Role of Plant-Growth-Promoting Rhizobacteria in Plant Machinery for Soil Heavy Metal Detoxification
Abstract
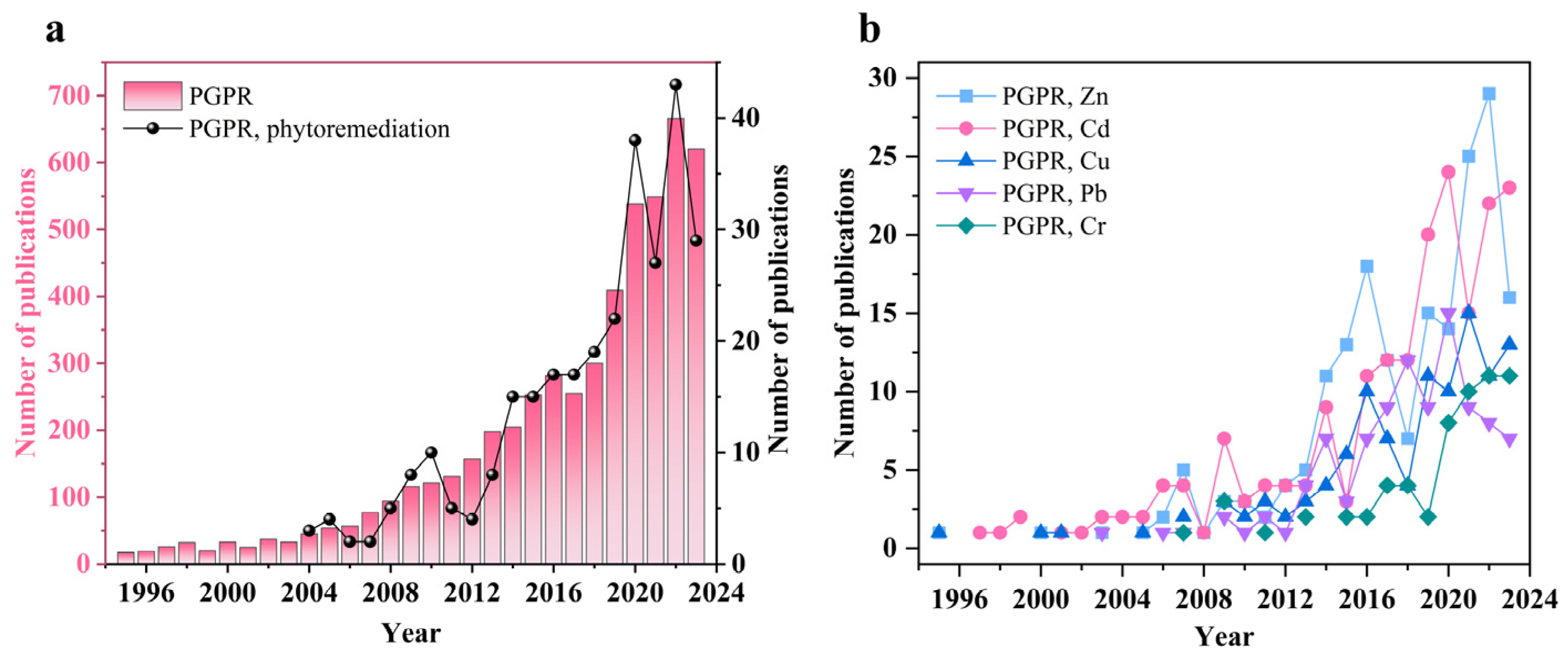
1. Introduction
2. Plant-Growth-Promoting Rhizobacteria (PGPR)
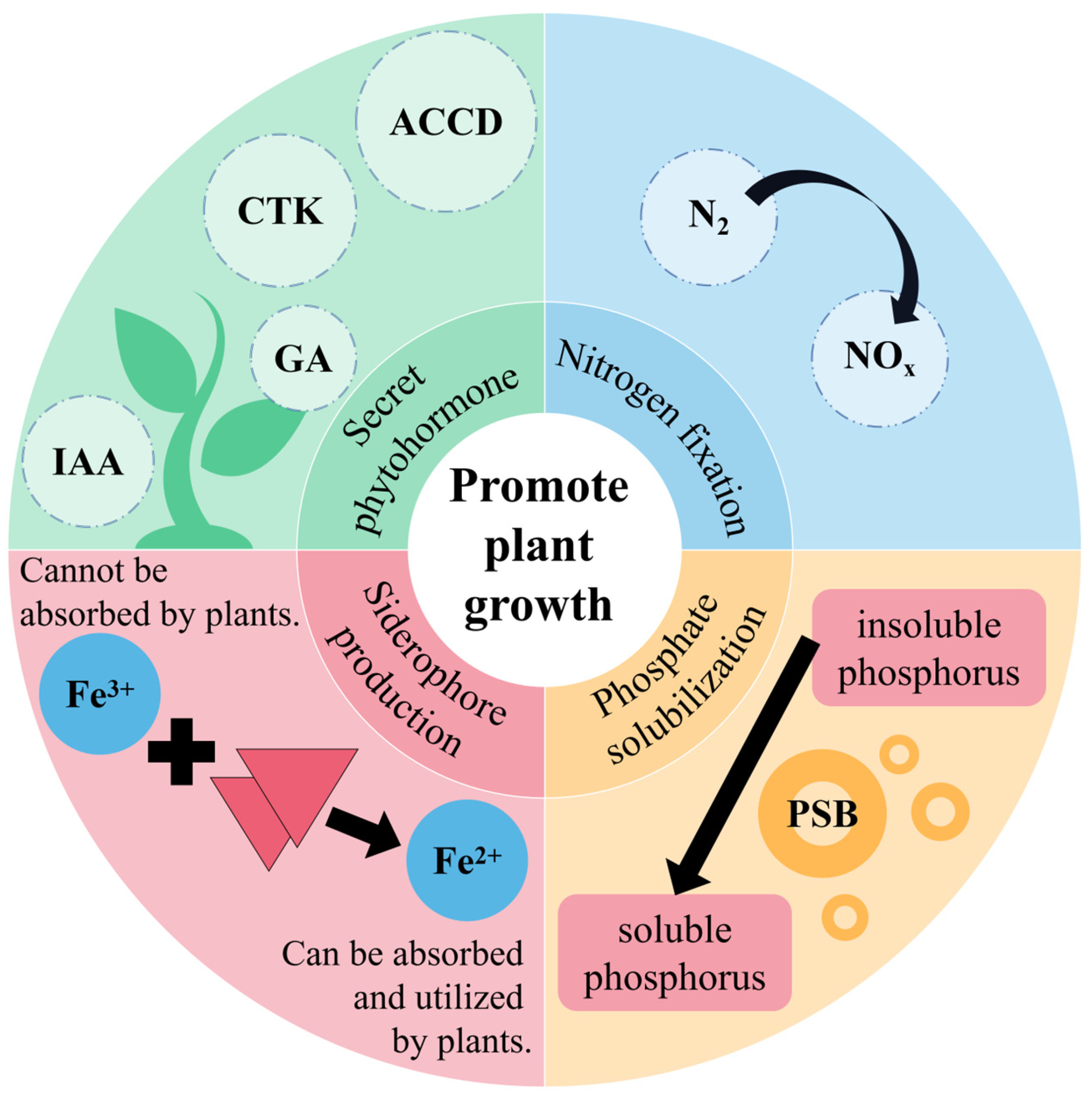
3. PGPR Can Promote Plant Growth
3.1. Biological Nitrogen Fixation
3.2. Phosphate Solubilization
3.3. Siderophore Production
3.4. Phytohormone Production
3.4.1. Auxin
3.4.2. Cytokinin
3.4.3. Aminocyclopropane-1-Carboxylate (ACC) Deaminase
3.4.4. Gibberellins
4. Interactions between PGPR and Heavy Metals
4.1. Chelation
4.1.1. Exopolysaccharide Production
4.1.2. Metallothionein Production
4.1.3. Soil Organic Acid Production
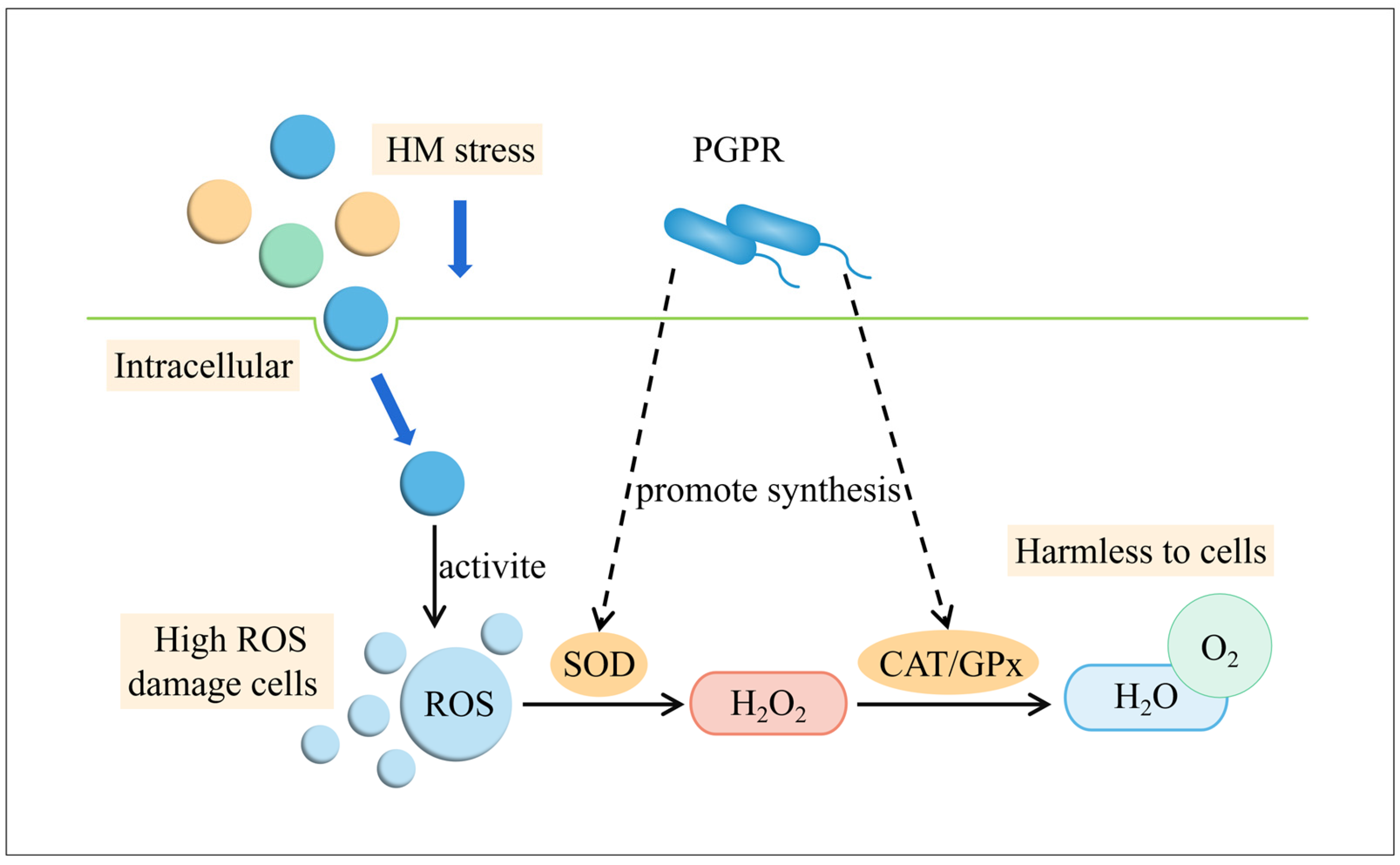
4.2. Induced Systematic Resistance
4.3. Transform Toxic Heavy Metals
5. Conclusions and Future Perspectives
6. Problems in the Practical Application of PGPR in Environmental Remediation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Wang, C. Natural and human factors affect the distribution of soil heavy metal pollution: A review. Water Air Soil Pollut. 2020, 231, 350. [Google Scholar] [CrossRef]
- Hou, D. Sustainable remediation in China: Elimination, immobilization, or dilution. Environ. Sci. Technol. 2021, 55, 15572–15574. [Google Scholar] [CrossRef] [PubMed]
- Keesstra, S.; Mol, G.; De Leeuw, J.; Okx, J.; De Cleen, M.; Visser, S. Soil-related sustainable development goals: Four concepts to make land degradation neutrality and restoration work. Land 2018, 7, 133. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Maxianová, A.; Winkler, J.; Adamcová, D.; Podlasek, A. Environmental consequences and the role of illegal waste dumps and their impact on land degradation. Land Use Policy 2019, 89, 104234. [Google Scholar] [CrossRef]
- Naila, A.; Meerdink, G.; Jayasena, V.; Sulaiman, A.Z.; Ajit, A.B.; Berta, G. A review on global metal accumulators—Mechanism, enhancement, commercial application, and research trend. Environ. Sci. Pollut. Res. 2019, 26, 26449–26471. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Padhan, D.; Rout, P.P.; Kundu, R.; Adhikary, S.; Padhi, P.P. Bioremediation of heavy metals and other toxic substances by microorganisms. In Soil Bioremediation: An Approach towards Sustainable Technology; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 285–329. [Google Scholar]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; Lade, H. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Pantoja-Guerra, M.; Valero-Valero, N.; Ramírez, C.A. Total auxin level in the soil–plant system as a modulating factor for the effectiveness of PGPR inocula: A review. Chem. Biol. Technol. Agric. 2023, 10, 6. [Google Scholar] [CrossRef]
- Dary, M.; Chamber-Pérez, M.; Palomares, A.; Pajuelo, E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef]
- De Souza, L.A.; de Andrade, S.A.L.; de Souza, S.C.R.; Schiavinato, M.A. Arbuscular mycorrhiza confers Pb tolerance in Calopogonium mucunoides. Acta Physiol. Plant. 2012, 34, 523–531. [Google Scholar] [CrossRef]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377. [Google Scholar] [CrossRef]
- Hartmann, A.; Rothballer, M.; Schmid, M. Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 2008, 312, 7–14. [Google Scholar] [CrossRef]
- Mccully, M.; Harper, J.; An, M.; Wu, H.; Kent, J.H. The rhizosphere: The key functional unit in plant/soil/microbial interactions in the field. implications for the understanding of allelopathic effects. Pol. J. Vet. Sci. 2005, 15, 493–498. [Google Scholar]
- Jha, C.K.; Saraf, M. Plant growth promoting rhizobacteria (PGPR): A review. J. Agric. Res. Dev. 2015, 5, 108–119. [Google Scholar]
- Barriuso, J.; Ramos Solano, B.; Lucas, J.A.; Lobo, A.P.; García-Villaraco, A.; Gutiérrez Mañero, F.J. Ecology, genetic diversity and screening strategies of plant growth promoting rhizobacteria (PGPR). In Plant-Bacteria Interactions: Strategies and Techniques to Promote Plant Growth; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–17. [Google Scholar]
- Penrose, D.M.; Glick, B.R. Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Can. J. Microbiol. 2001, 47, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the 4th International Conference on Plant Pathogenic Bacter, Station de Pathologie Vegetale et Phytobacteriologie, INRA, Angers, France, 2 September–27 August 1978; pp. 879–882. [Google Scholar]
- Liu, A.; Wang, W.; Zheng, X.; Chen, X.; Fu, W.; Wang, G.; Ji, J.; Jin, C.; Guan, C. Improvement of the Cd and Zn phytoremediation efficiency of rice (Oryza sativa) through the inoculation of a metal-resistant PGPR strain. Chemosphere 2022, 302, 134900. [Google Scholar] [CrossRef]
- Asadullah, A.B.; Javed, H. PGPR assisted bioremediation of heavy metals and nutrient accumulation in Zea mays under saline sodic soil. Pak. J. Bot. 2021, 53, 31–38. [Google Scholar]
- Wu, J.; Kamal, N.; Hao, H.; Qian, C.; Liu, Z.; Shao, Y.; Zhong, X.; Xu, B. Endophytic Bacillus megaterium BM18-2 mutated for cadmium accumulation and improving plant growth in Hybrid Pennisetum. Biotechnol. Rep. 2019, 24, e00374. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.; Liu, Z.; Qian, C.; Wu, J.; Zhong, X. Improving hybrid Pennisetum growth and cadmium phytoremediation potential by using Bacillus megaterium BM18-2 spores as biofertilizer. Microbiol. Res. 2021, 242, 126594. [Google Scholar] [CrossRef]
- Tirry, N.; Kouchou, A.; El Omari, B.; Ferioun, M.; El Ghachtouli, N. Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J. Genet. Eng. Biotechnol. 2021, 19, 149. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Tang, X.; Fan, X.; Yang, S.; Yao, L.; Li, Y.; Han, H. Isolation of urease-producing bacteria and their effects on reducing Cd and Pb accumulation in lettuce (Lactuca sativa L.). Environ. Sci. Pollut. Res. 2020, 27, 8707–8718. [Google Scholar] [CrossRef] [PubMed]
- Danish, S.; Kiran, S.; Fahad, S.; Ahmad, N.; Ali, M.A.; Tahir, F.A.; Rasheed, M.K.; Shahzad, K.; Li, X.; Wang, D. Alleviation of chromium toxicity in maize by Fe fortification and chromium tolerant ACC deaminase producing plant growth promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2019, 185, 109706. [Google Scholar] [CrossRef]
- Samreen, T.; Zahir, Z.A.; Naveed, M.; Asghar, M. Boron tolerant phosphorus solubilizing Bacillus spp. MN-54 improved canola growth in alkaline calcareous soils. Int. J. Agric. Biol. 2019, 21, 538–546. [Google Scholar]
- Abdelkrim, S.; Jebara, S.H.; Saadani, O.; Chiboub, M.; Abid, G.; Jebara, M. Effect of Pb-resistant plant growth-promoting rhizobacteria inoculation on growth and lead uptake by Lathyrus sativus. J. Basic Microbiol. 2018, 58, 579–589. [Google Scholar] [CrossRef]
- Gupta, P.; Kumar, V.; Usmani, Z.; Rani, R.; Chandra, A. Phosphate solubilization and chromium (VI) remediation potential of Klebsiella sp. strain CPSB4 isolated from the chromium contaminated agricultural soil. Chemosphere 2018, 192, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Pramanik, K.; Ghosh, P.K.; Soren, T.; Sarkar, A.; Dey, R.S.; Pandey, S.; Maiti, T.K. Characterization of Cd-resistant Klebsiella michiganensis MCC3089 and its potential for rice seedling growth promotion under Cd stress. Microbiol. Res. 2018, 210, 12–25. [Google Scholar] [CrossRef]
- Biswas, J.K.; Mondal, M.; Rinklebe, J.; Sarkar, S.K.; Chaudhuri, P.; Rai, M.; Shaheen, S.M.; Song, H.; Rizwan, M. Multi-metal resistance and plant growth promotion potential of a wastewater bacterium Pseudomonas aeruginosa and its synergistic benefits. Environ. Geochem. Health 2017, 39, 1583–1593. [Google Scholar] [CrossRef]
- Hansda, A.; Kumar, V. Cu-resistant Kocuria sp. CRB15: A potential PGPR isolated from the dry tailing of Rakha copper mine. 3 Biotech 2017, 7, 132. [Google Scholar] [CrossRef]
- Khan, W.U.; Yasin, N.A.; Ahmad, S.R.; Ali, A.; Ahmed, S.; Ahmad, A. Role of Ni-tolerant Bacillus spp. and Althea rosea L. in the phytoremediation of Ni-contaminated soils. Int. J. Phytoremediat. 2017, 19, 470–477. [Google Scholar] [CrossRef]
- Sobariu, D.L.; Fertu, D.I.T.; Diaconu, M.; Pavel, L.V.; Hlihor, R.-M.; Drăgoi, E.N.; Curteanu, S.; Lenz, M.; Corvini, P.F.-X.; Gavrilescu, M. Rhizobacteria and plant symbiosis in heavy metal uptake and its implications for soil bioremediation. New Biotechnol. 2017, 39, 125–134. [Google Scholar] [CrossRef]
- Hassan, W.; Bashir, S.; Ali, F.; Ijaz, M.; Hussain, M.; David, J. Role of ACC-deaminase and/or nitrogen fixing rhizobacteria in growth promotion of wheat (Triticum aestivum L.) under cadmium pollution. Environ. Earth Sci. 2016, 75, 267. [Google Scholar] [CrossRef]
- Pandey, N.; Bhatt, R. Role of soil associated Exiguobacterium in reducing arsenic toxicity and promoting plant growth in Vigna radiata. Eur. J. Soil Biol. 2016, 75, 142–150. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Rocha, I.; Oliveira, R.S.; Freitas, H. Serpentine bacteria influence metal translocation and bioconcentration of Brassica juncea and Ricinus communis grown in multi-metal polluted soils. Front. Plant Sci. 2015, 5, 757. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chi, J. Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 2014, 375, 205–214. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Ali, Q.; Ali, S.; Arif, M.S.; Hussain, S.; Rizvi, H. Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol. Environ. Saf. 2014, 104, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Yasmeen, T.; Riaz, M.; Arif, M.S.; Ali, S.; Raza, S.H. Proteus mirabilis alleviates zinc toxicity by preventing oxidative stress in maize (Zea mays) plants. Ecotoxicol. Environ. Saf. 2014, 110, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 2006, 64, 991–997. [Google Scholar] [CrossRef]
- Wagner, S.C. Biological Nitrogen Fixation. Nat. Educ. Knowl. 2011, 3, 15. [Google Scholar]
- Ohyama, T. Nitrogen as a major essential element of plants. Nitrogen Assim. Plants 2010, 37, 1–17. [Google Scholar]
- Stefan, B.; Rubio, L.M. State of the Art in Eukaryotic Nitrogenase Engineering. FEMS Microbiol. Lett. 2018, 365, fnx274. [Google Scholar]
- Hodge, A.; Robinson, D.; Fitter, A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Sukul, P.; Kumar, J.; Rani, A.; Abdillahi, A.M.; Rakesh, R.B.; Kumar, M.H. Functioning of plant growth promoting rhizobacteria (PGPR) and their mode of actions: An overview from chemistry point of view. Plant Arch. 2021, 21, 628–634. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Rovira, A. Microbial inoculation of plants: I. Establishment of free-living nitrogen-fixing bacteria in the rhizosphere and their effects on maize, tomato, and wheat. Plant Soil 1963, 19, 304–314. [Google Scholar] [CrossRef]
- Baldani, J.I.; Baldani, V.L. History on the biological nitrogen fixation research in graminaceous plants: Special emphasis on the Brazilian experience. An. Acad. Bras. Ciênc. 2005, 77, 549–579. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Costas, A.G.; Peters, J.W. Symbiotic Nitrogen Fixation and Challenges to Extending it to Non-Legumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef]
- Santos, K.F.; Moure, V.; Hauer, V.; Santos, A.; Donatti, L.; Galvão, C.; Pedrosa, F.; Souza, E.; Wassem, R.; Steffens, M. Wheat colonization by an Azospirillum brasilense ammonium-excreting strain reveals upregulation of nitrogenase and superior plant growth promotion. Plant Soil 2017, 415, 245–255. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Erat, M.; Erdoğan, Ü.; Dönmez, M.F. The influence of plant growth–promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. J. Plant Nutr. Soil Sci. 2007, 170, 288–295. [Google Scholar] [CrossRef]
- Dhiman, V.K.; Rana, N.; Dhiman, V.K.; Pandey, H.; Verma, P.; Singh, D. Effect of rhizobial isolates and nitrogen fertilizers on nursery performance, nodulation behavior and nitrogenase activity of Dalbergia sissoo Roxb. seedlings. Plant Stress 2022, 4, 100080. [Google Scholar] [CrossRef]
- Hao, X.; Taghavi, S.; Xie, P.; Orbach, M.; Alwathnani, H.; Rensing, C.; Wei, G. Phytoremediation of heavy and transition metals aided by legume-rhizobia symbiosis. Int. J. Phytoremediat. 2014, 16, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Bai, X.; Zhang, H.; Song, X.; Li, Z. Promotion of growth and metal accumulation of alfalfa by coinoculation with Sinorhizobium and Agrobacterium under copper and zinc stress. PeerJ 2019, 7, e6875. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.-Q.; Yan, X.-W.; Wei, G.-H.; Zhang, J.-H.; Fang, L.-C. Rhizobium inoculation enhances copper tolerance by affecting copper uptake and regulating the ascorbate-glutathione cycle and phytochelatin biosynthesis-related gene expression in Medicago sativa seedlings. Ecotoxicol. Environ. Saf. 2018, 162, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, Y.; Chen, M.; Huang, J.; Liu, F.; Xie, S.; Kong, L.; Pan, Y. Reduced cadmium toxicity in rapeseed via alteration of root properties and accelerated plant growth by a nitrogen-fixing bacterium. J. Hazard. Mater. 2023, 449, 131040. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Goswami, D.; Dhandhukia, P.; Thakker, J. Techniques to study microbial phytohormones. In Bacterial Metabolites in Sustainable Agroecosystem; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–27. [Google Scholar]
- Mehta, P.; Sharma, R.; Putatunda, C.; Walia, A. Endophytic fungi: Role in phosphate solubilization. In Advances in Endophytic Fungal Research: Present Status and Future Challenges; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 183–209. [Google Scholar]
- De Zutter, N.; Ameye, M.; Debode, J.; De Tender, C.; Ommeslag, S.; Verwaeren, J.; Vermeir, P.; Audenaert, K.; De Gelder, L. Shifts in the rhizobiome during consecutive in planta enrichment for phosphate-solubilizing bacteria differentially affect maize P status. Microb. Biotechnol. 2021, 14, 1594–1612. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W. Iron solutions: Acquisition strategies and signaling pathways in plants. Trends Plant Sci. 2003, 8, 188–193. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Brandan, C.P.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C. Biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary on common bean by native lipopeptide-producer Bacillus strains. Microbiol. Res. 2018, 211, 21–30. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Enhancement of plant growth and decontamination of nickel-spiked soil using PGPR. J. Basic Microbiol. 2009, 49, 195–204. [Google Scholar] [CrossRef]
- Tanimoto, E. Regulation of root growth by plant hormones—Roles for auxin and gibberellin. Crit. Rev. Plant Sci. 2005, 24, 249–265. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Hossen, F.A.; Ismail, M.R.; Hoque, A.; Islam, M.Z.; Shahidullah, S.; Meon, S. Efficiency of plant growth-promoting rhizobacteria (PGPR) for the enhancement of rice growth. Afr. J. Biotechnol. 2009, 8, 1247–1252. [Google Scholar]
- Teng, Z.; Shao, W.; Zhang, K.; Huo, Y.; Li, M. Characterization of phosphate solubilizing bacteria isolated from heavy metal contaminated soils and their potential for lead immobilization. J. Environ. Manag. 2019, 231, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Amara, U.; Khalid, R.; Hayat, R. Soil bacteria and phytohormones for sustainable crop production. In Bacterial Metabolites in Sustainable Agroecosystem; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 87–103. [Google Scholar]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Dodd, I.C. Phytohormone Mediation of Interactions between Plants and Non-Symbiotic Growth Promoting Bacteria under Edaphic Stresses. Front. Plant Sci. 2019, 10, 483140. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Biologically relevant metal ion-dependent hydroxyl radical generation An update. FEBS Lett. 1992, 307, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, J.; Ni, L.; Zhang, R.; Xia, A.; Jin, F. Conditional privatization of a public siderophore enables Pseudomonas aeruginosa to resist cheater invasion. Nat. Commun. 2018, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Kang, J.P.; Ahn, J.C.; Kim, Y.J.; Piao, C.H.; Yang, D.U.; Yang, D.C. Siderophore-producing rhizobacteria reduce heavy metal-induced oxidative stress in Panax ginseng Meyer. J. Ginseng Res. 2021, 45, 218–227. [Google Scholar] [CrossRef]
- Di Francesco, A.; Baraldi, E. How siderophore production can influence the biocontrol activity of Aureobasidium pullulans against Monilinia laxa on peaches. Biol. Control 2021, 152, 104456. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Kaur, R.; Handa, N.; Bakshi, P.; Sharma, P.; Ohri, P.; Bhardwaj, R. Reconnoitering the Efficacy of Plant Growth Promoting Rhizobacteria in Expediting Phytoremediation Potential of Heavy Metals. J. Plant Growth Regul. 2022, 42, 6474–6502. [Google Scholar] [CrossRef]
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl. Environ. Microbiol. 2009, 75, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, I.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid Auxin-Mediated Cell Expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Luo, S.; Wu, Y.; Ye, J.; Wang, Q.; Xu, X.; Pan, F.; Khan, K.Y.; Feng, Y.; Yang, X. The effects of the endophytic bacterium Pseudomonas fluorescens Sasm05 and IAA on the plant growth and cadmium uptake of Sedum alfredii Hance. Front. Microbiol. 2017, 8, 2538. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Charles, T.C.; Glick, B.R. Endophytic phytohormones and their role in plant growth promotion. In Functional Importance of the Plant Microbiome: Implications for Agriculture, Forestry and Bioenergy; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 89–105. [Google Scholar]
- Geries, L.; Elsadany, A.Y. Maximizing growth and productivity of onion (Allium cepa L.) by Spirulina platensis extract and nitrogen-fixing endophyte Pseudomonas stutzeri. Arch. Microbiol. 2021, 203, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lebrazi, S.; Fadil, M.; Chraibi, M.; Fikri-Benbrahim, K. Screening and optimization of indole-3-acetic acid production by Rhizobium sp. strain using response surface methodology. J. Genet. Eng. Biotechnol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J. Hazard. Mater. 2016, 320, 36–44. [Google Scholar] [CrossRef]
- Abbaszadeh-Dahaji, P.; Atajan, F.A.; Omidvari, M.; Tahan, V.; Kariman, K. Mitigation of copper stress in maize (Zea mays) and sunflower (Helianthus annuus) plants by copper-resistant Pseudomonas strains. Curr. Microbiol. 2021, 78, 1335–1343. [Google Scholar] [CrossRef]
- Carlos, M.-H.J.; Stefani, P.-V.Y.; Janette, A.-M.; Melani, M.-S.S.; Gabriela, P.-O. Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol. Res. 2016, 188, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.U. Construction of IAA-Deficient Mutants of Pseudomonas moraviensis and Their Comparative Effects with Wild Type Strains as Bio-inoculant on Wheat in Saline Sodic Soil. Geomicrobiol. J. 2019, 36, 376–384. [Google Scholar] [CrossRef]
- Li, S.M.; Zheng, H.X.; Zhang, X.S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2020, 40, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Tassi, E.; Pouget, J.; Petruzzelli, G.; Barbafieri, M. The effects of exogenous plant growth regulators in the phytoextraction of heavy metals. Chemosphere 2008, 71, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Jin, J.; Li, Q.; Zhao, C.; Nan, W.; Wang, X.; Ma, R.; Bi, Y. An intact cytokinin-signaling pathway is required for Bacillus sp. LZR216-promoted plant growth and root system architecture altereation in Arabidopsis thaliana seedlings. Plant Growth Regul. 2018, 84, 507–518. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka-Szelewa, E.; Bralska, M. Exogenously applied auxins and cytokinins ameliorate lead toxicity by inducing antioxidant defence system in green alga Acutodesmus obliquus. Plant Physiol. Biochem. 2018, 132, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zehra, A.; Sahito, Z.A.; Wang, W.; Chen, S.; Feng, Y.; He, Z.; Yang, X. Cytokinin-mediated shoot proliferation and its correlation with phytoremediation effects in Cd-hyperaccumulator ecotype of Sedum alfredii. Sci. Total Environ. 2024, 912, 168993. [Google Scholar] [CrossRef]
- Nieto, K.F.; Frankenberger, W.T. Influence of adenine, isopentyl alcohol and Azotobacter chroococcum on the vegetative growth of Zea mays. Plant Soil 1991, 135, 213–221. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Gracheva, N.V.; Grishenkova, N.N.; Dukhovskis, P.V.; Brazaitite, A.A. Cytokinin-like growth regulators mitigate toxic action of zinc and nickel ions on maize seedlings. Russ. J. Plant Physiol. 2007, 54, 381–387. [Google Scholar] [CrossRef]
- Al-Hakimi, A. Modification of cadmium toxicity in pea seedlings by kinetin. Plant Soil Environ. 2007, 53, 129–135. [Google Scholar] [CrossRef]
- Lin, Z.; Zhong, S.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Smet, D.; Van Der Straeten, D. Ethylene and Hormonal Cross Talk in Vegetative Growth and Development. Plant Physiol. 2015, 169, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Vaughan-Hirsch, J.; Poel, B.V.D. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Misra, S.; Chauhan, P.S. ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Camilios-Neto, D.; Bonato, P.; Wassem, R.; Tadra-Sfeir, M.Z.; Brusamarello-Santos, L.C.; Valdameri, G.; Donatti, L.; Faoro, H.; Weiss, V.A.; Chubatsu, L.S. Dual RNA-seq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes. BMC Genom. 2014, 15, 378. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.A.; Wu, H.; Wu, L.; Gao, X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 236091. [Google Scholar] [CrossRef]
- Singh, R.P.; Shelke, G.M.; Anil, K.; Jha, P.N. Biochemistry and genetics of ACC deaminase: A weapon to “stress ethylene” produced in plants. Front. Microbiol. 2015, 6, 937. [Google Scholar]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Springer: Dordrecht, The Netherlands, 2007; pp. 329–339. [Google Scholar]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Motesharezadeh, B.; Hosseini, H.M.; Alikhani, H.; Zolfaghari, A.A. Root-induced changes of Zn and Pb dynamics in the rhizosphere of sunflower with different plant growth promoting treatments in a heavily contaminated soil. Ecotoxicol. Environ. Saf. 2017, 147, 206. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R.; Bieber, J.E.; Schmidt-Dannert, M.G.; Nett, R.S.; Peters, R.J. A Third Class: Functional Gibberellin Biosynthetic Operon in Beta-Proteobacteria. Front. Microbiol. 2018, 9, 423779. [Google Scholar] [CrossRef] [PubMed]
- Joo, G.J.; Kang, S.M.; Hamayun, M.; Kim, S.K.; Na, C.I.; Shin, D.H.; Lee, I.J. Burkholderia sp. KCTC 11096BP as a newly isolated gibberellin producing bacterium. J. Microbiol. 2009, 47, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Bastián, F.; Cohen, A.; Piccoli, P.; Luna, V.; Bottini, R.; Baraldi, R.; Bottini, R. Production of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically-defined culture media. Plant Growth Regul. 1998, 24, 7–11. [Google Scholar] [CrossRef]
- Massoud, M.B.; Sakouhi, L.; Karmous, I.; Zhu, Y.; El Ferjani, E.; Sheehan, D.; Chaoui, A. Protective role of exogenous phytohormones on redox status in pea seedlings under copper stress. J. Plant Physiol. 2018, 221, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huan, Y.; Sun, J.; Lin, L.; Liao, M.a.; Wang, Z.; Liang, D.; Xia, H.; Lv, X.; Wang, J. Effects of exogenous gibberellic acid on growth and cadmium accumulation in Cyphomandra betacea seedlings. Environ. Prog. Sustain. Energy 2021, 40, e13655. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, X.; Guo, Z.; Peng, C.; Zeng, P.; Wang, X. Co-application of indole-3-acetic acid/gibberellin and oxalic acid for phytoextraction of cadmium and lead with Sedum alfredii Hance from contaminated soil. Chemosphere 2021, 285, 131420. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fang, H.H.P. Characterization of electrostatic binding sites of extracellular polymers by linear programming analysis of titration data. Biotechnol. Bioeng. 2010, 80, 806–811. [Google Scholar] [CrossRef]
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 9, 689–701. [Google Scholar] [CrossRef]
- Vimalnath, S.; Subramanian, S. Studies on the biosorption of Pb(II) ions from aqueous solution using extracellular polymeric substances (EPS) of Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2018, 172, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, R.; Szcze, A.; Czemierska, M.; Jarosz-Wiko Azka, A. Studies of cadmium(II), lead(II), nickel(II), cobalt(II) and chromium(VI) sorption on extracellular polymeric substances produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresour. Technol. 2017, 225, 113–120. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, Y.; Xiong, F. Sorption of Cu(II) and Cd(II) by extracellular polymeric substances (EPS) from Aspergillus fumigatus. Int. Biodeterior. Biodegrad. 2011, 65, 1012–1018. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q. Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour. Technol. 2014, 160, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Comte, S.; Guibaud, G.; Baudu, M. Biosorption properties of extracellular polymeric substances (EPS) resulting from activated sludge according to their type: Soluble or bound. Process Biochem. 2006, 41, 815–823. [Google Scholar] [CrossRef]
- Dong, B.; Liu, X.; Dai, L.; Dai, X. Changes of heavy metal speciation during high-solid anaerobic digestion of sewage sludge. Bioresour. Technol. 2013, 131, 152–158. [Google Scholar] [CrossRef]
- Kushwaha, S.; Sreedhar, B.; Sudhakar, P.P. A spectroscopic study for understanding the speciation of Cr on palm shell based adsorbents and their application for the remediation of chrome plating effluents. Bioresour. Technol. 2012, 116, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Z.; Shi, J.; Dong, H. Sulfur-based mixotrophic bio-reduction for efficient removal of chromium(VI) in groundwater. Geochim. Cosmochim. Acta 2019, 268, 296–309. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Chen, G.-J.; Bai, Y.-N.; Fu, L.; Qin, L.-P.; Zeng, R.J. Chromium isotope fractionation during Cr(VI) reduction in a methane-based hollow-fiber membrane biofilm reactor. Water Res. 2018, 130, 263–270. [Google Scholar] [CrossRef]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of Extracellular Polysaccharides and Biofilm Formation in Heavy Metal Resistance of Rhizobia. Materials 2016, 9, 418. [Google Scholar] [CrossRef]
- Bataineh, Z.M.; Heidger, P.M.; Thompson, S.A.; Timms, B.G. Immunocytochemical localization of metallothionein in the rat prostate gland. Prostate 1986, 9, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yang, J.-L.; Li, C.-H. Advances in metallotionein studies in forest trees. Plant Omics 2012, 5, 46–51. [Google Scholar]
- Cai, L.; Xu, Z.; Ren, M.; Guo, Q.; Hu, X.; Hu, G.; Wan, H.; Peng, P. Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong Province, China. Ecotoxicol. Environ. Saf. 2012, 78, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Atrian, S. Metallothionein protein evolution: A miniassay. JBIC J. Biol. Inorg. Chem. 2011, 16, 977–989. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 1–20. [Google Scholar] [CrossRef]
- Ehsanpour, A.A.; Zarei, S.; Abbaspour, J. The role of over expression of P5CS gene on proline, catalase, ascorbate peroxidase activity and lipid peroxidation of transgenic tobacco (Nicotiana tabacum L.) plant under in vitro drought stress. J. Cell Mol. Res. 2012, 4, 43–49. [Google Scholar]
- Murthy, S.; Bali, G.; Sarangi, S. Effect of lead on metallothionein concentration in leadresistant bacteria Bacillus cereus isolated from industrial effluent. Afr. J. Biotechnol. 2011, 10, 15966–15972. [Google Scholar] [CrossRef]
- Hussain, I.; Afzal, S.; Ashraf, M.A.; Rasheed, R.; Saleem, M.H.; Alatawi, A.; Ameen, F.; Fahad, S. Effect of metals or trace elements on wheat growth and its remediation in contaminated soil. J. Plant Growth Regul. 2023, 42, 2258–2282. [Google Scholar] [CrossRef]
- Israr, D.; Mustafa, G.; Khan, K.S.; Shahzad, M.; Ahmad, N.; Masood, S. Interactive effects of phosphorus and Pseudomonas putida on chickpea (Cicer arietinum L.) growth, nutrient uptake, antioxidant enzymes and organic acids exudation. Plant Physiol. Biochem. 2016, 108, 304–312. [Google Scholar] [CrossRef]
- Li, W.C.; Ye, Z.H.; Wong, M.H. Metal mobilization and production of short-chain organic acids by rhizosphere bacteria associated with a Cd/Zn hyperaccumulating plant, Sedum alfredii. Plant Soil 2010, 326, 453–467. [Google Scholar] [CrossRef]
- Tong, B.; Sun, T.; Sun, L. Low molecular weight organic acids in root exudates and cadmium accumulation in cadmium hyperaccumulator Solanum nigrum L. and nonhyperaccumulator Solanum lycopersicum L. Afr. J. Biotechnol. 2011, 10, 17180–17185. [Google Scholar]
- Yang, P.; Zhou, X.-F.; Wang, L.-L.; Li, Q.-S.; Zhou, T.; Chen, Y.-K.; Zhao, Z.-Y.; He, B.-Y. Effect of phosphate-solubilizing bacteria on the mobility of insoluble cadmium and metabolic analysis. Int. J. Environ. Res. Public Health 2018, 15, 1330. [Google Scholar] [CrossRef] [PubMed]
- Renella, G.; Landi, L.; Nannipieri, P. Degradation of low molecular weight organic acids complexed with heavy metals in soil. Geoderma 2004, 122, 311–315. [Google Scholar] [CrossRef]
- Krishnamurti, G.; Cieslinski, G.; Huang, P.; Van Rees, K. Kinetics of cadmium release from soils as influenced by organic acids: Implication in cadmium availability. J. Environ. Qual. 1997, 26, 271–277. [Google Scholar] [CrossRef]
- Yu, G.; Liu, J.; Long, Y.; Chen, Z.; Sunahara, G.I.; Jiang, P.; You, S.; Lin, H.; Xiao, H. Phytoextraction of cadmium-contaminated soils: Comparison of plant species and low molecular weight organic acids. Int. J. Phytoremediat. 2020, 22, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmud, J.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.B.; Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Hassen, A.; Saidi, N.; Cherif, M.; Boudabous, A. Effects of heavy metals on Pseudomonas aeruginosa and Bacillus thuringiensis. Bioresour. Technol. 1998, 65, 73–82. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediat. 2019, 21, 760–767. [Google Scholar] [CrossRef]
- Chai, M.-W.; Li, R.-L.; Shi, F.-C.; Liu, F.-C.; Pan, X.; Cao, D.; Wen, X. Effects of cadmium stress on growth, metal accumulation and organic acids of Spartina alterniflora Loisel. Afr. J. Biotechnol. 2012, 11, 6091–6099. [Google Scholar]
- Chen, H.-C.; Zhang, S.-L.; Wu, K.-J.; Li, R.; He, X.-R.; He, D.-N.; Huang, C.; Wei, H. The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. Under Cd stress. Ecotoxicol. Environ. Saf. 2020, 187, 109790. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Sharma, A.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ali, H.M.; Ahmad, P. Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 2019, 230, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Loon, L.; Bakker, P.; Pieterse, C. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef]
- Annapurna, K.; Kumar, A.; Kumar, L.V.; Govindasamy, V.; Bose, P.; Ramadoss, D. PGPR-induced systemic resistance (ISR) in plant disease management. In Bacteria in Agrobiology: Disease Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; pp. 405–425. [Google Scholar]
- Jorquera, M.A.; Maruyama, F.; Ogram, A.V.; Navarrete, O.U.; Lagos, L.M.; Inostroza, N.G.; Acuña, J.J.; Rilling, J.I.; de La Luz Mora, M. Rhizobacterial Community Structures Associated with Native Plants Grown in Chilean Extreme Environments. Microb. Ecol. 2016, 72, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Arumugam, S.; Vijayabharathi, R.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2014, 5, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Gururani, M.A.; Upadhyaya, C.P.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 2013, 32, 245–258. [Google Scholar] [CrossRef]
- Ju, W.; Liu, L.; Jin, X.; Duan, C.; Fang, L. Co-inoculation effect of plant-growth-promoting rhizobacteria and rhizobium on EDDS assisted phytoremediation of Cu contaminated soils. Chemosphere 2020, 254, 126724. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Rani, R.; Chandra, A.; Kumar, V. Potential applications of Pseudomonas sp. (strain CPSB21) to ameliorate Cr6+ stress and phytoremediation of tannery effluent contaminated agricultural soils. Sci. Rep. 2018, 8, 4860. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Jamwal, V.L.; Kohli, S.K.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Abdjtllah, E.F.; Hashem, A.; Ahmad, P. Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 2019, 217, 463–474. [Google Scholar] [CrossRef]
- Jing, Y.D.; He, Z.L.; Yang, X.E. Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J. Zhejiang Univ. B 2007, 8, 192–207. [Google Scholar] [CrossRef]
- Hamidpour, M.; Nemati, H.; Abbaszadeh Dahaji, P.; Roosta, H.R. Effects of plant growth-promoting bacteria on EDTA-assisted phytostabilization of heavy metals in a contaminated calcareous soil. Environ. Geochem. Health 2020, 42, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Khan, F.; Alqahtani, F.M.; Hashem, M.; Ahmad, F. Plant growth–promoting Rhizobacteria (PGPR) assisted bioremediation of Heavy Metal Toxicity. Appl. Biochem. Biotechnol. 2023, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Terry, L.N. Accumulation and volatilization of different chemical species of selenium by plants. Planta 1998, 206, 284–292. [Google Scholar] [CrossRef]
- Jeong, S.; Moon, H.S.; Nam, K.; Kim, J.Y.; Kim, T.S. Application of phosphate-solubilizing bacteria for enhancing bioavailability and phytoextraction of cadmium (Cd) from polluted soil. Chemosphere 2012, 88, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Prapagdee, B.; Chanprasert, M.; Mongkolsuk, S. Bioaugmentation with cadmium-resistant plant growth-promoting rhizobacteria to assist cadmium phytoextraction by Helianthus annuus. Chemosphere 2013, 92, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Juwarkar, A.A.; Nair, A.; Dubey, K.V.; Singh, S.; Devotta, S. Biosurfactant technology for remediation of cadmium and lead contaminated soils. Chemosphere 2007, 68, 1996–2002. [Google Scholar] [CrossRef]
- He, C.Q.; Tan, G.; Liang, X.; Du, W.; Chen, Y.; Zhi, G.; Zhu, Y. Effect of Zn-tolerant bacterial strains on growth and Zn accumulation in Orychophragmus violaceus. Appl. Soil Ecol. 2010, 44, 1–5. [Google Scholar] [CrossRef]
- Strigul, N.S.; Kravchenko, L.V. Mathematical modeling of PGPR inoculation into the rhizosphere. Environ. Model. Softw. 2006, 21, 1158–1171. [Google Scholar] [CrossRef]
- Guarino, F.; Miranda, A.; Castiglione, S.; Cicatelli, A. Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium. Chemosphere 2020, 251, 126310. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]


| Target Heavy Metals | PGPR | Test Plants | Plant-Growth-Promoting Traits/Mechanisms | References |
|---|---|---|---|---|
| Cd, Zn | Bacillus sp. | Oryza sativa | Secretes indole-3-acetic acid (IAA), 1-aminocyclopropane-1-carboxylate (ACC) deaminase, and siderophores; phosphate solubilization. | [19] |
| Ni, Pb, Cd and Cr | Pseudomonas putida | Zea mays | Increases the availability of Fe, Zn, K, and Ca. | [20] |
| Cd | Bacillus megaterium | Hybrid Pennisetum | Secretes IAA, siderophores, ACC deaminase (ACCD); phosphate solubilization and nitrogen fixation. | [21,22] |
| Cr | Pseudomonas sp. | Medicago sativa | Secretes IAA and siderophores; produces ammonia, cellulase, pectinase, chitinase, and ACCD; phosphate solubilization; nitrogen fixation. | [23] |
| Cd, Pb | Enterobacter bugandensis and Bacillus megaterium | Lactuca sativa L. | Secrete IAA and siderophores. | [24] |
| Cr | Agrobacterium fabrum | Zea mays | Secretes siderophores, IAA, and potassium; phosphate solubilization. | [25] |
| Ca | Bacillus spp. | Brassica napus L. | Secretes IAA, siderophores and ACCD; phosphate solubilization. | [26] |
| Pb | Luteibacter sp. and Variovorax sp. | Lathyrus sativus L. | Secrete IAA, siderophores, and HCN; phosphate solubilization. | [27] |
| Cr | Klebsiella sp. | - | Secretes IAA, ammonia, siderophores, and HCN. | [28] |
| Cd, Pb and As | Klebsiella michiganensis | Oryza sativa | Secretes IAA and ACCD; nitrogen fixation; phosphate solubilization. | [29] |
| As, Cd and Cr | Pseudomonas sp. | Lens culinaris | Secretes IAA. | [30] |
| Cu | Kocuria sp. | Saccharum spontaneum | Secretes IAA, product ammonia, and hydrogen cyanide (HCN); phosphate solubilization. | [31] |
| Ni | Bacillus spp. | Althea rosea L. | Secretes IAA; siderophore production; phosphate solubilization. | [32] |
| Cr, Cd | Azotobacter sp. | Lepidium sativum | Solubilizing of phosphorus; improves the dissolution and retention of iron in the growth medium; nitrogen fixation; produces plant hormones. | [33] |
| Cd | Azotobacter sp. | Triticum aestivum L. | Secretes IAA and ACCD; nitrogen fixation; phosphate solubilization. | [34] |
| As | Exiguobacterium sp. | Vigna radiata | Secretes IAA and EPS. | [35] |
| Ni, Zn and Fe | Psychrobacter sp. and Pseudomonas sp. | Brassica juncea and Ricinus communis | Secrete siderophores, ACCD, and IAA; phosphate solubilization. | [36] |
| Cd | Bradyrhizobium sp. | Lolium multiflorum Lam. | Secretes IAA, siderophores, and ACCD; phosphate solubilization. | [37] |
| Zn | Pseudomonas aeruginosa | Triticum aestivum L. | Secretes IAA, ACCD, and siderophores; phosphate solubilization. | [38] |
| Zn | Proteus mirabilis | Zea mays | Secretes IAA, siderophore, and ACCD; phosphate solubilization. | [39] |
| Cd | Ochrobactrum sp. | Oryza sativa | Secrete siderophores and ACCD. | [40] |
| Pb, As | Bacillus sp. | |||
| Ni | Bacillus subtilis | Brassica juncea | Secretes IAA; phosphate solubilization. | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, H.; Wang, Z.; Sha, W.; Song, S.; Qin, F.; Zhang, W. Role of Plant-Growth-Promoting Rhizobacteria in Plant Machinery for Soil Heavy Metal Detoxification. Microorganisms 2024, 12, 700. https://doi.org/10.3390/microorganisms12040700
Qin H, Wang Z, Sha W, Song S, Qin F, Zhang W. Role of Plant-Growth-Promoting Rhizobacteria in Plant Machinery for Soil Heavy Metal Detoxification. Microorganisms. 2024; 12(4):700. https://doi.org/10.3390/microorganisms12040700
Chicago/Turabian StyleQin, Haichen, Zixiao Wang, Wenya Sha, Shuhong Song, Fenju Qin, and Wenchao Zhang. 2024. "Role of Plant-Growth-Promoting Rhizobacteria in Plant Machinery for Soil Heavy Metal Detoxification" Microorganisms 12, no. 4: 700. https://doi.org/10.3390/microorganisms12040700
APA StyleQin, H., Wang, Z., Sha, W., Song, S., Qin, F., & Zhang, W. (2024). Role of Plant-Growth-Promoting Rhizobacteria in Plant Machinery for Soil Heavy Metal Detoxification. Microorganisms, 12(4), 700. https://doi.org/10.3390/microorganisms12040700






