: A Scoping Review" Microorganisms 12, no. 6: 1144. https://doi.org/10.3390/microorganisms12061144
APA StyleRiesenberger, B., Rodriguez, M., Marques, L., Cervantes, R., Gomes, B., Dias, M., Pena, P., Ribeiro, E., & Viegas, C. (2024). Filling the Knowledge Gap Regarding Microbial Occupational Exposure Assessment in Waste Water Treatment Plants: A Scoping Review. Microorganisms, 12(6), 1144. https://doi.org/10.3390/microorganisms12061144








