Abstract
Hepatitis C virus (HCV) remains a significant global health challenge. Approximately 50 million people were living with chronic hepatitis C based on the World Health Organization as of 2024, contributing extensively to global morbidity and mortality. The advent and approval of several direct-acting antiviral (DAA) regimens significantly improved HCV treatment, offering potentially high rates of cure for chronic hepatitis C. However, the promising aim of eventual HCV eradication remains challenging. Key challenges include the variability in DAA access across different regions, slightly variable response rates to DAAs across diverse patient populations and HCV genotypes/subtypes, and the emergence of resistance-associated substitutions (RASs), potentially conferring resistance to DAAs. Therefore, periodic reassessment of current HCV knowledge is needed. An up-to-date review on HCV is also necessitated based on the observed shifts in HCV epidemiological trends, continuous development and approval of therapeutic strategies, and changes in public health policies. Thus, the current comprehensive review aimed to integrate the latest knowledge on the epidemiology, pathophysiology, diagnostic approaches, treatment options and preventive strategies for HCV, with a particular focus on the current challenges associated with RASs and ongoing efforts in vaccine development. This review sought to provide healthcare professionals, researchers, and policymakers with the necessary insights to address the HCV burden more effectively. We aimed to highlight the progress made in managing and preventing HCV infection and to highlight the persistent barriers challenging the prevention of HCV infection. The overarching goal was to align with global health objectives towards reducing the burden of chronic hepatitis, aiming for its eventual elimination as a public health threat by 2030.
1. Introduction
Chronic hepatitis C remains a significant global health burden affecting a substantial number of individuals worldwide [1,2,3]. Based on the World Health Organization (WHO) estimates, there were about 50 million people living with chronic hepatitis C by the year 2023 worldwide, with an estimated 1 million new infections each year [3,4]. Additionally, about 3.26 million children were affected by chronic hepatitis C, highlighting its significant burden [5,6].
Following the first decade of the new millennium, the availability and approval of direct-acting antivirals (DAAs) marked a new era in the management of chronic hepatitis C [7,8]. The availability of these oral curative agents represented the culmination of over three decades of intensive research [9]. The DAAs are characterized by remarkable safety and effectiveness profiles, leading to achievement of sustained virologic response (SVR)—which marks the cure—in more than 90% of individuals with chronic hepatitis C [10,11,12].
The current approved therapeutic protocols for the treatment of chronic hepatitis C virus (HCV) infection issued by the Infectious Diseases Society of America (IDSA) and the American Association for the Study of Liver Diseases (AASLD)—titled “the HCV guidance”—involve combining various DAAs [13,14]. These DAAs can be classified as NS3 protease inhibitors (PIs, with the drug names having the suffix -previr), NS5A replication complex inhibitors (NS5AIs, with the drug names having the suffix -asvir), and NS5B polymerase nucleoside (NI) and non-nucleoside (NNI) inhibitors (with the drug names having the suffix -buvir) [15,16].
The first generation of DAAs comprises (1) NS5AIs including Daclatasvir, Ledipasvir, Elbasvir, and Ombitasvir; (2) PIs including Simeprevir, Grazoprevir, Asunaprevir, and Paritaprevir; and (3) the NNI represented by Dasabuvir [17,18]. The first-generation agents exhibit genotype-specific activity [19,20]. In contrast, the second-generation DAAs exhibit pan-genotypic activity with notable efficacy, including the NS5AIs Pibrentasvir and Velpatasvir, the PIs Glecaprevir and Voxilaprevir, and the NI Sofosbuvir [17,20].
Regarding the prevention of HCV and its possible eradication, significant challenges remain [21,22]. These challenges stem from the extensive genetic variability of HCV and the challenge of eliciting a protective immune response against the virus, with both factors representing a major obstacle to the development of an effective vaccine [23,24,25]. Nevertheless, optimism grew regarding the prospects of HCV elimination globally with the availability and success of DAAs as a curative strategy [16,26,27,28]. Thus, the promising goal of eliminating HCV as a public health threat relies on the “Treatment as Prevention” (TasP) strategy [29,30,31].
A major obstacle to the sustained success of DAAs as a curative treatment for HCV, let alone HCV eradication, is the emergence of HCV variants resistant to DAAs [32,33,34,35]. The presence of resistance-associated substitutions (RASs), which can occur naturally or develop during treatment, has the potential to reduce the effectiveness of treatment, with the possibility of forward transmission of drug-resistant variants, especially within high-risk populations [36,37,38].
Given the disparities in DAA access especially in low-income settings, variations in treatment responses among diverse populations, and the rise of RASs, a contemporary reassessment of HCV management is necessary. Additionally, the evolving epidemiological trends, ongoing advances in therapeutic modalities, and challenges in HCV prevention by vaccination necessitate an updated comprehensive review. Therefore, the aim of this comprehensive review was to provide an up-to-date source of information regarding HCV, including aspects of epidemiology, clinical features, diagnosis, treatment, and prevention, with a focus on addressing the challenges posed by DAA resistance and vaccine development efforts.
2. History of Hepatitis C
It became evident by the mid-1970s that a viral hepatitis agent, distinct from hepatitis A virus (HAV) and hepatitis B virus (HBV), was responsible for post-transfusion hepatitis, and this putative clinical entity was termed “non-A, non-B” hepatitis (NANBH) [16,39,40].
Subsequent investigation involving transfusion recipients revealed that acute clinical manifestations of NANBH were generally less severe than those of hepatitis; nevertheless, NANBH was capable of leading to grave outcomes, including cirrhosis and hepatic failure [41]. Experiments involving the inoculation of chimpanzees with blood from subjects afflicted by acute and chronic NANBH demonstrated a notable rise in hepatic transaminase; thus, these chimpanzees provided a crucial animal model to understand the nature of NANBH chronicity [42,43]. Subsequent investigations helped to characterize the nature of the NANBH culprit agent as a small, enveloped virus of less than 80 nm in diameter [44,45,46]; nevertheless, this agent was elusive to traditional viral culture and immunological identification techniques [47,48]. Progress was made through the serial passage of NANBH in chimpanzees, which provided critical pathological, physiological, and biochemical insights, alongside a valuable number of specimens for further study [49,50].
A breakthrough was achieved by the Michael Houghton team, which constructed a lambda phage library from cDNA of a high-titer chimpanzee plasma specimen [51]. The Michael Houghton team screened more than 1 million expression clones with serum from a chronic NANBH patient, which led to identification of a single positive cDNA clone, designated 5-1-1, marking the discovery of HCV [51]. This led to the development of initial assays for detecting anti-HCV antibodies, incorporating the 5-1-1 antigen [52]. As a result of this seminal work, the 2020 Nobel Prize in Medicine or Physiology was awarded to Harvey Alter, Michael Houghton, and Charles Rice for their seminal work in the discovery and characterization of HCV [53].
Characterization of the newly discovered virus revealed that the HCV genome comprises a positive-strand RNA of nearly 10,000 nucleotides, with a single open reading frame (ORF), closely related to the Flaviviridae family [54,55]. Identification of the 5′ and 3′ untranslated regions (UTRs) facilitated the production of a full-length cDNA clone, infectious upon intra-hepatic administration in chimpanzees [56].
Despite advancements in screening and the prevention of hepatitis C, it remains a significant global health concern [1,31]. Continuous investigations involving HCV life cycle, replication, mechanisms of persistence, and pathogenesis are still needed to unravel the complexity of this infection, which would be helpful in the eradication efforts [57]. The recent estimates point to slightly less than 1% of the global population as chronically infected with HCV, and while treatment efficacy and accessibility have tremendously improved, HCV infection continues to be a leading cause of morbidity and mortality worldwide [58].
3. HCV Origin and Classification
HCV is a member of the Hepacivirus genus within the Flaviviridae family, a classification delineated through seminal studies led by Peter Simmonds [59,60,61,62]. A notable review conducted by Peter Simmonds [63] meticulously examined the complex narrative surrounding the origins and evolutionary trajectory of HCV. The aforementioned review highlighted the challenges to pinpoint the ancient history of HCV in human populations and recommended a cautious interpretation of the term “origin”, particularly in reference to HCV global dissemination and diversification during the 20th century [63]. The absence of an animal virus closely related to HCV, despite exhaustive efforts, further complicates delineating the exact zoonotic source of HCV in humans [62,63,64].
In exploring the genetic heterogeneity of HCV, phylogenetic studies revealed a remarkable divergence within the virus, categorizing HCV into at least seven (or even eight) distinct genotypes, denoted by Arabic numerals [60,65,66,67] (Figure 1).
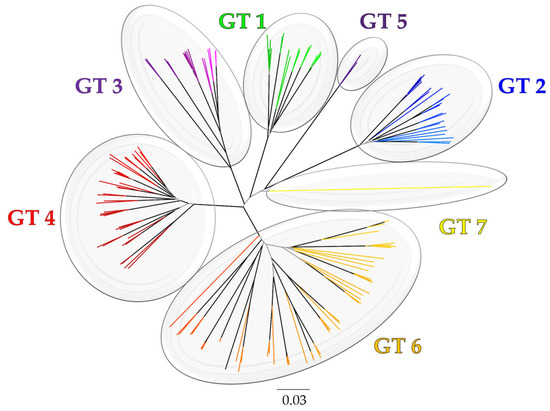
Figure 1.
The evolutionary history of HCV genotypes (GTs) based on (Sallam, 2017) [66]. The phylogeny was constructed using the neighbor-joining (NJ) method with bootstrap test for evaluation of topology (1000 replicates). Internal branches with bootstrap values ≥ 0.9 are highlighted in black. The evolutionary distances were computed using the TN93 method. The rate variation among sites was modelled with a gamma distribution (shape parameter = 4). The analysis involved 147 NS5B sequences (1495 bases) downloaded from the Los Alamos Hepatitis C sequence and immunology databases (https://hcv.lanl.gov/content/index, accessed on 30 April 2024). Evolutionary analyses were conducted in MEGA6 [68].
This genetic diversity extends within genotypes, which resulted in further subclassification of these genotypes into several subtypes identified by lowercase English letters [60,65]. These genotype and subtype classifications are based on variations in nucleotide sequences, with inter-genotypic differences exceeding 30% and intra-genotypic differences ranging between 20 and 25% [24,60,65]. Investigation of the evolutionary rate of HCV revealed a relatively rapid rate, estimated at 1.0–2.0 × 10−3 substitutions per site per year (s/s/y), comparable to other RNA viruses [69]. Given the extensive genetic divergence of HCV isolates, it is plausible that such variability underpins the observed differences in clinical manifestations and treatment responses among individuals infected with distinct HCV genotypes [70,71,72,73].
4. HCV Genome
The genomic architecture of HCV exemplifies a Flaviviridae member in being a positive-sense, single-stranded RNA genome, constituting a single ORF [74]. This ORF is responsible for the synthesis of a large single polyprotein, approximately 3000 amino acids in length, as delineated in the work by Li et al. and Scheel and Rice [75,76]. The processing of HCV polyprotein into its functional single proteins involves a series of cleavage events mediated by both host cellular proteases and virus-specific enzymes, yielding an array of structural and non-structural (NS) proteins critical for the viral life cycle [76,77].
The details of the HCV genomic regions can be further explained as follows. Positioned at the beginning of the HCV viral genome is the highly conserved 5′ UTR, which harbors an internal ribosomal entry site (IRES) facilitating the cap-independent translation of the viral genome [78]. This region precedes the coding sequences for structural proteins, including the core protein (C), the envelope glycoproteins (E1 and E2), and the ion-channel viroporin (p7) [78]. Notably, the C protein maintains a conserved sequence, whereas E1 and E2 glycoproteins exhibit significant sequence variability, a phenomenon attributed to the pressure of immune selection [70,79,80,81].
The HCV genome’s last segment encodes NS proteins, essential for viral replication and processing of the polyprotein [82]. The NS2 protein, a cysteine protease, facilitates the cleavage of NS3 from the NS2–NS3 junction [83]. NS3, together with NS4A, constitutes a serine protease complex essential for the cleavage of subsequent NS proteins, regulating the sequential processing of the viral proteome [76]. NS4B serves as a membrane anchor for the viral replication complex, while NS5A, alongside NS4B, contributes to the formation of the endoplasmic reticulum (ER) membranous web, a structure essential for viral replication dynamics [76,84]. Lastly, NS5B acts as an RNA-dependent RNA polymerase (RdRp), synthesizing the viral RNA genome and acting as the central replication factor of HCV [85,86]. Full representation of the HCV genome and viral proteins is illustrated in (Figure 2).
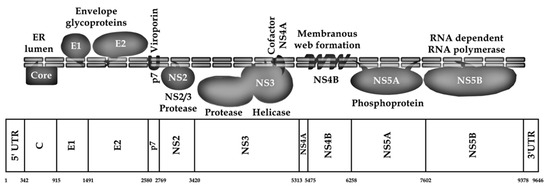
Figure 2.
Schematic illustration of HCV genome and polyprotein. The nucleotide positions are in accordance with strain H77 numbering (GenBank accession number: AF009606) based on (Sallam, 2017) [66].
5. HCV Pathogenesis, Pathology, and Immune Response
The hepatic tropism of HCV is related to its selective predilection to infect the hepatocytes as the primary site for its entry, replication, and assembly [87]. The primary mode of HCV transmission is identified as percutaneous, with documented cases of permucosal transmission as well [88]. Experiments showed that direct inoculation of HCV, either through intravenous injection of virions or intrahepatic introduction of genomic RNA, effectively initiates HCV infection [56].
Hepatic tropism of HCV is related to the preferential expression of specific proteins on hepatocytes acting to facilitate entry and replication of HCV. These include the low-density lipoprotein receptor (LDL-R) and scavenger receptor class B type I (SR-BI), the liver-specific microRNA miR-122 essential for HCV replication, and the unique lipoprotein synthesis pathways in the liver that support HCV virion production [89,90,91]. Additionally, the liver-specific distribution and dynamic interactions between viral components and more universally expressed host proteins including CD81, claudins, occludin, and epidermal growth factor receptor further highlight the role of the liver as the principal site for the HCV life cycle [92,93]. While the potential for productive HCV infection in extrahepatic cells remains under investigation for full elucidation, there is evidence supporting the detection of HCV replicative intermediates outside the liver, suggesting a minor yet evident extrahepatic replication compartment [94,95,96,97].
Following HCV infection, the host defense mechanisms, including the innate and adaptive immune responses, restrict the penetration of circulating HCV particles into hepatocytes [87,98]. HCV demonstrates an ability to adapt and evolve in response to the immunological challenges imposed by the host environment [99]. To circumvent the humoral responses, intrahepatic cell-to-cell transmission is utilized by the virus [100]. This complex dynamic interaction between HCV evasion strategies and the host immune response is the key feature of the outcome following HCV infection [101,102]. Understanding the mechanisms behind HCV intra-liver dissemination and its interaction with host immune defenses is crucial for developing targeted therapeutic strategies aimed at controlling viral spread and enhancing the efficacy of antiviral treatments [98].
The innate immune response plays a crucial role in fighting HCV infection, with the virus employing various strategies to circumvent this defense [100]. Central to the innate immune response against HCV is interferon (IFN) signaling, which constitutes early intrahepatic defenses [103]. Activation of IFN-regulatory factor 3 (IRF3) by viral infection triggers IFN-β production, subsequently activating the JAK-STAT signaling pathway, enhancing IFN-α synthesis, and stimulating the release of antiviral cytokines and chemokines [104]. The HCV NS3–NS4A complex disrupts IRF3 activation by cleaving key adapter protein Toll/IL-1 receptor domain-containing adaptor inducing IFN- β (TRIF), integral to the Toll-like receptor 3 signaling pathway [105]. This interference highlights the HCV ability to evade antiviral immunity, a process counteracted by NS3–NS4A protease inhibitors [106].
Additionally, the NS5A protein can disrupt IFN signaling through various mechanisms, including the stimulation of interleukin 8 (IL-8) production and inhibition of the antiviral proteins [107]. The involvement of natural killer (NK) and NKT cells, which are abundant in the liver, is significant for HCV control, with their activity potentially inhibited by HCV E2 protein interaction with CD81 and influenced by HLA and killer immunoglobulin-like receptor (KIR) interactions [108,109]. Moreover, robust CD4 and CD8 T-cell responses are essential for HCV control, although their failure to prevent chronicity of HCV infection remains poorly understood and needs further investigation [110]. HCV-specific T cells emerge swiftly during acute infection and can persist in individuals following HCV infection clearance [111]. In chronic infection, stronger CD8 T-cell responses correlate with reduced hepatitis C viral loads [112].
Spontaneous clearance of HCV has been correlated with specific major histocompatibility complex (MHC) class I and class II molecules [98]. Studies identified a positive association in terms of higher probability of virus clearance with certain alleles, including HLA-B27, HLA-B57, HLA-A11, HLA-A03, and HLA-Cw01, and a negative correlation in terms of higher possibility of HCV persistence with HLA-B18 and HLA-Cw04 [113,114,115,116,117,118]. Nevertheless, these associations may vary depending on the circulating HCV genotypes [119]. Although the exact mechanisms through which these alleles can influence HCV clearance remain largely undetermined, it is proposed that protective alleles may effectively present immunogenic and conserved epitopes to T cells, enhancing viral clearance [120]. Conversely, alleles associated with an increased risk of chronic infection might act as ligands for inhibitory receptors on NK cells, with supporting evidence suggesting a significant role for NK receptor polymorphisms in HCV clearance [108].
The correlation between specific MHC class II genes and HCV clearance adds further emphasis to the role of host genetics in the outcome of HCV infection [121,122]. Research indicates that individuals who end up clearing the HCV infection tend to target a broader range of MHC class II epitopes compared to individuals who develop chronic HCV infection, targeting a smaller number of epitopes, which suggests a potential discrepancy in antigen processing or epitope presentation [123].
Most acute HCV infections (55–85%) become chronic due to the virus’s effective evasion strategies, with spontaneous clearance being rare once chronicity is established [4,124,125]. Specifically, a large study by Bulteel et al. reported that spontaneous clearance of HCV occurred at an incidence rate of 0.36/100 person-years follow-up [125]. Thus, the majority of HCV-infected individuals progress to chronic infection despite robust T-cell responses [111]. The HCV persistence is facilitated by selection for mutations that evade immune responses while maintaining replicative fitness [126,127]. These mutations often occur in cytotoxic T-lymphocyte (CTL) epitopes and are associated with impaired MHC class I presentation or reduced TCR contact [128].
The humoral immune response also plays a critical role in controlling HCV infections, with neutralizing antibodies primarily targeting the E2 protein [129]. These antibodies can influence the viral fitness and sequence evolution during acute infection, suggesting their significant impact on viral dynamics [130]. Despite the high variability of HCV and mechanisms like glycan shielding and lipid hiding that aid in immune evasion, neutralizing antibodies decrease infection risk post-exposure and modulate disease progression [131,132,133].
The role of virus factors reflected in the extreme genetic diversity of HCV does not appear to significantly alter the clinical manifestations across different HCV genotypes; nevertheless, genotype-specific responses to different treatments and associations with conditions such as steatosis vary among different genotypes [24,134,135].
6. Epidemiology, Transmission, and Global Figures of Hepatitis C
HCV primarily leads to severe health complications such as liver failure and HCC, particularly through its progression to chronic infection [136]. The risk of these severely morbid conditions increases with age and the length of time a person has been infected with the virus [137,138]. The WHO reported that in 2022, around 242,000 deaths were attributed to hepatitis C, which highlights the significant burden of the disease [4].
The spread of HCV infection during the 20th century was significantly influenced by the increased production and global use of syringes, initially for medical purposes and subsequently for illicit drug use among injection drug users (IDUs) [139,140]. Before the recognition of blood-borne transmission, the widespread adoption of percutaneous injections played a crucial role in disseminating HCV globally, notably elevating infection rates by 5 to 20 times in regions with prevalent unsafe injection practices, particularly among IDUs [141,142]. Additionally, the transfusion of unscreened blood products further facilitated the spread of HCV before virus identification [143]. Thus, the widespread transmission of HCV throughout the 20th century was largely fueled by the increased production and use of syringes for both medical purposes and illicit drug use, highlighting the impact of healthcare practices on the spread of bloodborne pathogens [141].
Historically, before the 20th century, practices such as scarification rituals and circumcision likely maintained HCV transmission [144]. This hypothesis is supported by current evidence of HCV transmission through these practices in certain world regions (e.g., in Egypt) and molecular clock analyses of HCV RNA sequences [145,146].
Transmission of HCV occurs mainly through percutaneous exposure to infected blood [147]. HCV can also spread from mother to infant (vertical transmission) and less frequently via sexual contact, especially among men-who-have-sex-with-men (MSM) [148,149,150]. There is no risk of HCV transmission through intact skin; however, transmission can happen if infected blood contacts mucosal surfaces, such as the eyes [151].
The probability of HCV transmission is directly related to the volume of blood involved in transmission and the nature of exposure to the virus inoculum [152]. In acute or chronically infected individuals, the blood usually harbors 3 to 6 log copies of HCV RNA per mL [153,154]. Thus, the blood constitutes the main route for HCV transmission, with a lower likelihood of transmission if the blood is not viremic [155]. While HCV RNA can be detected in other body fluids, the potential of these fluids to transmit HCV infection remains unclear [156,157].
High-risk HCV transmission scenarios often involve substantial percutaneous exposures, such as through blood transfusions of contaminated blood [140,147]. Nevertheless, even small volumes of blood can transmit HCV if introduced percutaneously, especially in healthcare settings where strict infection control practices may not be adhered to consistently [158,159,160,161]. Overall, the per-act risk of HCV transmission through needlestick injuries has been reported at a rate of 1% to 2% [162,163]. Repeated, small-volume exposures to contaminated blood explain the high incidence of HCV among IDUs [164]. Non-medical percutaneous exposures, such as tattooing, body piercing, and wet cupping (hijama), are also associated with HCV transmission and are more prevalent in certain countries, though these factors can be confounded by other high-risk behaviors [165,166].
The role of sexual transmission in HCV is less conspicuous, as it appears to be rare among long-term monogamous couples with higher occurrence among MSM [167,168]. Transmission from an infected mother to her infant is less common, occurring in about 4% to 8% of cases [169].
Hepatitis C is a major global health challenge, being one of the primary causes of liver disease [170,171]. The WHO’s latest estimates report that there are approximately 50 million people infected with HCV globally, with around 1 million new infections each year and about 242,000 deaths annually attributed to HCV-related complications [4]. The prevalence of HCV varies significantly across different regions and among various age and risk groups [172,173]. In 2022, the highest number of total HCV infections was reported in Pakistan, followed by India, China, Russia, and the United States, while the prevalence was >3.0% in Gabon, Pakistan, Mongolia, Burundi, Ukraine, and Uzbekistan (Table 1 and Figure 3) [174].

Table 1.
The prevalence and total number of HCV infections in 2022 per country based on Polaris Observatory data [174].
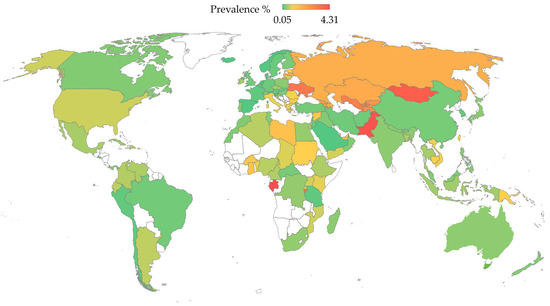
Figure 3.
The prevalence of HCV per country based on the Polaris Observatory data [174]. The map was generated in Microsoft Excel, powered by Bing, © Australian Bureau of Statistics, GeoNames, Microsoft, Navinfo, Open Places, OpenStreetMapTomTom, Zenrin. The map of Australia was generated by an older version of Microsoft Excel, powered by Bing, © GeoNames, Microsoft, Navinfo, TomTom, Wikipedia. We are neutral with regard to jurisdictional claims in this map.
Historically, Egypt has experienced the highest prevalence of HCV, particularly among individuals born before 1960, where rates among this particular age demographic had reached as high as 50% [175]. This high prevalence can be traced back to public health campaigns from the 1950s to the 1980s aimed at eradicating schistosomiasis [176]. These campaigns involved the mass intravenous administration of tartar emetic using equipment that was often reused and inadequately sterilized, leading to widespread HCV transmission [177].
Currently, China, India, and the United States represent countries with a high burden of HCV disease globally [2]. These three countries lead in the number of HCV cases, surpassing Egypt, which was among the top until 2019 [178,179].
7. Genetic Diversity of HCV
Shortly after the discovery of HCV, it became evident that distinct genetic strains of the virus existed in different geographical areas [180,181,182]. The HCV nomenclature standards—which were pioneered by the prominent virologist Peter Simmonds—have identified seven major genotypes of HCV, which are phylogenetically distinct, with further divisions into subtypes within these genotypes [60,65,183]. The HCV genotypes and subtypes display variations in their response to treatments. For example, genotypes 1 and 4 show less responsiveness to IFNs, while the lower susceptibility of genotype 3 to DAAs remains a challenging issue [184,185,186]. Additionally, HCV genotypes demonstrate variability in clinical outcomes beyond their influence on antiviral drug resistance. For example, genotype 3 is notably associated with an increased occurrence of liver steatosis, which involves significant fat accumulation within the liver as well as an increased progression to fibrosis and cirrhosis [187,188,189,190]. On the other hand, subtype 1b has been linked with a higher risk of HCC development [191,192].
The HCV genomic nucleotide difference among genotypes is higher than 30%, with approximately 15% genetic difference among subtypes [24,65]. It is important to point out that these genetic differences are not evenly distributed across the HCV genome since these differences are most pronounced in the E1, E2, and the V3 region of NS5A proteins, whereas the C protein shows less variability [24,193].
Despite the possible occurrence of recombination in HCV, inter-genotypic recombination is reported rarely; therefore, reconstruction of HCV phylogeny is considered reliable for the majority of sub-genomic HCV regions reflecting the clustering patterns of full genome analysis [194].
For the HCV genetic diversity at the global level, a notable variation has been reported [173]. In spite of this variability, genotype 1 is common globally, with phylodynamic studies showing that the global dissemination of this genotype took place between the 1940s and 1980s [195]. Evidence also suggested that subtype 1b may have spread earlier than subtype 1a [195]. Subtype 1a is frequently associated with IDUs, specifically in North America and Northern Europe, while subtype 1b is often associated with a history of blood product use [196,197].
Local epidemics of HCV often exhibit dominance of specific subtypes, largely due to founder effects rather than variations in how easily the virus is transmitted or the routes of transmission [198,199]. For example, the HCV epidemic in Egypt is predominantly driven by subtype 4a [200]. In contrast, West Africa demonstrates significant diversity within genotype 2, indicating it likely originated there [201]. In Southeast Asia, genotypes 3 and 6 predominate, especially among IDUs [202,203,204,205]. Meanwhile, genotype 5 is most frequently found in Southern Africa [206,207,208].
Regarding the origin of HCV in humans, recent discoveries of hepaciviruses in non-primates like dogs and horses show that these are distinctly different from HCV [209,210,211,212]. This suggests a complex and ancient evolution of HCV in humans, with origins that potentially date back hundreds or even thousands of years [63,145]. However, without older sequence data, the precise dating of HCV emergence remains challenging due to calibration uncertainties in molecular clock analyses [213].
8. Clinical and Histopathologic Features of Hepatitis C
Acute HCV infection often proceeds sub-clinically, with a significant proportion of cases remaining asymptomatic [214]. However, the onset of acute hepatitis C can manifest in a subset of infected individuals, with symptoms characteristic of acute viral hepatitis, including malaise, fatigue, anorexia, nausea, abdominal pain, jaundice, dark urine, and pale stools [215]. Notably, fulminant hepatitis, a severe and rapid deterioration of liver function, appears to occur rarely in the context of HCV [216,217].
The interval from HCV exposure to the appearance of symptoms or laboratory evidence of liver injury (incubation period) can vary widely, ranging from two to twenty weeks and typically manifest in seven weeks [218,219]. Frequently, the primary laboratory indication of acute HCV infection is elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), indicative of damaged hepatocytes [220].
Following HCV infection, viremia is rapidly detectable, rising to between 100,000 and 10 million IU/mL within a few weeks [153,221,222]. A decline in viral load often follows, occurring one to two weeks later and coinciding with a marked increase in ALT and AST levels [153,223]. This pattern reflects the immune system response to HCV infection, which mediates hepatocyte destruction and, importantly, influences the rate of spontaneous viral clearance [224,225]. Consequently, higher initial HCV viremic levels and more severe forms of acute hepatitis C are correlated with higher likelihoods of spontaneous clearance [226].
Spontaneous clearance of HCV typically manifests within six months of infection [227]. A higher probability of viral clearance has been observed in symptomatic individuals, females, and younger patients [228,229,230,231,232]. Conversely, spontaneous resolution of HCV infection is less likely to be observed among those co-infected with HIV, IDUs, or individuals of black ethnicity [233,234]. The identification of specific alleles near the IL28B gene, responsible for encoding interferon-lambda 3 (IFNλ3), has provided important insights into the genetic determinants of HCV infection outcomes [235,236,237]. These alleles are also predictive of spontaneous recovery from acute HCV [238,239]. Significantly, the distribution of this protective IL28B genotype (CC genotype as opposed to the CT/TT genotypes) exhibits marked racial variations, being more prevalent in Asian populations and notably less so in those of African descent [240].
In the vast majority of individuals (60% to 85%) who do not undergo spontaneous HCV infection resolution, chronic infection emerges as a highly variable condition, characterized by diverse clinical manifestations and progression rates [227,241]. Significant morbidity and mortality typically arise when the infection advances to cirrhosis or end-stage liver disease, which may further evolve into HCC [242,243,244].
Among chronically infected HCV patients, the levels of HCV RNA in the blood are relatively constant over time, generally fluctuating, and can reach over 1 million IU/mL [245]. Certain factors are associated with higher viral loads, including HIV co-infection, male gender, older age, and increased body mass index [246,247]. Conversely, individuals with concurrent HBV infection or more advanced liver disease tend to exhibit lower viral loads [248].
Chronic HCV infection induces a spectrum of histopathological changes in the liver, characterized primarily by variable levels of chronic inflammation and steatosis [249]. Frequent periportal lymphocytic infiltrates are noted but do not consistently predict the course of liver disease progression [249]. In chronic hepatitis C, a disparity in the development of significant fibrosis is noted where some individuals exhibit marked fibrotic changes after prolonged viral exposure, while others show minimal effects, the reason of which remains to be fully elucidated [247,250,251].
Fibrosis develops as a result of an imbalance in extracellular matrix dynamics, with collagen production exceeding its breakdown, initiating in the periportal zones [252]. This fibrosis process may stabilize or advance to more severe structural alterations, including the formation of septae that bridge adjacent lobules [253]. Progression beyond this stage culminates in cirrhosis, characterized by extensive scarring and nodular liver regeneration [254].
As cirrhosis advances, complications such as portal hypertension develop, and there is an escalated risk of HCC due to neoplastic transformations within the hepatic parenchyma [255,256]. Understanding this pathophysiological process that culminates in end-stage liver disease is necessary for timely and effective therapeutic interventions to halt or reverse the fibrosis processes in chronic HCV infection [257,258].
The potential for HCC in chronic HCV infection constitutes a significant risk, necessitating vigilant screening protocols for cirrhosis and HCC [259]. This proactive approach via HCV screening is crucial due to the typically asymptomatic progression of chronic HCV until the patients reach advanced stages [260].
The role of HCV in the direct induction of oncogenic processes remains under investigation [261,262,263]. There is growing evidence that the HCV C protein engages in oncogenic modulation by activating proto-oncogenes and suppressing apoptotic pathways [264,265]. However, it is also plausible that the chronic inflammation, which is the hallmark of persistent HCV infection, sufficiently promotes oncogenesis, leading to the development of HCC [266,267].
Finally, chronic HCV infection is implicated in a variety of extrahepatic manifestations predominantly inflammatory in nature [96,268]. These manifestations further complicate the clinical management of HCV, illustrating the need to address the broader systemic effects of chronic HCV infection [269].
9. Diagnosis of HCV
Infection by HCV should be considered in patients presenting with unexplained liver abnormalities [270]. Given the relatively common prevalence of chronic hepatitis C and the frequently asymptomatic nature of the disease, HCV testing is warranted in individuals displaying elevated liver transaminases or those with risk factors for HCV acquisition [271]. Importantly, the shared nature of risk factors between HCV and HIV necessitates vigilant screening protocols for this particular group, namely HIV-infected individuals [272]. Accordingly, HCV screening for all individuals diagnosed with HIV is advocated as a strategy aimed at early detection and management of HCV to prevent further liver damage and optimize patient outcomes [5].
The recent guidelines from the IDSA, the AASLD, and the U.S. Centers for Disease Control and Prevention (CDC) endorsed universal HCV screening in response to evolving epidemiological trends and the availability of effective treatments [13]. As of March 2020, it is recommended that all adults between the ages of 18 to 79 undergo HCV screening [5]. This policy was further expanded in April 2020 by the CDC to include a one-time screening for all adults aged 18 and older and for every pregnancy, barring settings where the HCV prevalence is below 0.1% [271].
The adoption of universal HCV screening represents a strategic evolution in public health policy, driven by a variety of factors [273]. The evident cost-effectiveness of widespread screening initiatives has established them as practical and essential public health interventions [274,275]. Additionally, universal screening significantly enhances the detection of HCV, which is particularly crucial given the often-silent progression of the infection [276]. The urgency for a universal screening protocol is emphasized by the increasing incidence of HCV, especially among younger populations, where the disease may otherwise go unnoticed until advanced stages [277,278]. The current availability of safe and cost-effective DAAs ensures that HCV can be treated efficiently following detection [279]. Effective treatment also contributes to halting the forward transmission of the virus, further reducing the HCV burden through TasP [280].
The initial diagnostic approach for HCV infection involves serologic testing through Enzyme Immunoassays (EIAs), which detects antibodies against HCV C, NS3, NS4, and NS5 proteins [281]. Despite its high specificity and sensitivity, EIAs’ results can occasionally yield false positives [281,282]. Therefore, positive serologic results necessitate further confirmation through HCV RNA testing to determine active infection [283,284].
Serological testing for HCV is diverse, employing a range of technologies from EIA and chemiluminescence immunoassay to rapid techniques such as agglutination and lateral flow assays [281,285]. Advanced methodologies include recombinant immunoblot assay (RIBA), electrochemical immunosensors, and nano-metal technologies utilizing gold nanoparticles and quantum dots [286,287,288]. Despite the technological advances in HCV screening, several challenges hinder its widespread application, particularly in resource-limited environments [281]. These issues include the prolonged turnaround times and the substantial costs associated with these tests, which are compounded by the bulky nature of the instrumentation and the necessity for skilled technicians [289]. Such constraints have motivated innovation in diagnostic approaches that balance accuracy and practicality, aiming to reduce both equipment costs and operational complexity while maintaining test sensitivity and specificity [290].
HCV RNA testing remains crucial for cases with elevated clinical suspicion, facilitated by methods like reverse transcriptase-polymerase chain reaction (RT-PCR), transcription-mediated amplification (TMA), reverse transcription loop-mediated isothermal amplification (RT-LAMP), and branched DNA (bDNA) assays [291,292,293,294]. These methods, targeting highly conserved regions of the HCV genome, are critical for confirming the chronic infection status and informing treatment strategies.
Genotyping of HCV was crucial for tailoring IFN-based therapy, and this involved utilization of reverse hybridization assays that amplify specific genomic regions (5′UTR or C) to determine genotype and occasionally subtype [281,295]. However, the most reliable method for HCV genotype determination employs genomic sequencing and phylogenetic analysis of the E1 or NS5B regions [296]. The role of HCV genotyping following the availability of pan-genotypic DAA regimens remains an issue to be further investigated [135].
Accurate staging of HCV-related liver disease, which historically relied on invasive liver biopsies, is essential [297]. Despite the detailed insights of liver biopsies, this invasive approach carries risks and limitations [298]. The shift towards non-invasive methods like serum markers and sonographic elastography is reshaping HCV management [299,300,301,302]. This allows more frequent and less invasive monitoring, providing a clearer assessment of liver fibrosis without the drawbacks associated with traditional biopsy methods [303].
10. Treatment of HCV
The therapeutic approach for HCV infection experienced a significant transformation, particularly with the advent of DAA, with a notable shift from IFN-based treatments to more effective and tolerable options with shorter durations of treatment [22]. Historically, the standard-of-care for HCV management involved pegylated IFN and ribavirin [304]. This option was often effective in achieving an SVR; nevertheless, it was complicated by its lengthy duration and often led to considerable side effects [305].
The introduction of DAAs marked a revolutionary step in HCV management, with cure rates above 95% and defining SVR, specifically undetectable HCV RNA 12 weeks post-treatment (SVR12), as a new clinical benchmark instead of 24 weeks [306,307]. Additionally, 8-week regimens have shown effectiveness in real-world studies, with a positive impact on treatment adherence and reduction in costs [308,309,310]. DAAs offer the enhancement of patient tolerability to treatment and allow for the customization of treatment regimens based on individual patient factors such as HCV genotype, stage of liver fibrosis, co-existing medical conditions, prior treatment history, and potential RASs [311,312]. Despite the clinical success of DAAs, a limitation is related to their high cost which is a critical consideration, especially in low- and middle-income countries [21].
Historically, the efficacy of IFN-based treatments was influenced by a range of factors, including patient demographics, viral characteristics, and genetic markers such as IL28B polymorphisms [304]. Additionally, IFN and ribavirin therapies were well-known for their broad and severe side effect profiles [313]. Common IFN adverse effects reported in large trials included fatigue, headache, nausea, insomnia, and pyrexia, along with more severe impacts such as anemia, neutropenia, and a range of psychiatric and immunological reactions [314]. Notably, ribavirin frequently caused hemolytic anemia, a challenging complication that often necessitated dose adjustments or discontinuation of therapy [315].
These side effects underline the challenges of the older treatment regimens and highlight the advantages of DAAs, which have fewer adverse effects and do not require the intensive monitoring and management that IFN-based therapies did [316]. Thus, the introduction of DAAs revolutionized the treatment of chronic HCV infection, marking a significant milestone in the management and possible eradication of the disease as a public health threat. The U.S. Food and Drug Administration (FDA) approved the first DAAs, telaprevir and boceprevir, in May 2011 [317]. Approval of other DAAs followed, which represented a major therapeutic breakthrough, marking a new era of hepatitis C treatment characterized by enhanced efficacy, tolerability, and shorter duration [318]. A timeline of DAAs’ development and approval is presented below:
2011: Introduction of boceprevir (Victrelis) and telaprevir (Incivek), pioneering the DAA classes with enhanced direct antiviral activity against HCV [319].
2013: Approval of sofosbuvir (Sovaldi), a landmark in DAA therapy characterized by high cure rates, reduced side effects, and shorter treatment durations [320].
2014: The FDA approved combination therapies such as ledipasvir/sofosbuvir (Harvoni), simplifying HCV treatment by eliminating the need for interferon and shortening the course of therapy [321].
2016: Introduction of pan-genotypic treatments such as elbasvir/grazoprevir (Zepatier) and sofosbuvir/velpatasvir (Epclusa), capable of treating all HCV genotypes effectively [322].
2017: Approval of glecaprevir/pibrentasvir (Mavyret), enhancing the treatment with shorter courses to achieve SVR and pan-genotypic high efficacy [323].
Thus, the introduction of DAA agents was a substantial progression from the previous treatment modalities [324]. The DAAs directly target specific steps within the HCV replication cycle. Clinical evidence robustly supports the efficacy of DAA regimens, with data indicating that over 95% of patients treated with DAAs achieve an SVR, which is clinically equated with virological cure [325,326,327,328,329,330]. A summary of four common, currently approved DAA regimens is presented in (Table 2).

Table 2.
List of commonly used direct-acting antiviral (DAA) regimens.
Innovative therapeutic strategies are being explored as well, to expand the treatment options for HCV especially for challenging cases (e.g., patients with HCC) as reviewed comprehensively by Medina et al. [343]. For example, agents such as ezetimibe that target cellular cholesterol, which is critical for viral entry, can offer a novel mechanism to prevent HCV entry into hepatocytes [344]. Moreover, clinical trials incorporating statins with DAAs or IFN are underway [345]. These trials aim to enhance the antiviral response by taking advantage of the effects of statins on lipid metabolism, which may also boost IFN effectiveness [343].
11. Prevention of HCV
Infection with HCV remains a challenging public health threat [57]. The burden of HCV necessitates various strategies for prevention [1]. Key preventive strategies to reduce the burden of HCV include improved screening, harm reduction in the context of IDU, prevention of healthcare-associated infections, and the use of TasP [346,347,348,349,350].
Universal HCV screening with concomitant treatment of the detected cases is recommended, considering the natural history of chronic hepatitis C where the disease is often asymptomatic until advanced liver disease develops [13]. Recommendations advocate for one-time screening for all individuals aged 18 years and routine screening for high-risk populations, including individuals with an IDU history and individuals on long-term hemodialysis [13,351]. Early diagnosis of HCV infection through widespread screening facilitates timely access to treatment [352]. Additionally, there is growing evidence supporting the cost-effectiveness of universal HCV screening even in countries with low HCV prevalence [353,354].
Regarding harm reduction, needle exchange programs (NEPs), opioid substitution therapy (OST), and educational programs have shown potential to reduce HCV transmission—in addition to other bloodborne viruses—among IDUs [355,356]. In healthcare settings, strict adherence to proper infection control practices is important to prevent HCV transmission, including proper sterilization of medical and dental equipment [357]. Importantly, the implementation of TasP appears crucial to reduce the burden of HCV infections [350]. Besides the dramatic improvement in the treatment of chronic HCV, the DAAs stimulated efforts towards its global elimination [358]. The absence of an effective HCV vaccine has led to the adoption of the TasP strategy [359]. TasP advocates for the widespread and prompt treatment of HCV infections to substantially lower HCV transmission within populations, thereby reducing the prevalence of hepatitis C [360].
However, the effective implementation of TasP faces significant challenges, particularly regarding the development of drug resistance [361]. RASs can occur naturally or develop during treatment, presenting a challenging obstacle and reducing treatment efficacy [17]. The presence of RASs may limit the scalability of treatment programs since therapies proven effective in clinical trials may exhibit diminished effectiveness in real-world applications due to these resistant strains [362].
Additionally, the spread of drug-resistant variants is particularly concerning in high-risk groups, such as IDUs, who are more prone to disseminating these variants [363,364]. Thus, there is a need for continuous surveillance and the development of novel therapeutic options that can bypass HCV resistance mechanisms [21].
12. Resistance-Associated Substitutions (RASs)
As mentioned earlier, the therapeutic strategy for chronic HCV infection has been revolutionized by the approval of DAAs, which present dramatically improved cure rates over IFN-based therapies across various HCV genotypes [365]. Nevertheless, the success story of DAAs as a curative therapy for HCV can be undermined by RASs conferring resistance [362]. This feared outcome is related to the high mutation rate inherent to HCV due to its error-prone replicase enzyme (RdRp) [366,367]. The RASs could arise from mutations occurring spontaneously due to the natural genetic diversity of the virus (natural resistance) or be induced under the selective pressure exerted by antiviral therapies (acquired resistance) [368,369,370]. Besides the issue of emerging RASs to DAAs, other factors contribute to treatment failure, including patient adherence to treatment and suboptimal treatment regimens [36].
The prevalence of RASs is influenced by both viral genotype and geographic factors [35,371]. In an early comprehensive analysis utilizing published GenBank data, the global prevalence of resistance-associated variants (RAVs) to DAAs was determined [372]. The study revealed that a significant proportion, 58.7% (854 out of 1455 sequences), harbored at least one dominant resistance variant, with notable geographic discrepancies [372]. Asia exhibited the highest frequency of RAVs at 74.1%, followed by Africa at 71.9%, America at 53.5%, and Europe at 51.4% [372]. Among the HCV genotypes, genotype 6 displayed the highest frequency of RAVs at 99%, a notably high prevalence compared to other genotypes. This was followed by genotype 2 at 87.9%, genotype 4 at 85.5%, subtype 1a at 56%, genotype 3 at 50%, and subtype 1b at 34.3%. The study also assessed the distribution of RAVs across different classes of DAAs. It was found that 40.0% of sequences contained RAVs associated with NS5A inhibitors, and 29.6% with NS3 inhibitors, highlighting a significant challenge in managing resistance to these therapies [372]. In contrast, resistance to NS5B NIs and NI-based combinations was notably lower, with less than 4% of sequences showing RAVs [372].
Defining the clinical significance of RASs remains a significant challenge [373]. For example, RASs identified in phenotypic assays during cell culture studies with selective pressure by DAAs do not always correspond to those emerging in clinical scenarios where failed treatment is manifested [373]. Thus, not all RASs detected through sequencing directly impact the efficacy of DAA therapies [374]. Furthermore, the significance of RAVs within the HCV quasispecies is a critical factor in understanding resistance dynamics [375]. Variants below a 15% frequency within the HCV quasispecies generally exert minimal influence on treatment outcomes [32]. This observation is essential for clinical practice, as it informs the threshold of variant detection that should concern clinicians, guiding more relevant approaches to the use of DAAs [373].
13. HCV Vaccination Challenges
Despite the revolutionary impact of DAAs on HCV treatment, the quest for a preventive hepatitis C vaccine continues to be a crucial public health issue [376]. While DAAs have remarkable effectiveness, these drugs carry a high cost burden and face distribution challenges that limit their accessibility, especially in low-income settings [377,378]. Furthermore, the curative treatments do not confer immunity against future infections, an issue of particular concern in populations engaged in high-risk behaviors (e.g., IDUs) [379]. A vaccine would dramatically reduce the incidence of new infections and hinder HCV transmission within communities, offering a sustainable and cost-effective strategy to mitigate the global HCV epidemic [380].
However, the development of an effective HCV vaccine is hampered by the high genetic diversity of HCV, which enables the effective escape from immune recognition [381,382]. This diversity complicates the identification of universal vaccine targets and restricts vaccine epitope design [383,384].
Promising vaccine strategies like the use of cyclic peptides, which hold potential for eliciting strong neutralizing antibody responses, face hurdles in development of the delivery systems necessary to maximize their immunogenicity [385]. Additionally, the absence of robust infection models that accurately mimic human HCV infection presents a significant barrier to assessing vaccine efficacy [23,386].
Recent advances in HCV vaccine research yielded promising results [383]. For example, experimental work with DNA and peptide-based vaccines in murine models has progressed, including a notable development involving a peptide vaccine derived from the HCV p7 protein [387]. Similarly, a DNA-based HCV vaccine has been effective in eliciting comprehensive T cell responses and memory, though it also stimulated a non-neutralizing antibody response [388]. The utility of messenger RNA (mRNA) vaccine technology, which gained momentum during the COVID-19 pandemic, represents a promising area to probe for HCV prevention [389]. However, significant challenges in the context of HCV vaccination remain, such as the extensive variability of HCV E proteins. Moreover, the low incidence of HCV in industrialized countries complicates conducting the clinical trials, which are often restricted to high-risk sub-populations. Nevertheless, ongoing research efforts, including a notable study by Patra et al., highlights the possibility of developing an mRNA vaccine platform to combat HCV effectively [390]. However, these efforts yielded promising early-stage vaccine candidates and simultaneously highlighted the need for ongoing research efforts to develop an HCV vaccine, which appears as a complex and lengthy journey [23].
14. Elimination of HCV by 2030
The WHO formulated a strategy aimed at mitigating the global health impact of hepatitis [391]. The WHO comprehensive hepatitis strategy targets a dramatic reduction in disease burden by 2030, endorsed universally by WHO member states. This strategy aims for a 90% reduction in new infections and a 65% decrease in mortality through an integrated approach that amplifies preventive measures such as TasP, expands the availability of diagnostic services, enhances access to antiviral therapies, and intensifies educational efforts [392,393].
In parallel, the Global HCV Elimination Coalition has implemented a multi-faceted approach to eradicate HCV by 2030 [394]. The revolutionary impact of DAAs is considered essential to these initiatives.
Additional strategies include the education and training of healthcare workers to ensure safe practices and effective patient education, which are essential for reducing new viral hepatitis cases, including HCV cases [395,396]. Integrating healthcare services to address the needs of marginalized populations, including IDUs, incarcerated individuals, and economically disadvantaged groups, is also important [397].
Together, these robust strategies necessitate that each country critically assess and adapt its healthcare frameworks to develop effective care to ensure comprehensive surveillance and management of hepatitis C cases [391]. This approach can reduce the incidence and mortality associated with hepatitis C and contribute to the ultimate goal of eliminating hepatitis C as a public health threat by 2030.
15. Conclusions, Future Perspectives, and Limitations
In this comprehensive review of HCV, the remarkable advances and persistent challenges in diagnosis, management, and prevention of hepatitis C were highlighted. The development of DAAs revolutionized the management of chronic hepatitis C, significantly enhancing both treatment efficacy and prevention capabilities via TasP. However, the emergence of RASs remains a critical concern. This issue emphasizes the need for ongoing surveillance and the development of new pan-genotypic therapies that can effectively address the potentially resistant variants of HCV.
The absence of effective HCV vaccines so far continues to hinder efforts toward the WHO 2030 goal of HCV elimination. Achieving this ambitious goal will require enhanced diagnostic accessibility to ensure early and accurate detection, broader accessibility and enhanced affordability of DAAs, and improved adherence to treatment alongside robust monitoring of RAS impacts.
Continuous research and innovation are imperative to mitigate the global burden of HCV. Future research is needed to better predict and counteract the evolving dynamics of HCV transmission and resistance to DAAs. Coordinated global efforts are still needed to achieve the ultimate goal of HCV eradication or to at least alleviate its negative impact on public health.
Finally, we acknowledge several limitations inherent in this review despite its comprehensive nature, as follows. First, the selection bias is expected especially in terms of inclusion of studies published in English, with risk of missing significant findings reported in other languages. Second, the skewed availability of data towards more researched populations and regions might underrepresent the epidemiology especially in terms of absent estimates on the HCV prevalence in several countries worldwide. Lastly, we must acknowledge key limitations concerning the novelty and reproducibility of this review. Although we aimed to compile and analyze the current and past literature on HCV, the nature of this review did not involve the presentation of novel experimental findings but rather an attempt to integrate the existing knowledge on HCV to highlight trends and gaps in current HCV research.
Author Contributions
Conceptualization, M.S.; methodology, M.S. and R.K.; software, M.S.; resources, M.S.; data curation, M.S. and R.K.; writing—original draft preparation, M.S.; writing—review and editing, M.S. and R.K.; visualization, M.S.; supervision, M.S.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author (M.S.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brunner, N.; Bruggmann, P. Trends of the Global Hepatitis C Disease Burden: Strategies to Achieve Elimination. J. Prev. Med. Public Health 2021, 54, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qi, J.L.; Wang, X.X.; Li, X.H.; Jin, R.; Liu, B.Y.; Liu, H.X.; Rao, H.Y. The burden of hepatitis C virus in the world, China, India, and the United States from 1990 to 2019. Front. Public Health 2023, 11, 1041201. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Milli, C.; Voller, F.; Silvestri, C. The Epidemiology of Chronic Hepatitis C: Where We Are Now. Livers 2024, 4, 172–181. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Hepatitis C—Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 19 April 2024).
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Donahue, K.; Doubeni, C.A.; Epling, J.W., Jr.; Kubik, M.; et al. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2020, 323, 970–975. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Updated Recommendations on Treatment of Adolescents and Children with Chronic HCV Infection, and HCV Simplified Service Delivery and Diagnostics. Available online: https://www.who.int/publications/i/item/9789240052734 (accessed on 15 May 2024).
- Welsch, C.; Jesudian, A.; Zeuzem, S.; Jacobson, I. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut 2012, 61 (Suppl. S1), i36–i46. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Naggie, S.; Rockstroh, J.K.; Matthews, G.V. Direct-Acting Antiviral Therapy for Treatment of Acute and Recent Hepatitis C Virus Infection: A Narrative Review. Clin. Infect. Dis. 2023, 77, S238–S244. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Marcellin, P.; Schinazi, R.F. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018, 38 (Suppl. S1), 7–13. [Google Scholar] [CrossRef] [PubMed]
- Falade-Nwulia, O.; Suarez-Cuervo, C.; Nelson, D.R.; Fried, M.W.; Segal, J.B.; Sulkowski, M.S. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann. Intern. Med. 2017, 166, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, V.; Banzi, R.; Cariani, E.; Chester, J.; Villa, E.; D’Amico, R.; Bertele, V.; Trenti, T. New Direct-Acting Antivirals for the Treatment of Patients With Hepatitis C Virus Infection: A Systematic Review of Randomized Controlled Trials. J. Clin. Exp. Hepatol. 2019, 9, 522–538. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Aronsohn, A.; Price, J.; Lo Re, V. Hepatitis C Guidance 2023 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin. Infect. Dis. 2023, ciad319. [Google Scholar] [CrossRef] [PubMed]
- AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin. Infect. Dis. 2018, 67, 1477–1492. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, D.; Penin, F. Hepatitis C virus proteins: From structure to function. Curr. Top. Microbiol. Immunol. 2013, 369, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Maasoumy, B. Breakthroughs in hepatitis C research: From discovery to cure. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.Y.M.; Rodrigo, C.; Cunningham, E.B.; Douglas, M.W.; Dietz, J.; Grebely, J.; Popping, S.; Sfalcin, J.A.; Parczewski, M.; Sarrazin, C.; et al. Characteristics of hepatitis C virus resistance in an international cohort after a decade of direct-acting antivirals. JHEP Rep. 2022, 4, 100462. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.C.; Nielsen, E.E.; Feinberg, J.; Katakam, K.K.; Fobian, K.; Hauser, G.; Poropat, G.; Djurisic, S.; Weiss, K.H.; Bjelakovic, M.; et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst. Rev. 2017, 9, Cd012143. [Google Scholar] [CrossRef] [PubMed]
- Geddawy, A.; Ibrahim, Y.F.; Elbahie, N.M.; Ibrahim, M.A. Direct Acting Anti-hepatitis C Virus Drugs: Clinical Pharmacology and Future Direction. J. Transl. Int. Med. 2017, 5, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.Y.M.; Ceccherini-Silberstein, F.; Dietz, J.; Popping, S.; Grebely, J.; Rodrigo, C.; Lennerstrand, J.; Douglas, M.W.; Parczewsk, M.; Harrigan, P.R.; et al. SHARED: An International Collaboration to Unravel Hepatitis C Resistance. Viruses 2021, 13, 1580. [Google Scholar] [CrossRef] [PubMed]
- Bartenschlager, R.; Baumert, T.F.; Bukh, J.; Houghton, M.; Lemon, S.M.; Lindenbach, B.D.; Lohmann, V.; Moradpour, D.; Pietschmann, T.; Rice, C.M.; et al. Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: Considerations for scientists and funding agencies. Virus Res. 2018, 248, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, R.; Farshadpour, F. Global elimination of hepatitis C virus infection: Progresses and the remaining challenges. World J. Hepatol. 2017, 9, 1239–1252. [Google Scholar] [CrossRef]
- Hartlage, A.S.; Kapoor, A. Hepatitis C Virus Vaccine Research: Time to Put Up or Shut Up. Viruses 2021, 13, 1596. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, N.; Moratorio, G.; Cristina, J.; Moreno, P. Hepatitis C virus genetic variability and evolution. World J. Hepatol. 2015, 7, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hakeem, M.S.; Shoukry, N.H. Protective immunity against hepatitis C: Many shades of gray. Front. Immunol. 2014, 5, 274. [Google Scholar] [CrossRef] [PubMed]
- Baumert, T.F.; Berg, T.; Lim, J.K.; Nelson, D.R. Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges. Gastroenterology 2019, 156, 431–445. [Google Scholar] [CrossRef]
- Lombardi, A.; Mondelli, M.U. Hepatitis C: Is eradication possible? Liver Int. 2019, 39, 416–426. [Google Scholar] [CrossRef]
- Dhiman, R.K.; Premkumar, M. Hepatitis C Virus Elimination by 2030: Conquering Mount Improbable. Clin. Liver Dis. 2020, 16, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Hellard, M.; Schroeder, S.E.; Pedrana, A.; Doyle, J.; Aitken, C. The Elimination of Hepatitis C as a Public Health Threat. Cold Spring Harb. Perspect. Med. 2020, 10, a036939. [Google Scholar] [CrossRef] [PubMed]
- Hagan, L.M.; Wolpe, P.R.; Schinazi, R.F. Treatment as prevention and cure towards global eradication of hepatitis C virus. Trends Microbiol. 2013, 21, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Bernal, L.A.; Soti, V. Hepatitis C Virus: Insights Into Its History, Treatment, Challenges, and Future Directions. Cureus 2023, 15, e43924. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J. Hepatol. 2016, 64, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M. Hepatitis C Virus Resistance to Direct-Acting Antiviral Drugs in Interferon-Free Regimens. Gastroenterology 2016, 151, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Ridruejo, E.; Pereson, M.J.; Flichman, D.M.; Di Lello, F.A. Hepatitis C virus treatment failure: Clinical utility for testing resistance-associated substitutions. World J. Hepatol. 2021, 13, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; Trinks, J.; Soriano, V. Hepatitis C virus resistance to the new direct-acting antivirals. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Hashmi, A.H.; Khan, A.; Asad Raza Kazmi, S.M.; Manzoor, S. Emergence and Persistence of Resistance-Associated Substitutions in HCV GT3 Patients Failing Direct-Acting Antivirals. Front. Pharmacol. 2022, 13, 894460. [Google Scholar] [CrossRef] [PubMed]
- Kyuregyan, K.K.; Kichatova, V.S.; Karlsen, A.A.; Isaeva, O.V.; Solonin, S.A.; Petkov, S.; Nielsen, M.; Isaguliants, M.G.; Mikhailov, M.I. Factors Influencing the Prevalence of Resistance-Associated Substitutions in NS5A Protein in Treatment-Naive Patients with Chronic Hepatitis C. Biomedicines 2020, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Mizokami, M.; Pianko, S.; Mangia, A.; Han, K.H.; Martin, R.; Svarovskaia, E.; Dvory-Sobol, H.; Doehle, B.; Hedskog, C.; et al. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: Prevalence and effect on treatment outcome. J. Hepatol. 2017, 66, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M. Hepatitis C Virus: 30 Years after Its Discovery. Cold Spring Harb. Perspect. Med. 2019, 9, a037069. [Google Scholar] [CrossRef] [PubMed]
- Feinstone, S.M.; Kapikian, A.Z.; Purcell, R.H.; Alter, H.J.; Holland, P.V. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N. Engl. J. Med. 1975, 292, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.J.; Kuo, G.; Bradley, D.W.; Bonino, F.; Saracco, G.; Lee, C.; Rosenblatt, J.; Choo, Q.L.; Houghton, M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet 1990, 335, 1–3. [Google Scholar] [CrossRef]
- Alter, H.J.; Purcell, R.H.; Holland, P.V.; Popper, H. Transmissible agent in non-A, non-B hepatitis. Lancet 1978, 1, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, F.B.; Gitnick, G.L.; Aach, R.D.; Szmuness, W.; Mosley, J.W.; Stevens, C.E.; Peters, R.L.; Weiner, J.M.; Werch, J.B.; Lander, J.J. Non-A, non-B hepatitis transmission in chimpanzees: A project of the transfusion-transmitted viruses study group. Intervirology 1978, 10, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.K.; Feinstone, S.M.; Purcell, R.H.; Alter, H.J.; London, W.T. Non-A, non-B hepatitis: Ultrastructural evidence for two agents in experimentally infected chimpanzees. Science 1979, 205, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.W. The agents of non-A, non-B viral hepatitis. J. Virol. Methods 1985, 10, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.W.; McCaustland, K.A.; Cook, E.H.; Schable, C.A.; Ebert, J.W.; Maynard, J.E. Posttransfusion non-A, non-B hepatitis in chimpanzees. Physicochemical evidence that the tubule-forming agent is a small, enveloped virus. Gastroenterology 1985, 88, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Sofi, A.A. The Discovery of Hepatitis Viruses: Agents and Disease. J. Clin. Exp. Hepatol. 2020, 10, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Pol, S.; Lagaye, S. The remarkable history of the hepatitis C virus. Genes. Immun. 2019, 20, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Bukh, J.; Forns, X.; Emerson, S.U.; Purcell, R.H. Studies of hepatitis C virus in chimpanzees and their importance for vaccine development. Intervirology 2001, 44, 132–142. [Google Scholar] [CrossRef]
- Prince, A.M.; Brotman, B. Biological and immunological aspects of hepatitis C virus infection in chimpanzees. Curr. Stud. Hematol. Blood Transfus. 1998, 62, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Choo, Q.L.; Kuo, G.; Weiner, A.J.; Overby, L.R.; Bradley, D.W.; Houghton, M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989, 244, 359–362. [Google Scholar] [CrossRef]
- Miyamura, T.; Saito, I.; Katayama, T.; Kikuchi, S.; Tateda, A.; Houghton, M.; Choo, Q.L.; Kuo, G. Detection of antibody against antigen expressed by molecularly cloned hepatitis C virus cDNA: Application to diagnosis and blood screening for posttransfusion hepatitis. Proc. Natl. Acad. Sci. USA 1990, 87, 983–987. [Google Scholar] [CrossRef]
- Ghany, M.G.; Lok, A.S.F.; Dienstag, J.L.; Feinstone, S.M.; Hoofnagle, J.H.; Jake Liang, T.; Seeff, L.B.; Cohen, D.E.; Bezerra, J.A.; Chung, R.T. The 2020 Nobel Prize for Medicine or Physiology for the Discovery of Hepatitis C Virus: A Triumph of Curiosity and Persistence. Hepatology 2021, 74, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H.; Purcell, R.H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc. Natl. Acad. Sci. USA 1990, 87, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Hijikata, M.; Nakagawa, M.; Ootsuyama, Y.; Muraiso, K.; Ohkoshi, S.; Shimotohno, K. Molecular structure of the Japanese hepatitis C viral genome. FEBS Lett. 1991, 280, 325–328. [Google Scholar] [CrossRef]
- Kolykhalov, A.A.; Agapov, E.V.; Blight, K.J.; Mihalik, K.; Feinstone, S.M.; Rice, C.M. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 1997, 277, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Taha, G.; Ezra, L.; Abu-Freha, N. Hepatitis C Elimination: Opportunities and Challenges in 2023. Viruses 2023, 15, 1413. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Silvestri, C.; Voller, F. Update on Hepatitis C Epidemiology: Unaware and Untreated Infected Population Could Be the Key to Elimination. SN Compr. Clin. Med. 2020, 2, 2808–2815. [Google Scholar] [CrossRef]
- Bukh, J.; Miller, R.H.; Purcell, R.H. Genetic heterogeneity of hepatitis C virus: Quasispecies and genotypes. Semin. Liver Dis. 1995, 15, 41–63. [Google Scholar] [CrossRef]
- Simmonds, P.; Bukh, J.; Combet, C.; Deleage, G.; Enomoto, N.; Feinstone, S.; Halfon, P.; Inchauspe, G.; Kuiken, C.; Maertens, G.; et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 2005, 42, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.R. Hepatitis C virus: Clades and properties. J. Gastroenterol. Hepatol. 2002, 17, S468–S470. [Google Scholar] [CrossRef] [PubMed]
- Hartlage, A.S.; Cullen, J.M.; Kapoor, A. The Strange, Expanding World of Animal Hepaciviruses. Annu. Rev. Virol. 2016, 3, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P. The origin of hepatitis C virus. Curr. Top. Microbiol. Immunol. 2013, 369, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pfaender, S.; Brown, R.J.; Pietschmann, T.; Steinmann, E. Natural reservoirs for homologs of hepatitis C virus. Emerg. Microbes Infect. 2014, 3, e21. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M. Phylogenetic Inference in the Epidemiologic and Evolutionary Investigation of HIV-1, HCV and HBV; Faculty of Medicine, Lund University: Lund, Sweden, 2017. [Google Scholar]
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J. Infect. Dis. 2018, 218, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Gray, R.R.; Parker, J.; Lemey, P.; Salemi, M.; Katzourakis, A.; Pybus, O.G. The mode and tempo of hepatitis C virus evolution within and among hosts. BMC Evol. Biol. 2011, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Torres-Puente, M.; Cuevas, J.M.; Jimenez-Hernandez, N.; Bracho, M.A.; Garcia-Robles, I.; Wrobel, B.; Carnicer, F.; Del Olmo, J.; Ortega, E.; Moya, A.; et al. Genetic variability in hepatitis C virus and its role in antiviral treatment response. J. Viral Hepat. 2008, 15, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Kohli, A.; Shaffer, A.; Sherman, A.; Kottilil, S. Treatment of hepatitis C: A systematic review. JAMA 2014, 312, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Zein, N.N. Clinical significance of hepatitis C virus genotypes. Clin. Microbiol. Rev. 2000, 13, 223–235. [Google Scholar] [CrossRef]
- Martinez, M.A.; Franco, S. Therapy Implications of Hepatitis C Virus Genetic Diversity. Viruses 2020, 13, 41. [Google Scholar] [CrossRef]
- Adams, R.L.; Pirakitikulr, N.; Pyle, A.M. Functional RNA structures throughout the Hepatitis C Virus genome. Curr. Opin. Virol. 2017, 24, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yamane, D.; Masaki, T.; Lemon, S.M. The yin and yang of hepatitis C: Synthesis and decay of hepatitis C virus RNA. Nat. Rev. Microbiol. 2015, 13, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Scheel, T.K.; Rice, C.M. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat. Med. 2013, 19, 837–849. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Javed, T.; Rehman, S.; Nawaz, Z.; Riazuddin, S. An overview of HCV molecular biology, replication and immune responses. Virol. J. 2011, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Niepmann, M.; Gerresheim, G.K. Hepatitis C Virus Translation Regulation. Int. J. Mol. Sci. 2020, 21, 2328. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.K.; Hijikata, M.; Iwamoto, A.; Alter, H.J.; Purcell, R.H.; Yoshikura, H. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J. Virol. 1994, 68, 1494–1500. [Google Scholar] [CrossRef]
- Prentoe, J.; Bukh, J. Hypervariable Region 1 in Envelope Protein 2 of Hepatitis C Virus: A Linchpin in Neutralizing Antibody Evasion and Viral Entry. Front. Immunol. 2018, 9, 2146. [Google Scholar] [CrossRef] [PubMed]
- Lapa, D.; Garbuglia, A.R.; Capobianchi, M.R.; Del Porto, P. Hepatitis C Virus Genetic Variability, Human Immune Response, and Genome Polymorphisms: Which Is the Interplay? Cells 2019, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, J. Hepatitis C virus proteins. World J. Gastroenterol. 2007, 13, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Boukadida, C.; Fritz, M.; Blumen, B.; Fogeron, M.L.; Penin, F.; Martin, A. NS2 proteases from hepatitis C virus and related hepaciviruses share composite active sites and previously unrecognized intrinsic proteolytic activities. PLoS Pathog. 2018, 14, e1006863. [Google Scholar] [CrossRef] [PubMed]
- Romero-Brey, I.; Merz, A.; Chiramel, A.; Lee, J.Y.; Chlanda, P.; Haselman, U.; Santarella-Mellwig, R.; Habermann, A.; Hoppe, S.; Kallis, S.; et al. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 2012, 8, e1003056. [Google Scholar] [CrossRef] [PubMed]
- Ranjith-Kumar, C.T.; Kao, C.C. Biochemical Activities of the HCV NS5B RNA-Dependent RNA Polymerase. In Hepatitis C Viruses: Genomes and Molecular Biology; Tan, S.L., Ed.; Horizon Bioscience: Norfolk, UK, 2006. [Google Scholar]
- Sesmero, E.; Thorpe, I.F. Using the Hepatitis C Virus RNA-Dependent RNA Polymerase as a Model to Understand Viral Polymerase Structure, Function and Dynamics. Viruses 2015, 7, 3974–3994. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Hahn, Y.S. Hepatocytes infected with hepatitis C virus change immunological features in the liver microenvironment. Clin. Mol. Hepatol. 2023, 29, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm. Rep. 1998, 47, 1–39. [Google Scholar]
- Sidorkiewicz, M. Hepatitis C Virus Uses Host Lipids to Its Own Advantage. Metabolites 2021, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Yang, C.H.; Lo, S.Y. Cellular factors involved in the hepatitis C virus life cycle. World J. Gastroenterol. 2021, 27, 4555–4581. [Google Scholar] [CrossRef]
- Fukuhara, T.; Matsuura, Y. Role of miR-122 and lipid metabolism in HCV infection. J. Gastroenterol. 2013, 48, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Fénéant, L.; Levy, S.; Cocquerel, L. CD81 and hepatitis C virus (HCV) infection. Viruses 2014, 6, 535–572. [Google Scholar] [CrossRef]
- Mailly, L.; Baumert, T.F. Hepatitis C virus infection and tight junction proteins: The ties that bind. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183296. [Google Scholar] [CrossRef] [PubMed]
- Russelli, G.; Pizzillo, P.; Iannolo, G.; Barbera, F.; Tuzzolino, F.; Liotta, R.; Traina, M.; Vizzini, G.; Gridelli, B.; Badami, E.; et al. HCV replication in gastrointestinal mucosa: Potential extra-hepatic viral reservoir and possible role in HCV infection recurrence after liver transplantation. PLoS ONE 2017, 12, e0181683. [Google Scholar] [CrossRef] [PubMed]
- Desombere, I.; Van Houtte, F.; Farhoudi, A.; Verhoye, L.; Buysschaert, C.; Gijbels, Y.; Couvent, S.; Swinnen, W.; Van Vlierberghe, H.; Elewaut, A.; et al. A Role for B Cells to Transmit Hepatitis C Virus Infection. Front. Immunol. 2021, 12, 775098. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Comarmond, C.; Domont, F.; Savey, L.; Desbois, A.C.; Saadoun, D. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther. Adv. Infect. Dis. 2016, 3, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Crovatto, M.; Pozzato, G.; Zorat, F.; Pussini, E.; Nascimben, F.; Baracetti, S.; Grando, M.G.; Mazzaro, C.; Reitano, M.; Modolo, M.L.; et al. Peripheral blood neutrophils from hepatitis C virus-infected patients are replication sites of the virus. Haematologica 2000, 85, 356–361. [Google Scholar] [PubMed]
- Chigbu, D.I.; Loonawat, R.; Sehgal, M.; Patel, D.; Jain, P. Hepatitis C Virus Infection: Host-Virus Interaction and Mechanisms of Viral Persistence. Cells 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Kemming, J.; Thimme, R.; Neumann-Haefelin, C. Adaptive Immune Response against Hepatitis C Virus. Int. J. Mol. Sci. 2020, 21, 5644. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.; Ray, R.B.; Ray, R. Strategies to Circumvent Host Innate Immune Response by Hepatitis C Virus. Cells 2019, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, N.S.; Vazquez, C.; Horner, S.M. Hepatitis C Virus. Strategies to Evade Antiviral Responses. Future Virol. 2014, 9, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Binder, M.; Bartenschlager, R. Failure of innate and adaptive immune responses in controlling hepatitis C virus infection. FEMS Microbiol. Rev. 2012, 36, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Schwerk, J.; Negash, A.; Savan, R.; Gale, M., Jr. Innate Immunity in Hepatitis C Virus Infection. Cold Spring Harb. Perspect. Med. 2021, 11, a036988. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Jiang, J.D.; Peng, Z. Recent advances in the anti-HCV mechanisms of interferon. Acta Pharm. Sin. B 2014, 4, 241–247. [Google Scholar] [CrossRef]
- Kaukinen, P.; Sillanpää, M.; Kotenko, S.; Lin, R.; Hiscott, J.; Melén, K.; Julkunen, I. Hepatitis C virus NS2 and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression. Virol. J. 2006, 3, 66. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.; Ramos, B.; Nunes, A.; Ribeiro, D. Hepatitis C Virus: Evading the Intracellular Innate Immunity. J. Clin. Med. 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Polyak, S.J.; Khabar, K.S.; Paschal, D.M.; Ezelle, H.J.; Duverlie, G.; Barber, G.N.; Levy, D.E.; Mukaida, N.; Gretch, D.R. Hepatitis C virus nonstructural 5A protein induces interleukin-8, leading to partial inhibition of the interferon-induced antiviral response. J. Virol. 2001, 75, 6095–6106. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.R.; Golden-Mason, L. Control of HCV Infection by Natural Killer Cells and Macrophages. Cold Spring Harb. Perspect. Med. 2020, 10, a037101. [Google Scholar] [CrossRef] [PubMed]
- Crotta, S.; Stilla, A.; Wack, A.; D’Andrea, A.; Nuti, S.; D’Oro, U.; Mosca, M.; Filliponi, F.; Brunetto, R.M.; Bonino, F.; et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 2002, 195, 35–41. [Google Scholar] [CrossRef]
- Burke, K.P.; Cox, A.L. Hepatitis C virus evasion of adaptive immune responses: A model for viral persistence. Immunol. Res. 2010, 47, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Luxenburger, H.; Neumann-Haefelin, C.; Thimme, R.; Boettler, T. HCV-Specific T Cell Responses During and After Chronic HCV Infection. Viruses 2018, 10, 645. [Google Scholar] [CrossRef]
- Lauer, G.M.; Ouchi, K.; Chung, R.T.; Nguyen, T.N.; Day, C.L.; Purkis, D.R.; Reiser, M.; Kim, A.Y.; Lucas, M.; Klenerman, P.; et al. Comprehensive analysis of CD8+-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J. Virol. 2002, 76, 6104–6113. [Google Scholar] [CrossRef] [PubMed]
- Ocal, S.; Selcuk, H.; Korkmaz, M.; Altun, R.; Yildirim, A.E.; Akbas, E. Effect of HLA on hepatitis C virus clearance and persistence in anti-HCV-positive end-stage renal disease patients. Saudi J. Gastroenterol. 2014, 20, 175–181. [Google Scholar] [CrossRef]
- Kim, A.Y.; Kuntzen, T.; Timm, J.; Nolan, B.E.; Baca, M.A.; Reyor, L.L.; Berical, A.C.; Feller, A.J.; Johnson, K.L.; Schulze zur Wiesch, J.; et al. Spontaneous control of HCV is associated with expression of HLA-B 57 and preservation of targeted epitopes. Gastroenterology 2011, 140, 686–696.e681. [Google Scholar] [CrossRef]
- Fitzmaurice, K.; Petrovic, D.; Ramamurthy, N.; Simmons, R.; Merani, S.; Gaudieri, S.; Sims, S.; Dempsey, E.; Freitas, E.; Lea, S.; et al. Molecular footprints reveal the impact of the protective HLA-A*03 allele in hepatitis C virus infection. Gut 2011, 60, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Haefelin, C.; Oniangue-Ndza, C.; Kuntzen, T.; Schmidt, J.; Nitschke, K.; Sidney, J.; Caillet-Saguy, C.; Binder, M.; Kersting, N.; Kemper, M.W.; et al. Human leukocyte antigen B27 selects for rare escape mutations that significantly impair hepatitis C virus replication and require compensatory mutations. Hepatology 2011, 54, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Thio, C.L.; Gao, X.; Goedert, J.J.; Vlahov, D.; Nelson, K.E.; Hilgartner, M.W.; O’Brien, S.J.; Karacki, P.; Astemborski, J.; Carrington, M.; et al. HLA-Cw*04 and hepatitis C virus persistence. J. Virol. 2002, 76, 4792–4797. [Google Scholar] [CrossRef] [PubMed]
- Kuniholm, M.H.; Kovacs, A.; Gao, X.; Xue, X.; Marti, D.; Thio, C.L.; Peters, M.G.; Terrault, N.A.; Greenblatt, R.M.; Goedert, J.J.; et al. Specific human leukocyte antigen class I and II alleles associated with hepatitis C virus viremia. Hepatology 2010, 51, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Bengsch, B.; Thimme, R.; Blum, H.E. Role of host genetic factors in the outcome of hepatitis C virus infection. Viruses 2009, 1, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Hoof, I.; van Baarle, D.; Keşmir, C.; Textor, J. HLA Preferences for Conserved Epitopes: A Potential Mechanism for Hepatitis C Clearance. Front. Immunol. 2015, 6, 552. [Google Scholar] [CrossRef] [PubMed]
- Mangia, A.; Gentile, R.; Cascavilla, I.; Margaglione, M.; Villani, M.R.; Stella, F.; Modola, G.; Agostiano, V.; Gaudiano, C.; Andriulli, A. HLA class II favors clearance of HCV infection and progression of the chronic liver damage. J. Hepatol. 1999, 30, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Yu, R.B.; Sun, N.X.; Wang, B.; Xu, Y.C.; Wu, G.L. Human leukocyte antigen class II DQB1*0301, DRB1*1101 alleles and spontaneous clearance of hepatitis C virus infection: A meta-analysis. World J. Gastroenterol. 2005, 11, 7302–7307. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Lauer, G.M.; Robbins, G.K.; McGovern, B.; Wurcel, A.G.; Gandhi, R.T.; Chung, R.T.; Walker, B.D. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J. Virol. 2002, 76, 12584–12595. [Google Scholar] [CrossRef]
- Maheshwari, A.; Ray, S.; Thuluvath, P.J. Acute hepatitis C. Lancet 2008, 372, 321–332. [Google Scholar] [CrossRef]
- Bulteel, N.; Partha Sarathy, P.; Forrest, E.; Stanley, A.J.; Innes, H.; Mills, P.R.; Valerio, H.; Gunson, R.N.; Aitken, C.; Morris, J.; et al. Factors associated with spontaneous clearance of chronic hepatitis C virus infection. J. Hepatol. 2016, 65, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Rehermann, B. Immune responses to HCV and other hepatitis viruses. Immunity 2014, 40, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Quarleri, J.F.; Oubiña, J.R. Hepatitis C virus strategies to evade the specific-T cell response: A possible mission favoring its persistence. Ann. Hepatol. 2016, 15, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Buchli, R.; Schiller, J.; Gao, J.; VanGundy, R.S.; Hildebrand, W.H.; Eckels, D.D. Natural epitope variants of the hepatitis C virus impair cytotoxic T lymphocyte activity. World J. Gastroenterol. 2010, 16, 1953–1969. [Google Scholar] [CrossRef] [PubMed]
- Cashman, S.B.; Marsden, B.D.; Dustin, L.B. The Humoral Immune Response to HCV: Understanding is Key to Vaccine Development. Front. Immunol. 2014, 5, 550. [Google Scholar] [CrossRef] [PubMed]
- Kuntzen, T.; Timm, J.; Berical, A.; Lewis-Ximenez, L.L.; Jones, A.; Nolan, B.; Schulze zur Wiesch, J.; Li, B.; Schneidewind, A.; Kim, A.Y.; et al. Viral sequence evolution in acute hepatitis C virus infection. J. Virol. 2007, 81, 11658–11668. [Google Scholar] [CrossRef] [PubMed]
- Nishio, A.; Hasan, S.; Park, H.; Park, N.; Salas, J.H.; Salinas, E.; Kardava, L.; Juneau, P.; Frumento, N.; Massaccesi, G.; et al. Serum neutralization activity declines but memory B cells persist after cure of chronic hepatitis C. Nat. Commun. 2022, 13, 5446. [Google Scholar] [CrossRef] [PubMed]
- Lavie, M.; Hanoulle, X.; Dubuisson, J. Glycan Shielding and Modulation of Hepatitis C Virus Neutralizing Antibodies. Front. Immunol. 2018, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Prieto, A.M.; Dorner, M. Immune Evasion Strategies during Chronic Hepatitis B and C Virus Infection. Vaccines 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- d’Avigdor, W.M.H.; Budzinska, M.A.; Lee, M.; Lam, R.; Kench, J.; Stapelberg, M.; McLennan, S.V.; Farrell, G.; George, J.; McCaughan, G.W.; et al. Virus Genotype-Dependent Transcriptional Alterations in Lipid Metabolism and Inflammation Pathways in the Hepatitis C Virus-infected Liver. Sci. Rep. 2019, 9, 10596. [Google Scholar] [CrossRef]
- Keikha, M.; Eslami, M.; Yousefi, B.; Ali-Hassanzadeh, M.; Kamali, A.; Yousefi, M.; Karbalaei, M. HCV genotypes and their determinative role in hepatitis C treatment. Virusdisease 2020, 31, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Roudot-Thoraval, F. Epidemiology of hepatitis C virus infection. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101596. [Google Scholar] [CrossRef] [PubMed]
- Zaltron, S.; Spinetti, A.; Biasi, L.; Baiguera, C.; Castelli, F. Chronic HCV infection: Epidemiological and clinical relevance. BMC Infect. Dis. 2012, 12, S2. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.; Price, J.C.; Tien, P.C. Hepatitis C Virus Infection in the Older Patient. Infect. Dis. Clin. N. Am. 2017, 31, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Mohamed, M.K.; Strickland, G.T.; Lavanchy, D.; Arthur, R.R.; Magder, L.S.; El Khoby, T.; Abdel-Wahab, Y.; Aly Ohn, E.S.; Anwar, W.; et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 2000, 355, 887–891. [Google Scholar] [CrossRef]
- Thursz, M.; Fontanet, A. HCV transmission in industrialized countries and resource-constrained areas. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Trickey, A.; Fraser, H.; Lim, A.G.; Peacock, A.; Colledge, S.; Walker, J.G.; Leung, J.; Grebely, J.; Larney, S.; Martin, N.K.; et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: A modelling study. Lancet Gastroenterol. Hepatol. 2019, 4, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Grebely, J.; Larney, S.; Peacock, A.; Colledge, S.; Leung, J.; Hickman, M.; Vickerman, P.; Blach, S.; Cunningham, E.B.; Dumchev, K.; et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction 2019, 114, 150–166. [Google Scholar] [CrossRef] [PubMed]
- van der Poel, C.L. Hepatitis C virus and blood transfusion: Past and present risks. J. Hepatol. 1999, 31 (Suppl. S1), 101–106. [Google Scholar] [CrossRef]
- Gürtler, L.G.; Eberle, J. Aspects on the history of transmission and favor of distribution of viruses by iatrogenic action: Perhaps an example of a paradigm of the worldwide spread of HIV. Med. Microbiol. Immunol. 2017, 206, 287–293. [Google Scholar] [CrossRef]
- Forni, D.; Cagliani, R.; Pontremoli, C.; Pozzoli, U.; Vertemara, J.; De Gioia, L.; Clerici, M.; Sironi, M. Evolutionary Analysis Provides Insight Into the Origin and Adaptation of HCV. Front. Microbiol. 2018, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Wahab, E.W.; Abdel Maksoud, A.; Shatat, H.Z.; Kotkat, A.M. Risky exposures and national estimate of HCV seroprevalence among school children in urban Egypt. Virusdisease 2016, 27, 351–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soza, A.; Riquelme, A.; Arrese, M. Routes of transmission of hepatitis C virus. Ann. Hepatol. 2010, 9, S30–S33. [Google Scholar] [CrossRef]
- El-Shabrawi, M.H.F.; Kamal, N.M.; Mogahed, E.A.; Elhusseini, M.A.; Aljabri, M.F. Perinatal transmission of hepatitis C virus: An update. Arch. Med. Sci. 2020, 16, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Nijmeijer, B.M.; Koopsen, J.; Schinkel, J.; Prins, M.; Geijtenbeek, T.B. Sexually transmitted hepatitis C virus infections: Current trends, and recent advances in understanding the spread in men who have sex with men. J. Int. AIDS Soc. 2019, 22 (Suppl. S6), e25348. [Google Scholar] [CrossRef] [PubMed]
- Tohme, R.A.; Holmberg, S.D. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology 2010, 52, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; De Pascalis, S.; Onorato, L.; Calò, F.; Sagnelli, C.; Sagnelli, E. Hepatitis B virus and hepatitis C virus infection in healthcare workers. World J. Hepatol. 2016, 8, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Yang, H.I.; Yuan, Y.; L’Italien, G.; Chen, C.J. Epidemiology and natural history of hepatitis C virus infection. World J. Gastroenterol. 2014, 20, 9270–9280. [Google Scholar]
- Hajarizadeh, B.; Grady, B.; Page, K.; Kim, A.Y.; McGovern, B.H.; Cox, A.L.; Rice, T.M.; Sacks-Davis, R.; Bruneau, J.; Morris, M.; et al. Patterns of hepatitis C virus RNA levels during acute infection: The InC3 study. PLoS ONE 2015, 10, e0122232. [Google Scholar] [CrossRef] [PubMed]
- Beld, M.; Penning, M.; McMorrow, M.; Gorgels, J.; van den Hoek, A.; Goudsmit, J. Different hepatitis C virus (HCV) RNA load profiles following seroconversion among injecting drug users without correlation with HCV genotype and serum alanine aminotransferase levels. J. Clin. Microbiol. 1998, 36, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Major, M.; Gutfraind, A.; Shekhtman, L.; Cui, Q.; Kachko, A.; Cotler, S.J.; Hajarizadeh, B.; Sacks-Davis, R.; Page, K.; Boodram, B.; et al. Modeling of patient virus titers suggests that availability of a vaccine could reduce hepatitis C virus transmission among injecting drug users. Sci. Transl. Med. 2018, 10, eaao4496. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Omata, K.; Satoh, T.; Miyasaka, T.; Arai, C.; Maeda, M.; Matsuno, T.; Miyamura, T. Quantitative detection of hepatitis C virus (HCV) RNA in saliva and gingival crevicular fluid of HCV-infected patients. J. Clin. Microbiol. 2005, 43, 4413–4417. [Google Scholar] [CrossRef] [PubMed]
- Pfaender, S.; Helfritz, F.A.; Siddharta, A.; Todt, D.; Behrendt, P.; Heyden, J.; Riebesehl, N.; Willmann, W.; Steinmann, J.; Münch, J.; et al. Environmental Stability and Infectivity of Hepatitis C Virus (HCV) in Different Human Body Fluids. Front. Microbiol. 2018, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.T.; Perz, J.F.; Bell, B.P. Viral hepatitis transmission in ambulatory health care settings. Clin. Infect. Dis. 2004, 38, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- De Carli, G.; Puro, V.; Ippolito, G. Risk of hepatitis C virus transmission following percutaneous exposure in healthcare workers. Infection 2003, 31 (Suppl. S2), 22–27. [Google Scholar] [PubMed]
- Yazdanpanah, Y.; De Carli, G.; Migueres, B.; Lot, F.; Campins, M.; Colombo, C.; Thomas, T.; Deuffic-Burban, S.; Prevot, M.H.; Domart, M.; et al. Risk factors for hepatitis C virus transmission to health care workers after occupational exposure: A European case-control study. Clin. Infect. Dis. 2005, 41, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Egro, F.M.; Nwaiwu, C.A.; Smith, S.; Harper, J.D.; Spiess, A.M. Seroconversion rates among health care workers exposed to hepatitis C virus-contaminated body fluids: The University of Pittsburgh 13-year experience. Am. J. Infect. Control 2017, 45, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Alfulayw, K.H.; Al-Otaibi, S.T.; Alqahtani, H.A. Factors associated with needlestick injuries among healthcare workers: Implications for prevention. BMC Health Serv. Res. 2021, 21, 1074. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Bruguera, M.; Puyuelo, T.; Barrera, J.M.; Sanchez Tapias, J.M.; Rodés, J. Risk of needle-stick injuries in the transmission of hepatitis C virus in hospital personnel. J. Hepatol. 1992, 16, 56–58. [Google Scholar] [CrossRef]
- Doerrbecker, J.; Behrendt, P.; Mateu-Gelabert, P.; Ciesek, S.; Riebesehl, N.; Wilhelm, C.; Steinmann, J.; Pietschmann, T.; Steinmann, E. Transmission of hepatitis C virus among people who inject drugs: Viral stability and association with drug preparation equipment. J. Infect. Dis. 2013, 207, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Tohme, R.A.; Holmberg, S.D. Transmission of hepatitis C virus infection through tattooing and piercing: A critical review. Clin. Infect. Dis. 2012, 54, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.; Batarseh, R.; Natsheh, A.; Abbadi, J.; Al-Fraihat, E.; Yaseen, A.; Kaddomi, D.; Khamees, N.; Mahafzah, A.; Şahin, G.Ö. An update on hepatitis C virus genotype distribution in Jordan: A 12-year retrospective study from a tertiary care teaching hospital in Amman. BMC Infect. Dis. 2019, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Dodge, J.L.; Murphy, E.L.; Tavis, J.E.; Kiss, A.; Levin, T.R.; Gish, R.G.; Busch, M.P.; Reingold, A.L.; Alter, M.J. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: The HCV partners study. Hepatology 2013, 57, 881–889. [Google Scholar] [CrossRef]
- Jin, F.; Matthews, G.V.; Grulich, A.E. Sexual transmission of hepatitis C virus among gay and bisexual men: A systematic review. Sex. Health 2017, 14, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Shebl, F.M.; El-Kamary, S.S.; Saleh, D.A.; Abdel-Hamid, M.; Mikhail, N.; Allam, A.; El-Arabi, H.; Elhenawy, I.; El-Kafrawy, S.; El-Daly, M.; et al. Prospective cohort study of mother-to-infant infection and clearance of hepatitis C in rural Egyptian villages. J. Med. Virol. 2009, 81, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Ryan, P.; Valencia, J.; Resino, S. The Challenging Road to Hepatitis C Virus Eradication. J. Clin. Med. 2021, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- De Siervi, S.; Cannito, S.; Turato, C. Chronic Liver Disease: Latest Research in Pathogenesis, Detection and Treatment. Int. J. Mol. Sci. 2023, 24, 10633. [Google Scholar] [CrossRef] [PubMed]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Humphreys, I.; Flaxman, A.; Brown, A.; Cooke, G.S.; Pybus, O.G.; Barnes, E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015, 61, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Polaris Observatory. Hepatitis C Countries/Territories—Distribution. Available online: https://cdafound.org/polaris-countries-distribution/ (accessed on 26 April 2024).
- Gomaa, A.; Allam, N.; Elsharkawy, A.; El Kassas, M.; Waked, I. Hepatitis C infection in Egypt: Prevalence, impact and management strategies. Hepat. Med. 2017, 9, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.H.; Chemaitelly, H.; Kouyoumjian, S.P.; Abu-Raddad, L.J. Characterizing the historical role of parenteral antischistosomal therapy in hepatitis C virus transmission in Egypt. Int. J. Epidemiol. 2020, 49, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Naficy, A.B.; Darwish, M.A.; Darwish, N.M.; Schisterman, E.; Clemens, J.D.; Edelman, R. Further evidence for association of hepatitis C infection with parenteral schistosomiasis treatment in Egypt. BMC Infect. Dis. 2002, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.S.; Andrieux-Meyer, I.; Applegate, T.L.; Atun, R.; Burry, J.R.; Cheinquer, H.; Dusheiko, G.; Feld, J.J.; Gore, C.; Griswold, M.G.; et al. Accelerating the elimination of viral hepatitis: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2019, 4, 135–184. [Google Scholar] [CrossRef] [PubMed]
- Elisabeth, M. Hepatitis C: Egypt makes “unprecedented progress” towards elimination. BMJ 2023, 383, p2353. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Holmes, E.C.; Cha, T.A.; Chan, S.W.; McOmish, F.; Irvine, B.; Beall, E.; Yap, P.L.; Kolberg, J.; Urdea, M.S. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J. Gen. Virol. 1993, 74 Pt 11, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Smith, D.B.; McOmish, F.; Yap, P.L.; Kolberg, J.; Urdea, M.S.; Holmes, E.C. Identification of genotypes of hepatitis C virus by sequence comparisons in the core, E1 and NS-5 regions. J. Gen. Virol. 1994, 75 Pt 5, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Mellor, J.; Holmes, E.C.; Jarvis, L.M.; Yap, P.L.; Simmonds, P. Investigation of the pattern of hepatitis C virus sequence diversity in different geographical regions: Implications for virus classification. The International HCV Collaborative Study Group. J. Gen. Virol. 1995, 76 Pt 10, 2493–2507. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, C.; Simmonds, P. Nomenclature and numbering of the hepatitis C virus. Methods Mol. Biol. 2009, 510, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Poveda, E.; Wyles, D.L.; Mena, Á.; Pedreira, J.D.; Castro-Iglesias, Á.; Cachay, E. Update on hepatitis C virus resistance to direct-acting antiviral agents. Antivir. Res. 2014, 108, 181–191. [Google Scholar] [CrossRef]
- Zeuzem, S.; Berg, T.; Moeller, B.; Hinrichsen, H.; Mauss, S.; Wedemeyer, H.; Sarrazin, C.; Hueppe, D.; Zehnter, E.; Manns, M.P. Expert opinion on the treatment of patients with chronic hepatitis C. J. Viral Hepat. 2009, 16, 75–90. [Google Scholar] [CrossRef]
- El-Shamy, A.; Hotta, H. Impact of hepatitis C virus heterogeneity on interferon sensitivity: An overview. World J. Gastroenterol. 2014, 20, 7555–7569. [Google Scholar] [CrossRef] [PubMed]
- Bochud, P.Y.; Cai, T.; Overbeck, K.; Bochud, M.; Dufour, J.F.; Müllhaupt, B.; Borovicka, J.; Heim, M.; Moradpour, D.; Cerny, A.; et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J. Hepatol. 2009, 51, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Patel, K.; Naggie, S. Genotype 3 Infection: The Last Stand of Hepatitis C Virus. Drugs 2017, 77, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Ilyas, J.; Duan, Z.; El-Serag, H.B. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology 2014, 60, 98–105. [Google Scholar] [CrossRef]
- Sharma, P.; Balan, V.; Hernandez, J.; Rosati, M.; Williams, J.; Rodriguez-Luna, H.; Schwartz, J.; Harrison, E.; Anderson, M.; Byrne, T.; et al. Hepatic Steatosis in Hepatitis C Virus Genotype 3 Infection: Does It Correlate with Body Mass Index, Fibrosis, and HCV Risk Factors? Dig. Dis. Sci. 2004, 49, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Bruno, S.; Mondelli, M.U.; Maisonneuve, P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: A meta-analysis. J. Hepatol. 2009, 50, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Crosignani, A.; Maisonneuve, P.; Rossi, S.; Silini, E.; Mondelli, M.U. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: A seventeen-year prospective cohort study. Hepatology 2007, 46, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, L.; Li, G.; Libin, P.; Piampongsant, S.; Vandamme, A.M.; Theys, K. Genetic Diversity and Selective Pressure in Hepatitis C Virus Genotypes 1-6: Significance for Direct-Acting Antiviral Treatment and Drug Resistance. Viruses 2015, 7, 5018–5039. [Google Scholar] [CrossRef] [PubMed]
- González-Candelas, F.; López-Labrador, F.X.; Bracho, M.A. Recombination in hepatitis C virus. Viruses 2011, 3, 2006–2024. [Google Scholar] [CrossRef] [PubMed]
- Magiorkinis, G.; Magiorkinis, E.; Paraskevis, D.; Ho, S.Y.; Shapiro, B.; Pybus, O.G.; Allain, J.P.; Hatzakis, A. The global spread of hepatitis C virus 1a and 1b: A phylodynamic and phylogeographic analysis. PLoS Med. 2009, 6, e1000198. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Rashid, Z.Z.; Wong, K.K.; Abdullah, S.A.; Rahman, M.M. Hepatitis C genotype and associated risks factors of patients at University Kebangsaan Malaysia Medical Centre. Pak. J. Med. Sci. 2013, 29, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Kartashev, V.; Döring, M.; Nieto, L.; Coletta, E.; Kaiser, R.; Sierra, S. New findings in HCV genotype distribution in selected West European, Russian and Israeli regions. J. Clin. Virol. 2016, 81, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Preciado, M.V.; Valva, P.; Escobar-Gutierrez, A.; Rahal, P.; Ruiz-Tovar, K.; Yamasaki, L.; Vazquez-Chacon, C.; Martinez-Guarneros, A.; Carpio-Pedroza, J.C.; Fonseca-Coronado, S.; et al. Hepatitis C virus molecular evolution: Transmission, disease progression and antiviral therapy. World J. Gastroenterol. 2014, 20, 15992–16013. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Stoddard, M.B.; Wang, S.; Blair, L.M.; Giorgi, E.E.; Parrish, E.H.; Learn, G.H.; Hraber, P.; Goepfert, P.A.; Saag, M.S.; et al. Elucidation of hepatitis C virus transmission and early diversification by single genome sequencing. PLoS Pathog. 2012, 8, e1002880. [Google Scholar] [CrossRef] [PubMed]
- Mohamoud, Y.A.; Mumtaz, G.R.; Riome, S.; Miller, D.; Abu-Raddad, L.J. The epidemiology of hepatitis C virus in Egypt: A systematic review and data synthesis. BMC Infect. Dis. 2013, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Pepin, J.; Frost, E.; Deslandes, S.; Labbé, A.C.; Pybus, O.G. Phylogeography and molecular epidemiology of hepatitis C virus genotype 2 in Africa. J. Gen. Virol. 2009, 90, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Irekeola, A.A.; Malek, N.A.; Wada, Y.; Mustaffa, N.; Muhamad, N.I.; Shueb, R.H. Prevalence of HCV genotypes and subtypes in Southeast Asia: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, Y.; Wu, W.; Feng, R.; Wu, Z.; Cun, W.; Dong, S. Hepatitis C virus genotype diversity among intravenous drug users in Yunnan Province, Southwestern China. PLoS ONE 2013, 8, e82598. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Lin, H.H.; Liu, Y.C.; Lee, S.S.; Chen, Y.L.; Hung, C.C.; Ko, W.C.; Huang, C.K.; Lai, C.H.; Chen, Y.S.; et al. Extremely high prevalence and genetic diversity of hepatitis C virus infection among HIV-infected injection drug users in Taiwan. Clin. Infect. Dis. 2008, 46, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M. Hepatitis C virus genetic variability: Pathogenic and clinical implications. Clin. Liver Dis. 2003, 7, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.G.; Willems, B.; Vincelette, J.; Bernier, L.; Côté, J.; Delage, G. Biological and clinicopathological features associated with hepatitis C virus type 5 infections. J. Hepatol. 1996, 24, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Gededzha, M.P.; Selabe, S.G.; Blackard, J.T.; Kyaw, T.; Mphahlele, M.J. Near full-length genome analysis of HCV genotype 5 strains from South Africa. Infect. Genet. Evol. 2014, 21, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Verbeeck, J.; Maes, P.; Lemey, P.; Pybus, O.G.; Wollants, E.; Song, E.; Nevens, F.; Fevery, J.; Delport, W.; Van der Merwe, S.; et al. Investigating the origin and spread of hepatitis C virus genotype 5a. J. Virol. 2006, 80, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Thézé, J.; Lowes, S.; Parker, J.; Pybus, O.G. Evolutionary and Phylogenetic Analysis of the Hepaciviruses and Pegiviruses. Genome Biol. Evol. 2015, 7, 2996–3008. [Google Scholar] [CrossRef][Green Version]
- Lyons, S.; Kapoor, A.; Sharp, C.; Schneider, B.S.; Wolfe, N.D.; Culshaw, G.; Corcoran, B.; McGorum, B.C.; Simmonds, P. Nonprimate hepaciviruses in domestic horses, United kingdom. Emerg. Infect. Dis. 2012, 18, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Moon, H.W.; Sung, H.W.; Kwon, H.M. First identification and phylogenetic analysis of equine hepacivirus in Korea. Infect. Genet. Evol. 2017, 49, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Scheel, T.K.; Simmonds, P.; Kapoor, A. Surveying the global virome: Identification and characterization of HCV-related animal hepaciviruses. Antivir. Res. 2015, 115, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Warnock, R.C.; Parham, J.F.; Joyce, W.G.; Lyson, T.R.; Donoghue, P.C. Calibration uncertainty in molecular dating analyses: There is no substitute for the prior evaluation of time priors. Proc. Biol. Sci. 2015, 282, 20141013. [Google Scholar] [CrossRef]
- Ozaras, R.; Tahan, V. Acute hepatitis C: Prevention and treatment. Expert. Rev. Anti Infect. Ther. 2009, 7, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Masia, R.; Misdraji, J. 11—Liver and Bile Duct Infections. In Diagnostic Pathology of Infectious Disease, 2nd ed.; Kradin, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 272–322. [Google Scholar] [CrossRef]
- Younis, B.B.; Arshad, R.; Khurhsid, S.; Masood, J.; Nazir, F.; Tahira, M. Fulminant hepatic failure (FHF) due to acute hepatitis C. Pak. J. Med. Sci. 2015, 31, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Gordon, F.D.; Anastopoulos, H.; Khettry, U.; Loda, M.; Jenkins, R.L.; Lewis, W.D.; Trey, C. Hepatitis C infection: A rare cause of fulminant hepatic failure. Am. J. Gastroenterol. 1995, 90, 117–120. [Google Scholar] [PubMed]
- Hoofnagle, J.H. Hepatitis C: The clinical spectrum of disease. Hepatology 1997, 26, 15s–20s. [Google Scholar] [CrossRef] [PubMed]
- Baldo, V.; Baldovin, T.; Trivello, R.; Floreani, A. Epidemiology of HCV Infection. Curr. Pharm. Des. 2008, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, O.; Kiyici, M.; Yilmaz, Y.; Ulukaya, E.; Yerci, O. Clinical significance of activity of ALT enzyme in patients with hepatitis C virus. World J. Gastroenterol. 2007, 13, 5481–5485. [Google Scholar] [CrossRef] [PubMed]
- Glynn, S.A.; Wright, D.J.; Kleinman, S.H.; Hirschkorn, D.; Tu, Y.; Heldebrant, C.; Smith, R.; Giachetti, C.; Gallarda, J.; Busch, M.P. Dynamics of viremia in early hepatitis C virus infection. Transfusion 2005, 45, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Hajarizadeh, B.; Grebely, J.; Dore, G.J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Chan, T.M.; Sanchez-Pescador, R.; Urdea, M.; Lok, A.S. Correlation between serum HCV RNA and aminotransferase levels in patients with chronic HCV infection. Dig. Dis. Sci. 1996, 41, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Dustin, L.B.; Cashman, S.B.; Laidlaw, S.M. Immune control and failure in HCV infection--tipping the balance. J. Leukoc. Biol. 2014, 96, 535–548. [Google Scholar] [CrossRef]
- Bowen, D.G.; Walker, C.M. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 2005, 436, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Grebely, J.; Prins, M.; Hellard, M.; Cox, A.L.; Osburn, W.O.; Lauer, G.; Page, K.; Lloyd, A.R.; Dore, G.J. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: Towards a vaccine. Lancet Infect. Dis. 2012, 12, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H. Course and outcome of hepatitis C. Hepatology 2002, 36, S21–S29. [Google Scholar] [CrossRef] [PubMed]
- Kenny-Walsh, E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N. Engl. J. Med. 1999, 340, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Inoue, G.; Horiike, N.; Michitaka, K.; Onji, M. Hepatitis C virus clearance is prominent in women in an endemic area. J. Gastroenterol. Hepatol. 2000, 15, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Sata, M.; Suzuki, H.; Noguchi, S.; Tanikawa, K. Higher elimination rate of hepatitis C virus among women. J. Viral Hepat. 1996, 3, 317–321. [Google Scholar] [CrossRef]
- Zhang, M.; Rosenberg, P.S.; Brown, D.L.; Preiss, L.; Konkle, B.A.; Eyster, M.E.; Goedert, J.J. Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood 2006, 107, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Messick, K.; Sanders, J.C.; Goedert, J.J.; Eyster, M.E. Hepatitis C viral clearance and antibody reactivity patterns in persons with haemophilia and other congenital bleeding disorders. Haemophilia 2001, 7, 568–574. [Google Scholar] [CrossRef]
- Grebely, J.; Raffa, J.D.; Lai, C.; Krajden, M.; Conway, B.; Tyndall, M.W. Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users. Can. J. Gastroenterol. 2007, 21, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, B.A.; Lewis, J.D.; Reddy, K.R.; Bellamy, S.L.; Porter, S.B.; Weinrieb, R.M.; Stieritz, D.D.; Chang, K.M. Influence of alcohol use, race, and viral coinfections on spontaneous HCV clearance in a US veteran population. Hepatology 2004, 40, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Vendemiale, G.; Altomare, E.; Serviddio, G. The impact of interferon lambda 3 gene polymorphism on natural course and treatment of hepatitis C. Clin. Dev. Immunol. 2012, 2012, 849373. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nishida, N.; Sugiyama, M.; Kurosaki, M.; Matsuura, K.; Sakamoto, N.; Nakagawa, M.; Korenaga, M.; Hino, K.; Hige, S.; et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 2009, 41, 1105–1109. [Google Scholar] [CrossRef]
- Susser, S.; Herrmann, E.; Lange, C.; Hamdi, N.; Müller, T.; Berg, T.; Perner, D.; Zeuzem, S.; Sarrazin, C. Predictive value of interferon-lambda gene polymorphisms for treatment response in chronic hepatitis C. PLoS ONE 2014, 9, e112592. [Google Scholar] [CrossRef]
- Beinhardt, S.; Aberle, J.H.; Strasser, M.; Dulic-Lakovic, E.; Maieron, A.; Kreil, A.; Rutter, K.; Staettermayer, A.F.; Datz, C.; Scherzer, T.M.; et al. Serum level of IP-10 increases predictive value of IL28B polymorphisms for spontaneous clearance of acute HCV infection. Gastroenterology 2012, 142, 78–85.e72. [Google Scholar] [CrossRef]
- Thomas, D.L.; Thio, C.L.; Martin, M.P.; Qi, Y.; Ge, D.; O’Huigin, C.; Kidd, J.; Kidd, K.; Khakoo, S.I.; Alexander, G.; et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009, 461, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.S.; Wang, H.; Geng, X.P. Two IL28B polymorphisms are associated with the treatment response of different genotypes of hepatitis C in different racial populations: A meta-analysis. Exp. Ther. Med. 2012, 3, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 2009, 49, 1335–1374. [Google Scholar] [CrossRef]
- Shen, C.; Jiang, X.; Li, M.; Luo, Y. Hepatitis Virus and Hepatocellular Carcinoma: Recent Advances. Cancers 2023, 15, 533. [Google Scholar] [CrossRef]
- Axley, P.; Ahmed, Z.; Ravi, S.; Singal, A.K. Hepatitis C Virus and Hepatocellular Carcinoma: A Narrative Review. J. Clin. Transl. Hepatol. 2018, 6, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Khullar, V.; Firpi, R.J. Hepatitis C cirrhosis: New perspectives for diagnosis and treatment. World J. Hepatol. 2015, 7, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Herrmann, E. Dynamics of hepatitis C virus infection. Ann. Hepatol. 2002, 1, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, S.M.; Preiss, L.R.; Eyster, M.E.; Goedert, J.J. Correlates of high hepatitis C virus RNA load in a cohort of HIV-negative and HIV-positive individuals with haemophilia. J. Viral Hepat. 2011, 18, 161–169. [Google Scholar] [CrossRef]
- McCaughan, G.W.; George, J. Fibrosis progression in chronic hepatitis C virus infection. Gut 2004, 53, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, J.; Wen, B.; Luo, S.; Lin, Y.; Ou, W.; Guo, F.; Tang, P.; Liu, W.; Qu, X. HBV/HCV dual infection impacts viral load, antibody response, and cytokine expression differently from HBV or HCV single infection. Sci. Rep. 2016, 6, 39409. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Ward, S.C.; Thung, S.N. Liver pathology of hepatitis C, beyond grading and staging of the disease. World J. Gastroenterol. 2016, 22, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Zeremski, M.; Dimova, R.B.; Pillardy, J.; de Jong, Y.P.; Jacobson, I.M.; Talal, A.H. Fibrosis Progression in Patients With Chronic Hepatitis C Virus Infection. J. Infect. Dis. 2016, 214, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Gkouvatsos, K.; Pantopoulos, K. Chronic hepatitis C and liver fibrosis. World J. Gastroenterol. 2014, 20, 11033–11053. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Ray, R.B. Mechanisms Underlying Hepatitis C Virus-Associated Hepatic Fibrosis. Cells 2019, 8, 1249. [Google Scholar] [CrossRef] [PubMed]
- Reungoat, E.; Grigorov, B.; Zoulim, F.; Pécheur, E.I. Molecular Crosstalk between the Hepatitis C Virus and the Extracellular Matrix in Liver Fibrogenesis and Early Carcinogenesis. Cancers 2021, 13, 2270. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Saxena, R. Regression of Hepatic Fibrosis and Evolution of Cirrhosis: A Concise Review. Adv. Anat. Pathol. 2021, 28, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Fuchs, B.C.; Bardeesy, N.; Baumert, T.F.; Chung, R.T. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J. Hepatol. 2014, 61, S79–S90. [Google Scholar] [CrossRef] [PubMed]
- Spengler, U.; Nattermann, J. Immunopathogenesis in hepatitis C virus cirrhosis. Clin. Sci. 2007, 112, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.J. Understanding the Complexities of Cirrhosis. Clin. Ther. 2015, 37, 1822–1836. [Google Scholar] [CrossRef]
- Haep, N.; Florentino, R.M.; Squires, J.E.; Bell, A.; Soto-Gutierrez, A. The Inside-Out of End-Stage Liver Disease: Hepatocytes are the Keystone. Semin. Liver Dis. 2021, 41, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, C.O.; Brunetto, M.R. Surveillance for hepatocellular carcinoma in chronic viral hepatitis: Is it time to personalize it? World J. Gastroenterol. 2021, 27, 5536–5554. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Paul, S. Viral hepatitis: Past, present, and future. World J. Gastroenterol. 2022, 28, 1405–1429. [Google Scholar] [CrossRef] [PubMed]
- Virzì, A.; Roca Suarez, A.A.; Baumert, T.F.; Lupberger, J. Oncogenic Signaling Induced by HCV Infection. Viruses 2018, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- Vescovo, T.; Refolo, G.; Vitagliano, G.; Fimia, G.M.; Piacentini, M. Molecular mechanisms of hepatitis C virus–induced hepatocellular carcinoma. Clin. Microbiol. Infect. 2016, 22, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Roca Suarez, A.A.; Wrensch, F.; Baumert, T.F.; Lupberger, J. Hepatitis C Virus and Hepatocellular Carcinoma: When the Host Loses Its Grip. Int. J. Mol. Sci. 2020, 21, 3057. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, S.; Shokri, S.; Taherkhani, R.; Farshadpour, F. Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Pascut, D.; Hoang, M.; Nguyen, N.N.Q.; Pratama, M.Y.; Tiribelli, C. HCV Proteins Modulate the Host Cell miRNA Expression Contributing to Hepatitis C Pathogenesis and Hepatocellular Carcinoma Development. Cancers 2021, 13, 2485. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Gupta, P.; Irshad, K. Molecular basis of hepatocellular carcinoma induced by hepatitis C virus infection. World J. Hepatol. 2017, 9, 1305–1314. [Google Scholar] [CrossRef]
- Mitchell, J.K.; McGivern, D.R. Mechanisms of hepatocarcinogenesis in chronic hepatitis C. Hepat. Oncol. 2014, 1, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Kuna, L.; Jakab, J.; Smolic, R.; Wu, G.Y.; Smolic, M. HCV Extrahepatic Manifestations. J. Clin. Transl. Hepatol. 2019, 7, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Marcell, L.; Kottilil, S. Systemic manifestations of hepatitis C infection. Infect. Agent. Cancer 2016, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Kao, J.H. Acute hepatitis C virus infection: Clinical update and remaining challenges. Clin. Mol. Hepatol. 2023, 29, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Schillie, S.; Wester, C.; Osborne, M.; Wesolowski, L.; Ryerson, A.B. CDC Recommendations for Hepatitis C Screening Among Adults—United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–17. [Google Scholar] [CrossRef]
- European AIDS Treatment Network (NEAT) Acute Hepatitis C Infection Consensus Panel. Acute hepatitis C in HIV-infected individuals: Recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS 2011, 25, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, R. Screening for Hepatitis C Virus: How Universal Is Universal? Clin. Ther. 2020, 42, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, H.; Kim, B.K.; Chang, Y.; Jang, J.Y.; Kim, D.Y. Cost-effectiveness of chronic hepatitis C screening and treatment. Clin. Mol. Hepatol. 2022, 28, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, F.; Buti, M.; Domínguez-Hernández, R.; Casado, M.A.; Esteban, R. Is the universal population Hepatitis C virus screening a cost-effective strategy? A systematic review of the economic evidence. Rev. Esp. Quim. 2020, 33, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Shahid, I.; Alzahrani, A.R.; Al-Ghamdi, S.S.; Alanazi, I.M.; Rehman, S.; Hassan, S. Hepatitis C Diagnosis: Simplified Solutions, Predictive Barriers, and Future Promises. Diagnostics 2021, 11, 1253. [Google Scholar] [CrossRef] [PubMed]
- Assoumou, S.A.; Tasillo, A.; Leff, J.A.; Schackman, B.R.; Drainoni, M.L.; Horsburgh, C.R.; Barry, M.A.; Regis, C.; Kim, A.Y.; Marshall, A.; et al. Cost-Effectiveness of One-Time Hepatitis C Screening Strategies Among Adolescents and Young Adults in Primary Care Settings. Clin. Infect. Dis. 2018, 66, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, E.M.; Sharon, M.J.; Davis, S.M.; Lander, O.M.; Burrell, C.N. Centers for Disease Control and Prevention Recommendations for Hepatitis C Testing: The Need to Adopt Universal Screening in an Appalachian Emergency Department. Acad. Emerg. Med. 2020, 27, 844–852. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, J.; Yang, L. Cost-effectiveness of new antiviral treatments for non-genotype 1 hepatitis C virus infection in China: A societal perspective. BMJ Glob. Health 2020, 5, e003194. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.; Cooke, G.S.; Nayagam, S.; Thursz, M.; Hallett, T.B. Scaling up prevention and treatment towards the elimination of hepatitis C: A global mathematical model. Lancet 2019, 393, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Warkad, S.D.; Song, K.S.; Pal, D.; Nimse, S.B. Developments in the HCV Screening Technologies Based on the Detection of Antigens and Antibodies. Sensors 2019, 19, 4257. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, W.; Amini, A.; Boeras, D.; Falconer, J.; Kelly, H.; Peeling, R.; Varsaneux, O.; Tucker, J.D.; Easterbrook, P. Diagnostic accuracy of tests to detect Hepatitis C antibody: A meta-analysis and review of the literature. BMC Infect. Dis. 2017, 17, 695. [Google Scholar] [CrossRef] [PubMed]
- Kamili, S.; Drobeniuc, J.; Araujo, A.C.; Hayden, T.M. Laboratory diagnostics for hepatitis C virus infection. Clin. Infect. Dis. 2012, 55 (Suppl. S1), S43–S48. [Google Scholar] [CrossRef]
- Gupta, E.; Bajpai, M.; Choudhary, A. Hepatitis C virus: Screening, diagnosis, and interpretation of laboratory assays. Asian J. Transfus. Sci. 2014, 8, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.S. Laboratory assays for diagnosis and management of hepatitis C virus infection. J. Clin. Microbiol. 2002, 40, 4407–4412. [Google Scholar] [CrossRef] [PubMed]
- Uliana, C.V.; Riccardi, C.S.; Yamanaka, H. Diagnostic tests for hepatitis C: Recent trends in electrochemical immunosensor and genosensor analysis. World J. Gastroenterol. 2014, 20, 15476–15491. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Sykes, E.A.; Jennings, T.L.; Chan, W.C. Rapid screening of genetic biomarkers of infectious agents using quantum dot barcodes. ACS Nano 2011, 5, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Safi, M.A. Hepatitis C: An Overview of Various Laboratory Assays with their Mode of Diagnostic Cooperation. Clin. Lab. 2017, 63, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, K.; Smart, G. HCV antigen testing for the diagnosis of hepatitis C infection: A cost-efficient algorithm. Clin. Lab. 2014, 60, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Shenge, J.A.; Osiowy, C. Rapid Diagnostics for Hepatitis B and C Viruses in Low- and Middle-Income Countries. Front. Virol. 2021, 1, 742722. [Google Scholar] [CrossRef]
- Meng, S.; Li, J. A novel duplex real-time reverse transcriptase-polymerase chain reaction assay for the detection of hepatitis C viral RNA with armored RNA as internal control. Virol. J. 2010, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Zhang, J.; Hu, J.S.; Chen, H.T.; Du, L.; Wu, L.Q.; Ding, Y.Z.; Xiong, S.H.; Huang, X.C.; Zhang, Y.H.; et al. Rapid detection of hepatitis C virus RNA by a reverse transcription loop-mediated isothermal amplification assay. FEMS Immunol. Med. Microbiol. 2011, 63, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.O.; Perez, R.M.; Silva, I.S.; Lemos, L.B.; Simonetti, J.P.; Medina-Pestana, J.O.; Silva, A.E.; Ferraz, M.L. Transcription-mediated amplification (TMA) for the assessment of viremia in hemodialysis patients with hepatitis C. J. Med. Virol. 2012, 84, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Tsongalis, G.J. Branched DNA technology in molecular diagnostics. Am. J. Clin. Pathol. 2006, 126, 448–453. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef]
- Daniel, H.D.; David, J.; Raghuraman, S.; Gnanamony, M.; Chandy, G.M.; Sridharan, G.; Abraham, P. Comparison of Three Different Hepatitis C Virus Genotyping Methods: 5’NCR PCR-RFLP, Core Type-Specific PCR, and NS5b Sequencing in a Tertiary Care Hospital in South India. J. Clin. Lab. Anal. 2017, 31, e22045. [Google Scholar] [CrossRef] [PubMed]
- Flemming, J.A.; Hurlbut, D.J.; Mussari, B.; Hookey, L.C. Liver biopsies for chronic hepatitis C: Should nonultrasound-guided biopsies be abandoned? Can. J. Gastroenterol. 2009, 23, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Seeff, L.B.; Everson, G.T.; Morgan, T.R.; Curto, T.M.; Lee, W.M.; Ghany, M.G.; Shiffman, M.L.; Fontana, R.J.; Di Bisceglie, A.M.; Bonkovsky, H.L.; et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin. Gastroenterol. Hepatol. 2010, 8, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Abu-Freha, N.; Mathew Jacob, B.; Elhoashla, A.; Afawi, Z.; Abu-Hammad, T.; Elsana, F.; Paz, S.; Etzion, O. Chronic hepatitis C: Diagnosis and treatment made easy. Eur. J. Gen. Pr. 2022, 28, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.M.; Crespo, G.; Navasa, M.; Forns, X. Noninvasive assessment of liver fibrosis. Hepatology 2011, 53, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Florea, M.; Serban, T.; Tirpe, G.R.; Tirpe, A.; Lupsor-Platon, M. Noninvasive Assessment of Hepatitis C Virus Infected Patients Using Vibration-Controlled Transient Elastography. J. Clin. Med. 2021, 10, 2575. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Oura, K.; Yoneyama, H.; Shi, T.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Nomura, T.; Morishita, A.; Tsutsui, K.; et al. Albumin-bilirubin score indicates liver fibrosis staging and prognosis in patients with chronic hepatitis C. Hepatol. Res. 2019, 49, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Schiavon Lde, L.; Narciso-Schiavon, J.L.; de Carvalho-Filho, R.J. Non-invasive diagnosis of liver fibrosis in chronic hepatitis C. World J. Gastroenterol. 2014, 20, 2854–2866. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Yu, M.L. Evolution of interferon-based therapy for chronic hepatitis C. Hepat. Res. Treat. 2010, 2010, 140953. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Obel, N.; Christensen, P.B.; Kjær, M.; Laursen, A.L.; Krarup, H.B.; Møller, A.; Schlichting, P.; Bukh, J.; Weis, N. Effectiveness of treatment with pegylated interferon and ribavirin in an unselected population of patients with chronic hepatitis C: A Danish nationwide cohort study. BMC Infect. Dis. 2011, 11, 177. [Google Scholar] [CrossRef]
- Lynch, E.N.; Russo, F.P. Outcomes and Follow-Up after Hepatitis C Eradication with Direct-Acting Antivirals. J. Clin. Med. 2023, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.F.; Bastiampillai, A.J.; Manini, M.; Monico, S. Twelve week post-treatment undetectable hepatitis C virus (HCV)-RNA by PCR assay predicts a sustained virological response to anti-HCV therapy independently from immunological status of the infected patients. J. Antimicrob. Chemother. 2013, 68, 974–975. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Awasthi, A.; Katiyar, H.; Rungta, S.; Deep, A.; Kumar, V.; Shalimar; Kumar, A.; Tiwari, P.; Goel, A. Eight versus twelve weeks of sofosbuvir-velpatasvir in treatment-naïve non-cirrhotic patients with chronic hepatitis C virus infection: Study protocol for a multicentric, open labelled, randomized, non-inferiority trial (RESOLVE trial). PLoS ONE 2023, 18, e0285725. [Google Scholar] [CrossRef] [PubMed]
- Zarębska-Michaluk, D.; Piekarska, A.; Jaroszewicz, J.; Klapaczyński, J.; Sitko, M.; Tudrujek-Zdunek, M.; Tomasiewicz, K.; Belica-Wdowik, T.; Pabjan, P.; Lorenc, B.; et al. Efficacy of 8- versus 12-week treatment with ledipasvir/sofosbuvir in chronic hepatitis C patients eligible for 8-week regimen in a real-world setting. Arch. Med. Sci. 2022, 18, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Reau, N.; Cheng, W.H.; Shao, Q.; Marx, S.E.; Brooks, H.; Martinez, A. Real-World Effectiveness of 8-Week Glecaprevir/Pibrentasvir in Treatment-Naïve, Compensated Cirrhotic HCV Patients. Infect. Dis. Ther. 2023, 12, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Elbadry, M.; Moussa, A.M.; Eltabbakh, M.; Al Balakosy, A.; Abdalgaber, M.; Abdeen, N.; El Sheemy, R.Y.; Afify, S.; El-Kassas, M. The art of managing hepatitis C virus in special population groups: A paradigm shift. Egypt. Liver J. 2022, 12, 61. [Google Scholar] [CrossRef]
- Bourlière, M.; Pietri, O. Hepatitis C virus therapy: No one will be left behind. Int. J. Antimicrob. Agents 2019, 53, 755–760. [Google Scholar] [CrossRef]
- Shiffman, M.L. Side effects of medical therapy for chronic hepatitis C. Ann. Hepatol. 2004, 3, 5–10. [Google Scholar] [PubMed]
- Manns, M.P.; Wedemeyer, H.; Cornberg, M. Treating viral hepatitis C: Efficacy, side effects, and complications. Gut 2006, 55, 1350–1359. [Google Scholar] [CrossRef]
- Dieterich, D.T.; Spivak, J.L. Hematologic disorders associated with hepatitis C virus infection and their management. Clin. Infect. Dis. 2003, 37, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.H.; Matielo, C.E.L.; Curvelo, L.A.; Rocco, R.A.; Felga, G.; Della Guardia, B.; Boteon, Y.L. Update on the management and treatment of viral hepatitis. World J. Gastroenterol. 2021, 27, 3249–3261. [Google Scholar] [CrossRef]
- Pan, Q.; Peppelenbosch, M.P.; Janssen, H.L.; de Knegt, R.J. Telaprevir/boceprevir era: From bench to bed and back. World J. Gastroenterol. 2012, 18, 6183–6188. [Google Scholar] [CrossRef] [PubMed]
- Nyalakonda, H.; Utay, N.S. A new era of therapy for hepatitis C virus infection. Curr. Opin. Infect. Dis. 2015, 28, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Wilby, K.J.; Partovi, N.; Ford, J.A.; Greanya, E.; Yoshida, E.M. Review of boceprevir and telaprevir for the treatment of chronic hepatitis C. Can. J. Gastroenterol. 2012, 26, 205–210. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, T.; Savini, C.; Seyedkazemi, S. Sofosbuvir, a Significant Paradigm Change in HCV Treatment. J. Clin. Transl. Hepatol. 2015, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, D.; Hughes, G. Ledipasvir/Sofosbuvir (harvoni): Improving options for hepatitis C virus infection. Pharm. Ther. 2015, 40, 256–276. [Google Scholar]
- Al-Salama, Z.T.; Deeks, E.D. Elbasvir/Grazoprevir: A Review in Chronic HCV Genotypes 1 and 4. Drugs 2017, 77, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Lampertico, P.; Carrión, J.A.; Curry, M.; Turnes, J.; Cornberg, M.; Negro, F.; Brown, A.; Persico, M.; Wick, N.; Porcalla, A.; et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: A meta-analysis. J. Hepatol. 2020, 72, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Brzdęk, M.; Zarębska-Michaluk, D.; Invernizzi, F.; Cilla, M.; Dobrowolska, K.; Flisiak, R. Decade of optimizing therapy with direct-acting antiviral drugs and the changing profile of patients with chronic hepatitis C. World J. Gastroenterol. 2023, 29, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Orkin, C.; Iser, D.M.; Zamora, F.X.; Nelson, M.; Stephan, C.; Massetto, B.; Gaggar, A.; Ni, L.; Svarovskaia, E.; et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): A multicentre, open-label, non-randomised, phase 3 study. Lancet 2015, 385, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef]
- Lawitz, E.; Mangia, A.; Wyles, D.; Rodriguez-Torres, M.; Hassanein, T.; Gordon, S.C.; Schultz, M.; Davis, M.N.; Kayali, Z.; Reddy, K.R.; et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 2013, 368, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, J.; Cerri, K.; Valentine, W. Achieving sustained virologic response in hepatitis C: A systematic review of the clinical, economic and quality of life benefits. BMC Infect. Dis. 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Messina, V.; Gentilucci, U.V.; Adinolfi, L.E.; Ascione, A.; Barbarini, G.; Barlattani, A.; Cariti, G.; Cozzolongo, R.; Fimiani, B.; et al. Sustained virological response in patients with HCV treated with daclatasvir plus sofosbuvir, with or without ribavirin: A large, field-practice study. Drugs Context 2020, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alshuwaykh, O.; Kwo, P.Y. Current and future strategies for the treatment of chronic hepatitis C. Clin. Mol. Hepatol. 2021, 27, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Foster, G.R.; Wang, S.; Asatryan, A.; Gane, E.; Feld, J.J.; Asselah, T.; Bourlière, M.; Ruane, P.J.; Wedemeyer, H.; et al. Glecaprevir-Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N. Engl. J. Med. 2018, 378, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Wyles, D.; Poordad, F.; Wang, S.; Alric, L.; Felizarta, F.; Kwo, P.Y.; Maliakkal, B.; Agarwal, K.; Hassanein, T.; Weilert, F.; et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: A partially randomized phase 3 clinical trial. Hepatology 2018, 67, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Kowdley, K.V.; Zadeikis, N.; Wang, S.; Hassanein, T.; Horsmans, Y.; Colombo, M.; Calinas, F.; Aguilar, H.; de Ledinghen, V.; et al. Efficacy of Glecaprevir/Pibrentasvir for 8 or 12 Weeks in Patients With Hepatitis C Virus Genotype 2, 4, 5, or 6 Infection Without Cirrhosis. Clin. Gastroenterol. Hepatol. 2018, 16, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Forns, X.; Lee, S.S.; Valdes, J.; Lens, S.; Ghalib, R.; Aguilar, H.; Felizarta, F.; Hassanein, T.; Hinrichsen, H.; Rincon, D.; et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): A single-arm, open-label, multicentre phase 3 trial. Lancet Infect. Dis. 2017, 17, 1062–1068. [Google Scholar] [CrossRef]
- Feld, J.J.; Jacobson, I.M.; Hézode, C.; Asselah, T.; Ruane, P.J.; Gruener, N.; Abergel, A.; Mangia, A.; Lai, C.L.; Chan, H.L.; et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N. Engl. J. Med. 2015, 373, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R.; Afdhal, N.; Roberts, S.K.; Bräu, N.; Gane, E.J.; Pianko, S.; Lawitz, E.; Thompson, A.; Shiffman, M.L.; Cooper, C.; et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N. Engl. J. Med. 2015, 373, 2608–2617. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Gordon, S.C.; Reddy, K.R.; Rossaro, L.; Bernstein, D.E.; Lawitz, E.; Shiffman, M.L.; Schiff, E.; Ghalib, R.; Ryan, M.; et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N. Engl. J. Med. 2014, 370, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.C.; Huang, C.F.; Cheng, P.N.; Tseng, K.C.; Chen, C.Y.; Kuo, H.T.; Huang, Y.H.; Tai, C.M.; Peng, C.Y.; Bair, M.J.; et al. Ledipasvir/sofosbuvir for HCV genotype 1, 2, 4-6 infection: Real-world evidence from a nationwide registry in Taiwan. J. Formos. Med. Assoc. 2022, 121, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Shiha, G.; Esmat, G.; Hassany, M.; Soliman, R.; Elbasiony, M.; Fouad, R.; Elsharkawy, A.; Hammad, R.; Abdel-Razek, W.; Zakareya, T.; et al. Ledipasvir/sofosbuvir with or without ribavirin for 8 or 12 weeks for the treatment of HCV genotype 4 infection: Results from a randomised phase III study in Egypt. Gut 2019, 68, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Abergel, A.; Asselah, T.; Metivier, S.; Kersey, K.; Jiang, D.; Mo, H.; Pang, P.S.; Samuel, D.; Loustaud-Ratti, V. Ledipasvir-sofosbuvir in patients with hepatitis C virus genotype 5 infection: An open-label, multicentre, single-arm, phase 2 study. Lancet Infect. Dis. 2016, 16, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Trinh, H.; Do, S.; Nguyen, T.; Nguyen, P.; Henry, L. Open Label Study of 8 vs. 12 Weeks of Ledipasvir/Sofosbuvir in Genotype 6 Treatment Naïve or Experienced Patients. Am. J. Gastroenterol. 2017, 112, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Ghalib, R.; Reddy, K.R.; Pockros, P.J.; Ben Ari, Z.; Zhao, Y.; Brown, D.D.; Wan, S.; DiNubile, M.J.; Nguyen, B.Y.; et al. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann. Intern. Med. 2015, 163, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; García, A.H.; Crespo, F.I.; Toro, F.I.; Mayora, S.J.; De Sanctis, J.B. A Synopsis of Hepatitis C Virus Treatments and Future Perspectives. Curr. Issues Mol. Biol. 2023, 45, 8255–8276. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.J.; Cypel, M.; Kumar, D.; Dahari, H.; Pinto Ribeiro, R.V.; Marks, N.; Kamkar, N.; Bahinskaya, I.; Onofrio, F.Q.; Zahoor, M.A.; et al. Short-course, direct-acting antivirals and ezetimibe to prevent HCV infection in recipients of organs from HCV-infected donors: A phase 3, single-centre, open-label study. Lancet Gastroenterol. Hepatol. 2020, 5, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.Z.; Sabri, N.A.; Zaki, H.M.; Shaheen, S.M. Clinical effects of simvastatin in chronic hepatitis C patients receiving sofosbuvir/daclatasvir combination. A randomized, placebo-controlled, double-blinded study. Clin. Exp. Hepatol. 2020, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, E.J.; Patel, P.R. Opportunities for Enhanced Prevention and Control of Hepatitis C Through Improved Screening and Testing Efforts. J. Infect. Dis. 2024, 229, S350–S356. [Google Scholar] [CrossRef] [PubMed]
- Delile, J.-M.; de Ledinghen, V.; Jauffret-Roustide, M.; Roux, P.; Reiller, B.; Foucher, J.; Dhumeaux, D. Hepatitis C virus prevention and care for drug injectors: The French approach. Hepatol. Med. Policy 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Høj, S.B.; Minoyan, N.; Artenie, A.A.; Grebely, J.; Bruneau, J. The role of prevention strategies in achieving HCV elimination in Canada: What are the remaining challenges? Can. Liver J. 2018, 1, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.W.; Valdiserri, R.O.; Koh, H.K. Hepatitis C virus prevention, care, and treatment: From policy to practice. Clin. Infect. Dis. 2012, 55 (Suppl. S1), S58–S63. [Google Scholar] [CrossRef] [PubMed]
- van Santen, D.K.; Sacks-Davis, R.; Stewart, A.; Boyd, A.; Young, J.; van der Valk, M.; Smit, C.; Rauch, A.; Braun, D.L.; Jarrin, I.; et al. Treatment as prevention effect of direct-acting antivirals on primary hepatitis C virus incidence: Findings from a multinational cohort between 2010 and 2019. eClinicalMedicine 2023, 56, 101810. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Hepatitis C Work Group. KDIGO 2018 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int. Suppl. 2018, 8, 91–165. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Ramers, C.B.; Dillon, J.F.; Feld, J.J.; Lazarus, J.V. Simplification of Care for Chronic Hepatitis C Virus Infection. Semin. Liver Dis. 2020, 40, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Kim, K.A.; Choi, G.H.; Jang, E.S.; Ki, M.; Choi, H.Y.; Jeong, S.H. A cost-effectiveness study of universal screening for hepatitis C virus infection in South Korea: A societal perspective. Clin. Mol. Hepatol. 2022, 28, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Ide, K.; Kawasaki, Y.; Tanaka-Mizuno, S.; Seto, K.; Iwane, S.; Eguchi, Y.; Kawakami, K. Estimating the cost-effectiveness of screening for hepatitis C virus infection in Japan. Hepatol. Res. 2020, 50, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Platt, L.; Minozzi, S.; Reed, J.; Vickerman, P.; Hagan, H.; French, C.; Jordan, A.; Degenhardt, L.; Hope, V.; Hutchinson, S.; et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: Findings from a Cochrane Review and meta-analysis. Addiction 2018, 113, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.; Le Gall, J.M.; Debrus, M.; Protopopescu, C.; Ndiaye, K.; Demoulin, B.; Lions, C.; Haas, A.; Mora, M.; Spire, B.; et al. Innovative community-based educational face-to-face intervention to reduce HIV, hepatitis C virus and other blood-borne infectious risks in difficult-to-reach people who inject drugs: Results from the ANRS-AERLI intervention study. Addiction 2016, 111, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Raad, I.I.; Chaftari, A.M.; Torres, H.A.; Ayoub, E.M.; Narouz, L.I.; Bartek, J.; Hachem, R. Challenge of hepatitis C in Egypt and hepatitis B in Mauritania. World J. Hepatol. 2018, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Q.; Park, J.S. Revamping hepatitis C global eradication efforts: Towards simplified and enhanced screening, prevention, and treatment. Transl. Gastroenterol. Hepatol. 2024, 9. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Xuan, J.W.; Tang, H.; Ye, X.G.; Xu, P.; Lee, I.H.; Hu, S.L. Hepatitis C virus cure with direct acting antivirals: Clinical, economic, societal and patient value for China. World J. Hepatol. 2019, 11, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.H.; Mahmud, S.; Chemaitelly, H.; Abu-Raddad, L.J. Treatment as prevention for hepatitis C virus in the Middle East and North Africa: A modeling study. Front. Public Health 2023, 11, 1187786. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, C.C.; Baumert, T.F. Addressing the Challenges of Hepatitis C Virus Resistance and Treatment Failure. Viruses 2016, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Malandris, K.; Kalopitas, G.; Theocharidou, E.; Germanidis, G. The Role of RASs /RVs in the Current Management of HCV. Viruses 2021, 13, 2096. [Google Scholar] [CrossRef]
- van Buuren, N.; Tellinghuisen, T.L.; Richardson, C.D.; Kirkegaard, K. Transmission genetics of drug-resistant hepatitis C virus. eLife 2018, 7, e32579. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lin, H.J.; Chen, Y.J.; Lee, C.M.; Wang, S.F.; Chang, K.Y.; Chen, T.L.; Liu, H.F.; Chen, Y.M. Molecular epidemiology of HCV genotypes among injection drug users in Taiwan: Full-length sequences of two new subtype 6w strains and a recombinant form_2b6w. J. Med. Virol. 2010, 82, 57–68. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Sulkowski, M.S. Chronic Hepatitis C: Advances in Therapy and the Remaining Challenges. Med. Clin. N. Am. 2023, 107, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.M.; Li, H.; Wang, S.; Stoddard, M.B.; Learn, G.H.; Korber, B.T.; Bhattacharya, T.; Guedj, J.; Parrish, E.H.; Hahn, B.H.; et al. Quantifying the diversification of hepatitis C virus (HCV) during primary infection: Estimates of the in vivo mutation rate. PLoS Pathog. 2012, 8, e1002881. [Google Scholar] [CrossRef] [PubMed]
- Farci, P. New insights into the HCV quasispecies and compartmentalization. Semin. Liver Dis. 2011, 31, 356–374. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, V.C.; Cento, V.; Mirabelli, C.; Artese, A.; Costa, G.; Alcaro, S.; Perno, C.F.; Ceccherini-Silberstein, F. Hepatitis C virus genetic variability and the presence of NS5B resistance-associated mutations as natural polymorphisms in selected genotypes could affect the response to NS5B inhibitors. Antimicrob. Agents Chemother. 2014, 58, 2781–2797. [Google Scholar] [CrossRef] [PubMed]
- Leggewie, M.; Sreenu, V.B.; Abdelrahman, T.; Leitch, E.C.; Wilkie, G.S.; Klymenko, T.; Muir, D.; Thursz, M.; Main, J.; Thomson, E.C. Natural NS3 resistance polymorphisms occur frequently prior to treatment in HIV-positive patients with acute hepatitis C. AIDS 2013, 27, 2485–2488. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Gupta, E. Emerging resistance to directly-acting antiviral therapy in treatment of chronic Hepatitis C infection-A brief review of literature. J. Fam. Med. Prim. Care 2020, 9, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.M.; Bhardwaj, N.; Hedskog, C.; Chodavarapu, K.; Camus, G.; McNally, J.; Brainard, D.; Miller, M.D.; Mo, H.; Svarovskaia, E.; et al. Global epidemiology of HCV subtypes and resistance-associated substitutions evaluated by sequencing-based subtype analyses. J. Hepatol. 2017, 67, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Li, H.; Ren, H.; Hu, P. Global prevalence of pre-existing HCV variants resistant to direct-acting antiviral agents (DAAs): Mining the GenBank HCV genome data. Sci. Rep. 2016, 6, 20310. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C. Treatment failure with DAA therapy: Importance of resistance. J. Hepatol. 2021, 74, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C.; Cooper, C.L.; Manns, M.P.; Reddy, K.R.; Kowdley, K.V.; Roberts, S.K.; Dvory-Sobol, H.; Svarovskia, E.; Martin, R.; Camus, G.; et al. No impact of resistance-associated substitutions on the efficacy of sofosbuvir, velpatasvir, and voxilaprevir for 12 weeks in HCV DAA-experienced patients. J. Hepatol. 2018, 69, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Perales, C. Quasispecies dynamics and clinical significance of hepatitis C virus (HCV) antiviral resistance. Int. J. Antimicrob. Agents 2020, 56, 105562. [Google Scholar] [CrossRef]
- Cox, A.L. Challenges and Promise of a Hepatitis C Virus Vaccine. Cold Spring Harb. Perspect. Med. 2020, 10, a036947. [Google Scholar] [CrossRef] [PubMed]
- Henry, B. Drug Pricing & Challenges to Hepatitis C Treatment Access. J. Health Biomed. Law. 2018, 14, 265–283. [Google Scholar] [PubMed]
- Graham, C.S.; Swan, T. A path to eradication of hepatitis C in low- and middle-income countries. Antivir. Res. 2015, 119, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, J.H.; Mathur, P. Hepatitis C Virus Infection in Persons Who Inject Drugs in the Middle East and North Africa: Intervention Strategies. Viruses 2021, 13, 1363. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; Barnes, E.; Cox, A.L. Approaches, Progress, and Challenges to Hepatitis C Vaccine Development. Gastroenterology 2019, 156, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.G.; Walker, C.M. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J. Exp. Med. 2005, 201, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.A.; Leung, P.; Gaudieri, S.; Deshpande, P.; Cameron, B.; Walker, M.; Chopra, A.; Lloyd, A.R.; Luciani, F. Transmitted/Founder Viruses Rapidly Escape from CD8+ T Cell Responses in Acute Hepatitis C Virus Infection. J. Virol. 2015, 89, 5478–5490. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.J. Current progress in development of hepatitis C virus vaccines. Nat. Med. 2013, 19, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Adugna, A. Therapeutic strategies and promising vaccine for hepatitis C virus infection. Immun. Inflamm. Dis. 2023, 11, e977. [Google Scholar] [CrossRef]
- Schlotthauer, F.; McGregor, J.; Drummer, H.E. To Include or Occlude: Rational Engineering of HCV Vaccines for Humoral Immunity. Viruses 2021, 13, 805. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.D.; Urbanowicz, R.A.; Tarr, A.W.; Ball, J.K. Hepatitis C Virus Vaccine: Challenges and Prospects. Vaccines 2020, 8, 90. [Google Scholar] [CrossRef]
- Filskov, J.; Andersen, P.; Agger, E.M.; Bukh, J. HCV p7 as a novel vaccine-target inducing multifunctional CD4+ and CD8+ T-cells targeting liver cells expressing the viral antigen. Sci. Rep. 2019, 9, 14085. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.Q.; Pérez, P.; Ljungberg, K.; Sorzano CÓ, S.; Gómez, C.E.; Liljeström, P.; Esteban, M.; García-Arriaza, J. Potent Anti-hepatitis C Virus (HCV) T Cell Immune Responses Induced in Mice Vaccinated with DNA-Launched RNA Replicons and Modified Vaccinia Virus Ankara-HCV. J. Virol. 2019, 93, e00055-19. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Correa, J.D.; Chies, J.A.B. The COVID-19 Pandemic Affected Hepatitis C Virus Circulation and Genotypic Frequencies-Implications for Hepatitis C Prevention, Treatment and Research. Epidemiologia 2024, 5, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.; Meyer, K.; Haga, Y.; Reagan, E.K.; Weissman, D.; Ray, R. Hepatitis C virus E1 and modified E2 delivered from an mRNA vaccine induces protective immunity. NPJ Vaccines 2023, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Schröeder, S.E.; Pedrana, A.; Scott, N.; Wilson, D.; Kuschel, C.; Aufegger, L.; Atun, R.; Baptista-Leite, R.; Butsashvili, M.; El-Sayed, M.; et al. Innovative strategies for the elimination of viral hepatitis at a national level: A country case series. Liver Int. 2019, 39, 1818–1836. [Google Scholar] [CrossRef] [PubMed]
- Waheed, Y.; Siddiq, M.; Jamil, Z.; Najmi, M.H. Hepatitis elimination by 2030: Progress and challenges. World J. Gastroenterol. 2018, 24, 4959–4961. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; El-Sayed, M.H.; Kao, J.H.; Lazarus, J.V.; Lemoine, M.; Lok, A.S.; Zoulim, F. Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 533–542. [Google Scholar] [CrossRef] [PubMed]
- The Coalition for Global Hepatitis Elimination. About the Coalition. Available online: https://www.globalhep.org/about/about-coalition (accessed on 28 April 2024).
- Janjua, N.Z.; Butt, Z.A.; Mahmood, B.; Altaf, A. Towards safe injection practices for prevention of hepatitis C transmission in South Asia: Challenges and progress. World J. Gastroenterol. 2016, 22, 5837–5852. [Google Scholar] [CrossRef] [PubMed]
- Othman, B.; Barakat, M.; Omar, A.; Al-Rawashdeh, A.; Qashou, Y.; Zrieq, R.; Al-Najjar, M.A.A. Evaluation of hepatitis B knowledge, practices, and beliefs among the Jordanian population: A cross-sectional study. PLoS ONE 2022, 17, e0277186. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaccio, M.; Villes, V.; Perfect, C.; El Kaim, J.L.; Donatelli, M.; James, C.; Easterbrook, P.; Delabre, R.M. Need for integration of hepatitis C (HCV) services in community-based settings for people who inject drugs: Results from a global values and preferences survey. Harm Reduct. J. 2023, 20, 15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).