Influence of Two Hexose Transporters on Substrate Affinity and Pathogenicity in Magnaporthe oryzae
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, Media and Culturing Methods
2.2. Knockout MoHXT1, MoHXT2 and MoHXT3 Using CRISPR-Cas9
2.3. Complementation Assay of MoHXTs Mutants
2.4. Yeast Mutant Complementation Experiment
2.5. Analysis of Conidial Morphology, Conidial Germination and Appressoria Formation
2.6. Pathogenicity Assay
2.7. Phylogenetic Analysis, Domain Architecture and Molecular Docking
2.8. Expression of MoHXT2/MoHXT3 in Xenopus oocytes
2.9. Rapid Site-Directed Mutagenesis
2.10. Transcriptome Sequencing and Analysis
3. Results
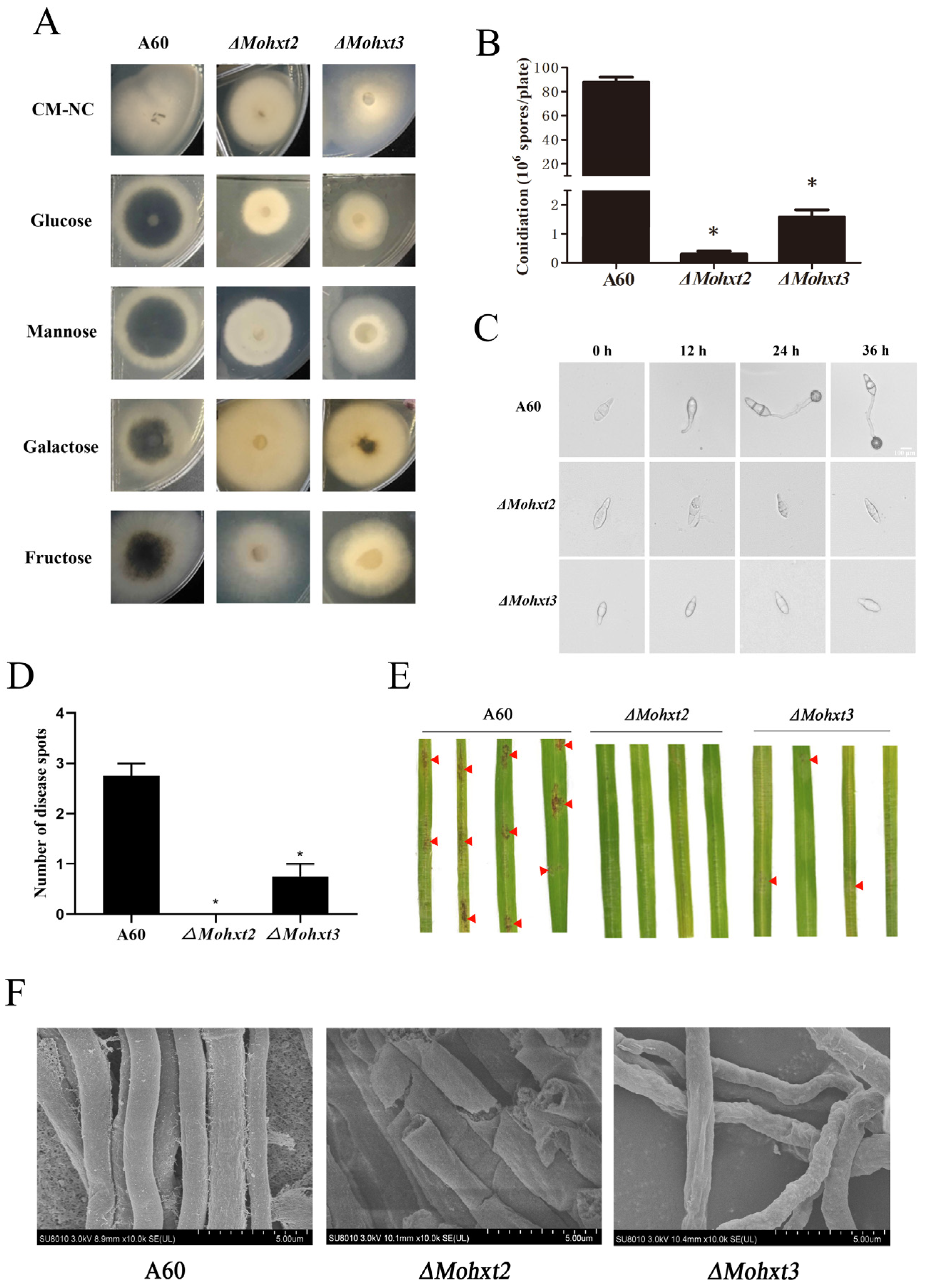
3.1. Knockout of MoHXT2 and MoHXT3, but Not MoHXT1, Affects Melanin Deposition
3.2. MoHXT2 and MoHXT3 Are Required for Conidiation, Appressorium Development, Pathogenicity and Cell Wall Integrity
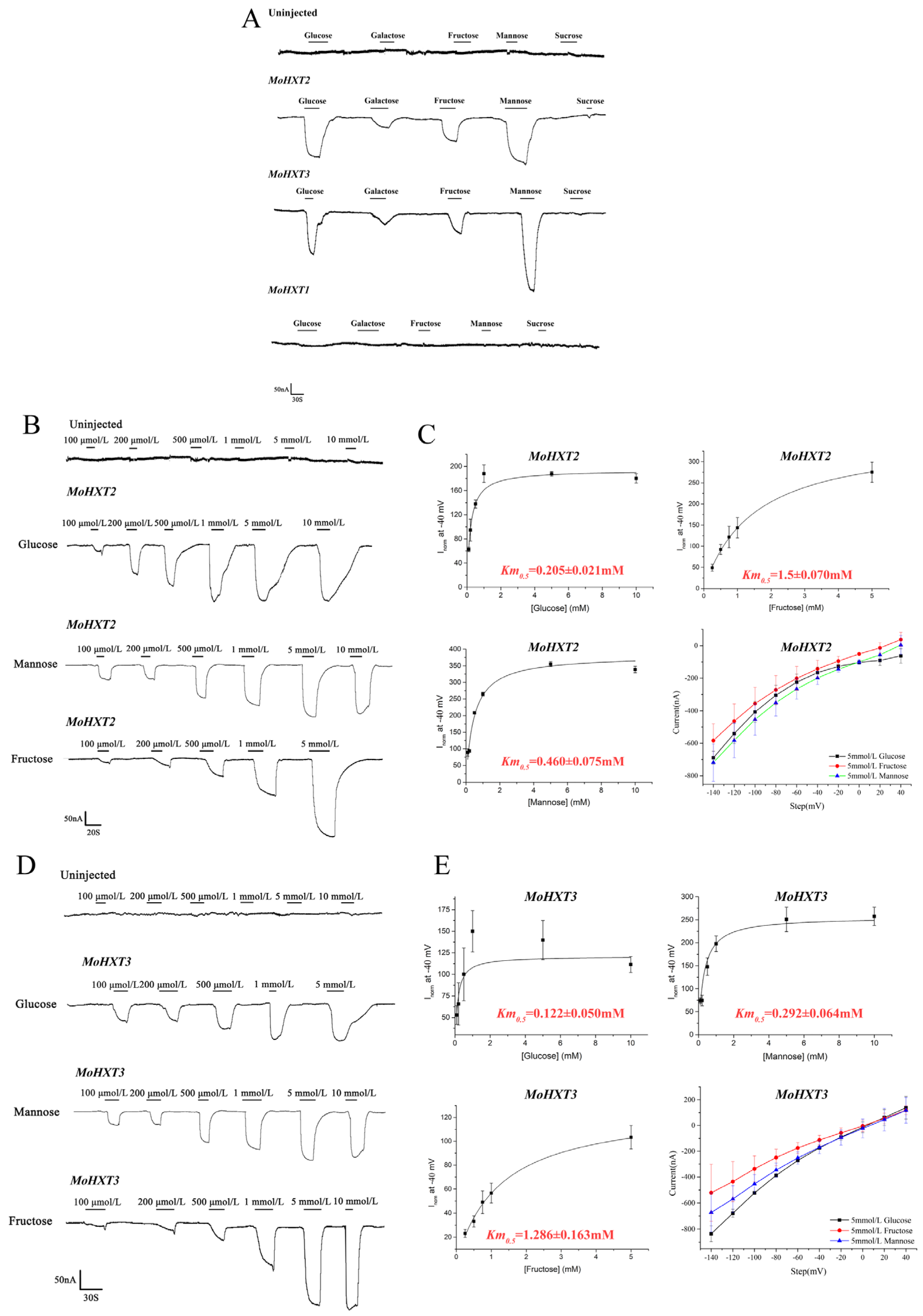
3.3. Determination of the Substrate Affinity of MoHXT2/MoHXT3 in X. oocytes
3.4. MoHXT2 and MoHXT3 Restore Sugar Transport Ability in a Monosaccharide Transport-Defected Yeast Strain
3.5. Modeling and Docking of MoHXT2 and Hexoses
3.6. Verified Amino Acid Sites Affected MoHXT2 Transport Activity
3.7. Transcription Profiles of Hexose Transporters in ΔMohxt2 and ΔMohxt3 Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.H.; Song, Y.H.; Ruan, Y.L. Sugar conundrum in plant-pathogen interactions: Roles of invertase and sugar transporters depend on pathosystems. J. Exp. Bot. 2022, 73, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.J. Living the sweet life: How does a plant pathogenic fungus acquire sugar from plants? PLoS Biol. 2010, 8, e1000308. [Google Scholar] [CrossRef] [PubMed]
- Voegele, R.T.; Struck, C.; Hahn, M.; Mendgen, K. The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc. Natl. Acad. Sci. USA 2001, 98, 8133–8138. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Lin, X.; Yao, M.; Liu, P.; Guo, J.; Huang, L.; Voegele, R.T.; Kang, Z.; Liu, J. Hexose transporter PsHXT1-mediated sugar uptake is required for pathogenicity of wheat stripe rust. Plant Biotechnol. J. 2020, 18, 2367–2369. [Google Scholar] [CrossRef] [PubMed]
- Lingner, U.; Munch, S.; Deising, H.B.; Sauer, N. Hexose transporters of a hemibiotrophic plant pathogen: Functional variations and regulatory differences at different stages of infection. J. Biol. Chem. 2011, 286, 20913–20922. [Google Scholar] [CrossRef] [PubMed]
- Wittek, A.; Dreyer, I.; Al-Rasheid, K.; Sauer, N.; Hedrich, R.; Geiger, D. The fungal UmSrt1 and maize ZmSUT1 sucrose transporters battle for plant sugar resources. J. Integr. Plant Biol. 2017, 59, 422–435. [Google Scholar] [CrossRef]
- Pujol-Gimenez, J.; Barrenetxe, J.; Gonzalez-Muniesa, P.; Lostao, M.P. The facilitative glucose transporter GLUT12: What do we know and what would we like to know? J. Physiol. Biochem. 2013, 69, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wieczorke, R.; Krampe, S.; Weierstall, T.; Freidel, K.; Hollenberg, C.P.; Boles, E. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999, 464, 123–128. [Google Scholar] [CrossRef]
- Boles, E.; Hollenberg, C.P. The molecular genetics of hexose transport in yeasts. FEMS Microbiol. Rev. 1997, 21, 85–111. [Google Scholar] [CrossRef]
- Maier, A.; Volker, B.; Boles, E.; Fuhrmann, G.F. Characterisation of glucose transport in Saccharomyces cerevisiae with plasma membrane vesicles (countertransport) and intact cells (initial uptake) with single Hxt1, Hxt2, Hxt3, Hxt4, Hxt6, Hxt7 or Gal2 transporters. FEMS Yeast Res. 2002, 2, 539–550. [Google Scholar] [CrossRef]
- Wei, H.; Vienken, K.; Weber, R.; Bunting, S.; Requena, N.; Fischer, R. A putative high affinity hexose transporter, hxtA, of Aspergillus nidulans is induced in vegetative hyphae upon starvation and in ascogenous hyphae during cleistothecium formation. Fungal Genet. Biol. 2004, 41, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Madi, L.; McBride, S.A.; Bailey, L.A.; Ebbole, D.J. rco-3, a gene involved in glucose transport and conidiation in Neurospora crassa. Genetics 1997, 146, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Wang, R.; Ning, Y.; Shi, X.; He, F.; Zhang, C.; Fan, J.; Jiang, N.; Zhang, Y.; Zhang, T.; Hu, Y.; et al. Immunity to Rice Blast Disease by Suppression of Effector-Triggered Necrosis. Curr. Biol. 2016, 26, 2399–2411. [Google Scholar] [CrossRef]
- Hong, Y.; Cai, R.; Guo, J.; Zhong, Z.; Bao, J.; Wang, Z.; Chen, X.; Zhou, J.; Lu, G.D. Carbon catabolite repressor MoCreA is required for the asexual development and pathogenicity of the rice blast fungus. Fungal Genet. Biol. 2021, 146, 103496. [Google Scholar] [CrossRef]
- Deng, Y.Z.; Naqvi, N.I. Metabolic Basis of Pathogenesis and Host Adaptation in Rice Blast. Annu. Rev. Microbiol. 2019, 73, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Hirabuchi, A.; Fujisawa, S.; Mitsuoka, C.; Terauchi, R.; Takano, Y. MoST1 encoding a hexose transporter-like protein is involved in both conidiation and mycelial melanization of Magnaporthe oryzae. FEMS Microbiol. Lett. 2014, 352, 104–113. [Google Scholar] [CrossRef]
- Xu, J.J.; Chen, J.; Zheng, K.L.; Lu, G.D. The bioinformatic analysis of the hexose transporters in Magnaporthe Oryzae. WuYi Sci. J. 2014, 30, 187–194. [Google Scholar]
- Liu, M.; Zhang, Z. Endocytosis Detection in Magnaporthe oryzae. Bio-Protocol 2019, 9, e3322. [Google Scholar] [CrossRef]
- Foster, A.J.; Martin-Urdiroz, M.; Yan, X.; Wright, H.S.; Soanes, D.M.; Talbot, N.J. CRISPR-Cas9 ribonucleoprotein-mediated co-editing and counterselection in the rice blast fungus. Sci. Rep. 2018, 8, 14355. [Google Scholar] [CrossRef]
- Talbot, N.J.; Salch, Y.P.; Ma, M.; Hamer, J.E. Karyotypic Variation within Clonal Lineages of the Rice Blast Fungus, Magnaporthe grisea. Appl. Environ. Microb. 1993, 59, 585–593. [Google Scholar] [CrossRef]
- Ding, S.L.; Liu, W.; Iliuk, A.; Ribot, C.; Vallet, J.; Tao, A.; Wang, Y.; Lebrun, M.H.; Xu, J.R. The tig1 histone deacetylase complex regulates infectious growth in the rice blast fungus Magnaporthe oryzae. Plant Cell 2010, 22, 2495–2508. [Google Scholar] [CrossRef]
- Lin, F.; Chen, S.; Que, Z.; Wang, L.; Liu, X.; Pan, Q. Blast Resistance Gene Pi37 Encodes a Nucleotide Binding Site-Leucine-Rich Repeat Protein and Is a Member of a Resistance Gene Cluster on Rice Chromosome 1. Genetics 2007, 177, 1871–1880. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Y.; Ren, Z.F.; Ganeteg, U.; Yao, G.K.; Li, Z.L.; Huang, T.; Li, J.H.; Tian, Y.Q.; Lin, F.; et al. AtLHT1 Transporter Can Facilitate the Uptake and Translocation of a Glycinergic-Chlorantraniliprole Conjugate in Arabidopsis thaliana. J. Agric. Food Chem. 2018, 66, 12527–12535. [Google Scholar] [CrossRef]
- Heredia, C.F. Impairment by hexoses of the utilization of maltose by Saccharomyces cerevisiae. Biochim. Biophys. Acta 1998, 1425, 151–158. [Google Scholar] [CrossRef]
- Raja, M.; Puntheeranurak, T.; Gruber, H.J.; Hinterdorfer, P.; Kinne, R.K.H. The role of transporter ectodomains in drug recognition and binding: Phlorizin and the sodium–glucose cotransporter. MedChemComm 2016, 7, 1056–1068. [Google Scholar] [CrossRef][Green Version]
- Chen, D.; Kamran, M.; Chen, S.; Xing, J.; Qu, Z.; Liu, C.; Ren, Z.; Cai, X.; Chen, X.; Xu, J. Two nucleotide sugar transporters are important for cell wall integrity and full virulence of Magnaporthe oryzae. Mol. Plant Pathol. 2023, 24, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, F.; He, B.; Hu, J.; Dai, Y.; Chen, W.; Yi, M.; Zhang, H.; Ye, Y.; Cui, Z.; et al. Targeting Magnaporthe oryzae effector MoErs1 and host papain-like protease OsRD21 interaction to combat rice blast. Nat. Plants 2024. [Google Scholar] [CrossRef] [PubMed]

| Receptor | Ligand | Docking Scores a (Kcal/mol) | Key Residues |
|---|---|---|---|
| MoHXT2 | D-Glucose | −4.46 | Met145/Ala89/Lys421 |
| MoHXT2 | D-Fructose | −5.11 | Met145/Lys421/Ala420 |
| MoHXT2 | D-Galactose | −4.37 | Met145/Arg97/Ser148/Glu149 |
| MoHXT2 | D-Mannose | −5.10 | Gln164/Gln299/Gln300 |
| MoHXT2 | Phlorizin dihydrate | −7.02 | Met145/Gln149/Arg156 |
| Combination of Comparisons | Enriched KEGG Terms | Number of Genes | p Value |
|---|---|---|---|
| A60 vs. ΔMoHXT2 | Metabolic pathways | 78 | 1.2 × 10−0.5 |
| Starch and sucrose metabolism | 11 | 0.018 | |
| Glutathione metabolism | 7 | 0.028 | |
| Phenylalanine metabolism | 6 | 0.031 | |
| Total: 102 | |||
| A60 vs. ΔMoHXT3 | Metabolic pathways | 72 | 4 × 10−0.6 |
| Tyrosine metabolism | 12 | 7.1 × 10−0.6 | |
| Valine, leucine and isoleucine degradation | 9 | 0.0015 | |
| Biosynthesis of secondary metabolites | 34 | 0.0024 | |
| Tryptophan metabolism | 8 | 0.0025 | |
| Fatty acid degradation | 7 | 0.0025 | |
| Amino sugar and nucleotide sugar metabolism | 10 | 0.0047 | |
| Glutathione metabolism | 7 | 0.0048 | |
| Phenvlalanine metabolism | 6 | 0.0069 | |
| Ubiquinone and other terpenoid–quinone biosynthesis | 4 | 0.007 | |
| Biosynthesis of antibiotics | 23 | 0.026 | |
| Glycosphingolipid biosynthesis | 3 | 0.029 | |
| Total: 195 | |||
| ΔMoHXT2 vs. ΔMoHXT3 | Metabolic pathways | 43 | 0.00038 |
| Tyrosine metabolism | 7 | 0.0028 | |
| Taurine and hypotaurine metabolism | 3 | 0.042 | |
| Total: 53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Guo, D.; Luo, X.; Chen, R.; Wang, W.; Xu, H.; Chen, S.; Lin, F. Influence of Two Hexose Transporters on Substrate Affinity and Pathogenicity in Magnaporthe oryzae. Microorganisms 2024, 12, 681. https://doi.org/10.3390/microorganisms12040681
Huang T, Guo D, Luo X, Chen R, Wang W, Xu H, Chen S, Lin F. Influence of Two Hexose Transporters on Substrate Affinity and Pathogenicity in Magnaporthe oryzae. Microorganisms. 2024; 12(4):681. https://doi.org/10.3390/microorganisms12040681
Chicago/Turabian StyleHuang, Tinghong, Dekang Guo, Xiao Luo, Ronghua Chen, Wenjuan Wang, Hanhong Xu, Shen Chen, and Fei Lin. 2024. "Influence of Two Hexose Transporters on Substrate Affinity and Pathogenicity in Magnaporthe oryzae" Microorganisms 12, no. 4: 681. https://doi.org/10.3390/microorganisms12040681
APA StyleHuang, T., Guo, D., Luo, X., Chen, R., Wang, W., Xu, H., Chen, S., & Lin, F. (2024). Influence of Two Hexose Transporters on Substrate Affinity and Pathogenicity in Magnaporthe oryzae. Microorganisms, 12(4), 681. https://doi.org/10.3390/microorganisms12040681






