Yeast Diversity in Honey and Pollen Samples from Stingless Bees in the State of Bahia, Brazil: Use of the MALDI-TOF MS/Genbank Proteomic Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Dilution, Plating, and Isolation
2.3. Preservation and Reactivation of Yeasts
2.4. Protein Extraction
2.5. Identification of Yeast Species by MALDI-TOF MS
2.5.1. Acquisition of Mass Spectra
2.5.2. Statistical/Molecular Analysis of Mass Spectra
3. Results
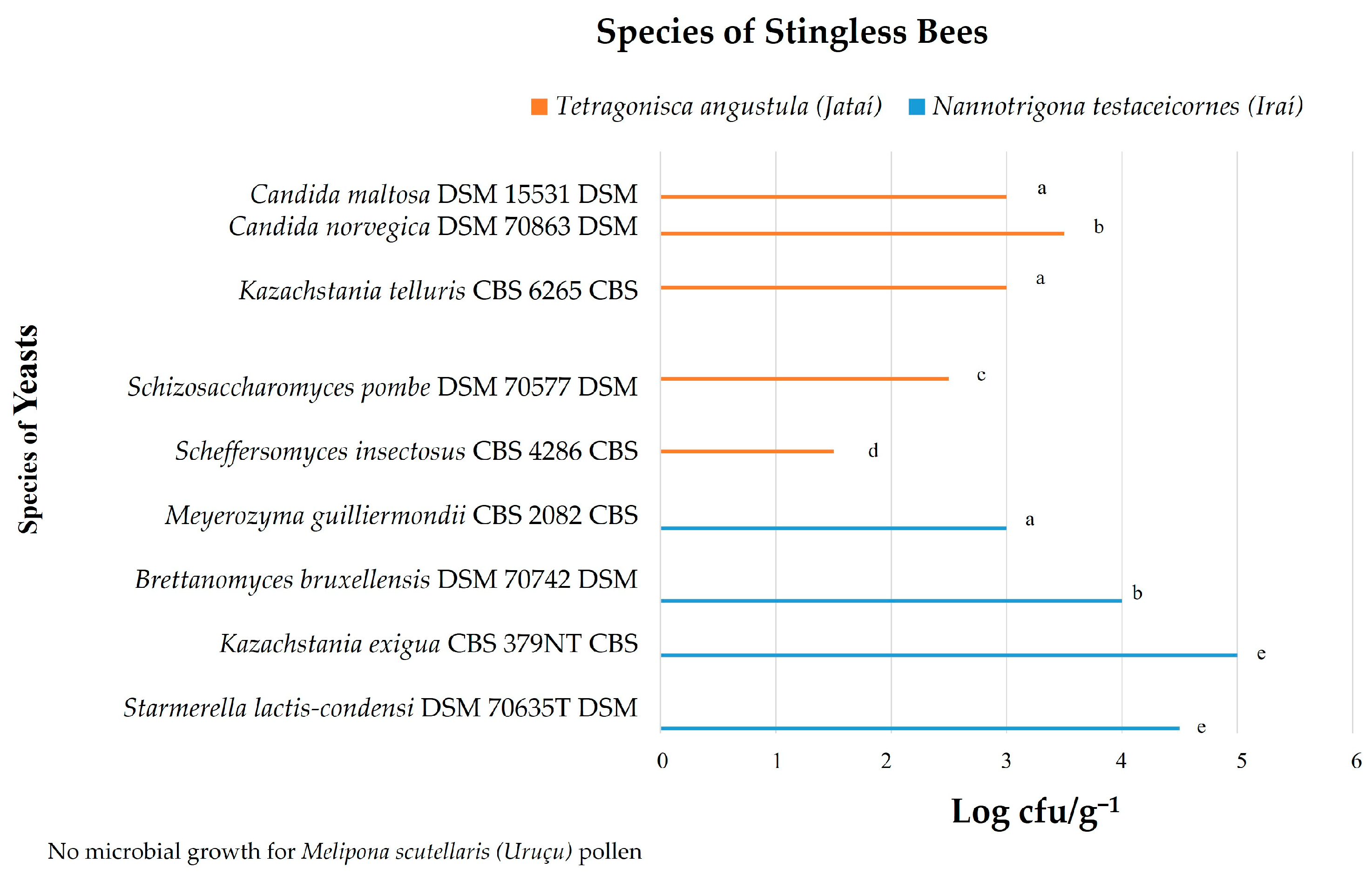
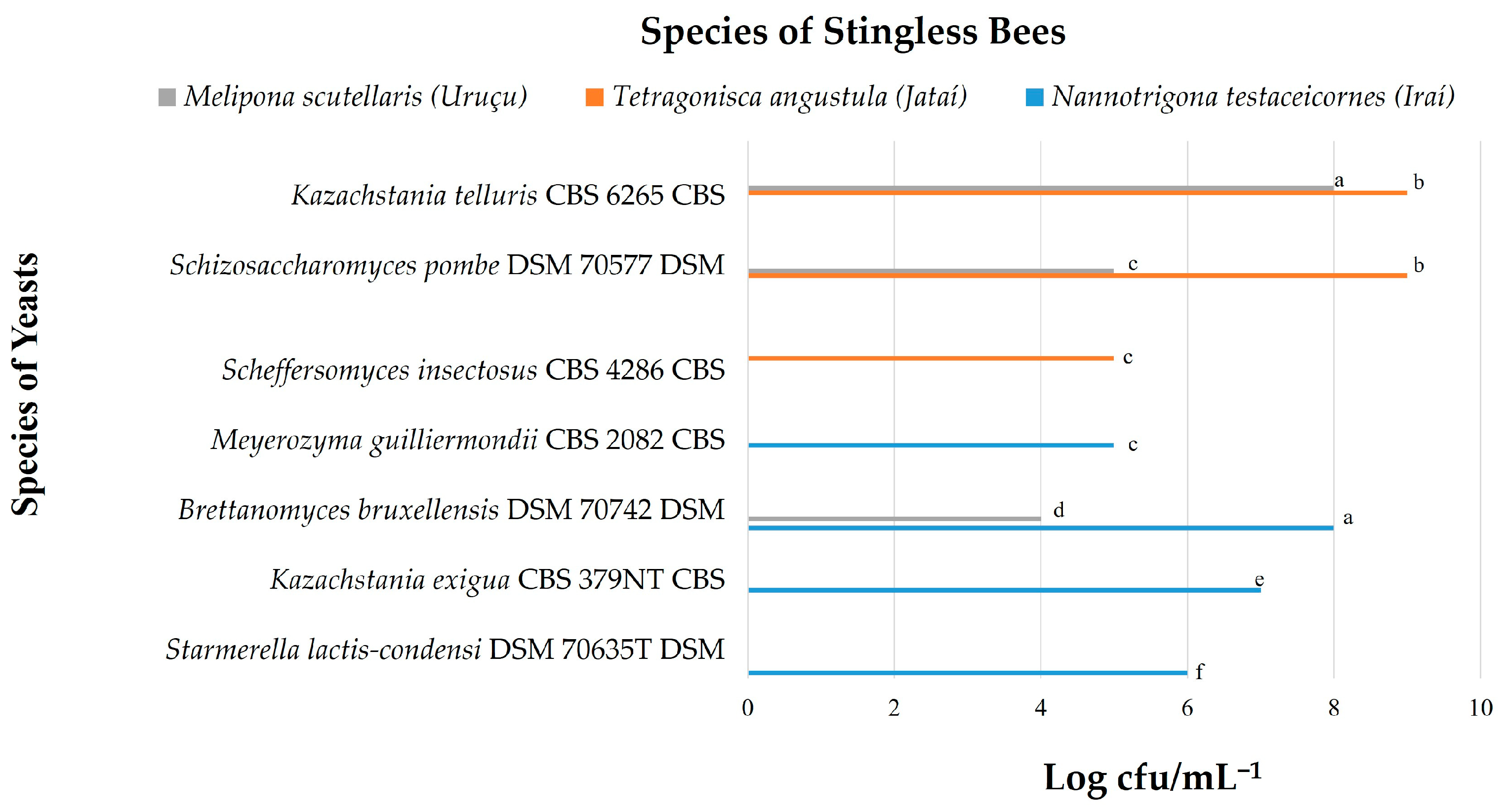
3.1. Yeast Count
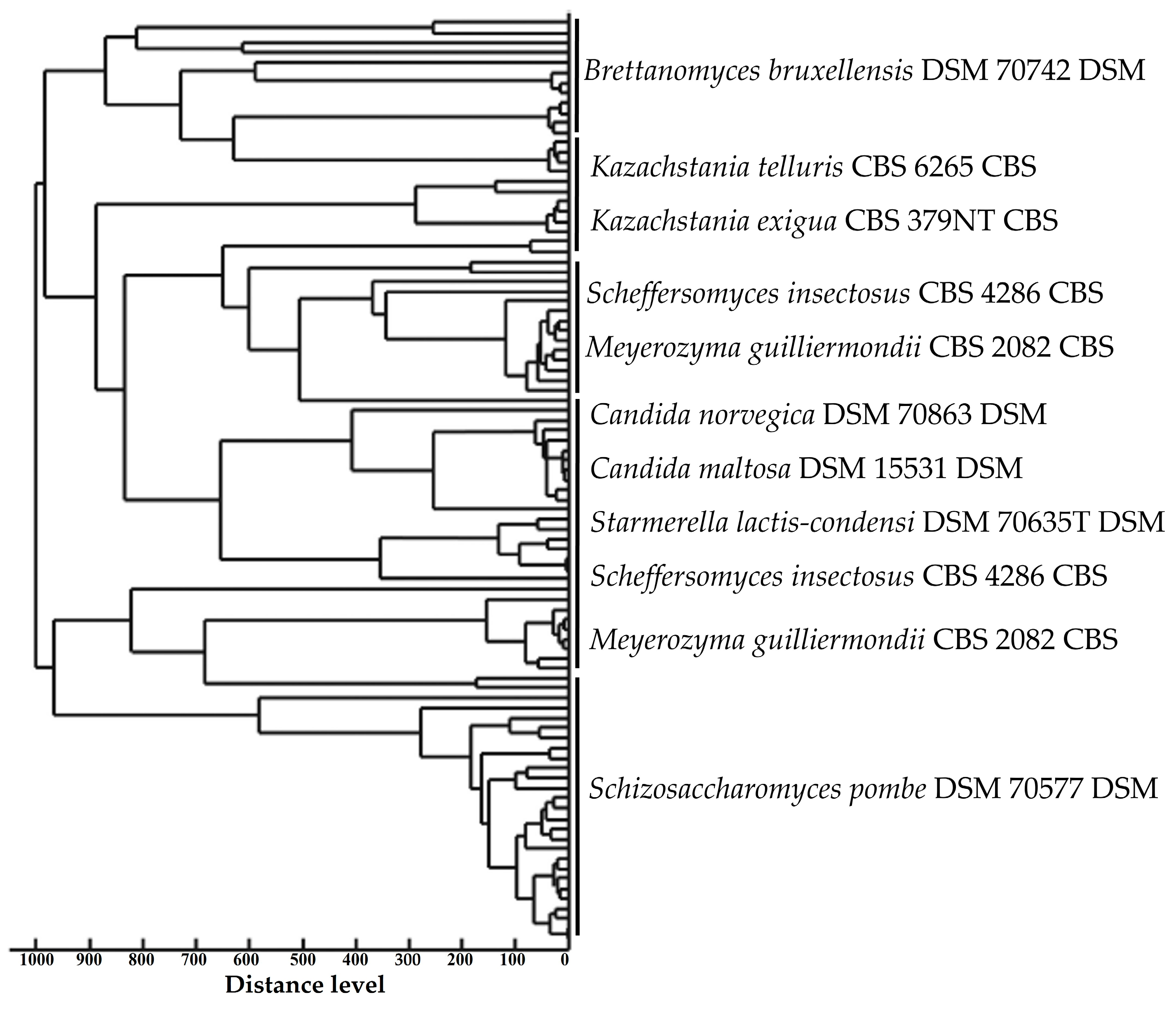
3.2. Yeast Proteomic Identification Using MALDI-TOF MS/Genbank
4. Discussion
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hrncir, M.; Jarau, S.; Barth, F.G. Stingless Bees (Meliponini): Senses and Behavior. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2016, 202, 597–601. [Google Scholar] [CrossRef]
- Rocha, V.M.; Portela, R.D.; dos Anjos, J.P.; de Souza, C.O.; Umsza-Guez, M.A. Stingless Bee Propolis: Composition, Biological Activities and Its Applications in the Food Industry. Food Prod. Process. Nutr. 2023, 5, 29. [Google Scholar] [CrossRef]
- Souza, E.C.A.; Menezes, C.; Flach, A. Stingless Bee Honey (Hymenoptera, Apidae, Meliponini): A Review of Quality Control, Chemical Profile, and Biological Potential. Apidologie 2021, 52, 113–132. [Google Scholar] [CrossRef]
- Rocha, V.M.; Portela, R.W.; Lacerda, L.E.; Sokolonski, A.R.; de Souza, C.O.; dos Anjos, J.P.; Nascimento, R.Q.; Umsza-Guez, M.A. Propolis from Different Brazilian Stingless Bee Species: Phenolic Composition and Antimicrobial Activity. Food Prod. Process. Nutr. 2024, 21, 6–17. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and Health in Honey Bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Schmitt, A.J.; Thomas, J.B.; Carter, C.J. Review: Nectar Biology: From Molecules to Ecosystems. Plant Sci. 2017, 262, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Villas-Bôas, J. Manual de Aproveitamento Integral dos Produtos das Abelhas Nativas Sem Ferrão; Instituto Sociedade, População e Natureza: Brasília, Brzail, 2018; ISBN 9788563288080. [Google Scholar]
- Simone-Finstrom, M.; Spivak, M. Propolis and Bee Health: The Natural History and Significance of Resin Use by Honey Bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Elias-Santos, D.; Fialho, M.D.C.Q.; Vitorino, R.; Oliveira, L.L.; Zanuncio, J.C.; Serrão, J.E. Proteome of the Head and Thorax Salivary Glands in the Stingless Bee Melipona Quadrifasciata Anthidioides. Apidologie 2013, 44, 684–698. [Google Scholar] [CrossRef]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical Profiles of Stingless Bee (Apidae: Meliponini) Honey from South East Asia (Thailand). Food Chem. 2016, 192, 149–155. [Google Scholar] [CrossRef] [PubMed]
- de Almeida-Muradian, L.B.; Stramm, K.M.; Estevinho, L.M. Efficiency of the FT-IR ATR Spectrometry for the Prediction of the Physicochemical Characteristics of Melipona subnitida Honey and Study of the Temperature’s Effect on Those Properties. Int. J. Food Sci. Technol. 2014, 49, 188–195. [Google Scholar] [CrossRef]
- Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Physicochemical Profiles, Minerals and Bioactive Compounds of Stingless Bee Honey (Meliponinae). J. Food Compos. Anal. 2016, 50, 61–69. [Google Scholar] [CrossRef]
- Ávila, S.; Beux, M.R.; Ribani, R.H.; Zambiazi, R.C. Stingless Bee Honey: Quality Parameters, Bioactive Compounds, Health-Promotion Properties and Modification Detection Strategies. Trends Food Sci. Technol. 2018, 81, 37–50. [Google Scholar] [CrossRef]
- Da Silva, R.N.A.; Magalhães-Guedes, K.T.; de Souza, C.O.; Alves, R.M.dO.; Umsza-Guez, M.A. Microbiological and Physical-Chemical Characteristics of Pollen and Honey from Stingless Bees: A Review. Food Prod. Process. Nutr. 2024, 2, 1–12. [Google Scholar]
- De Paula, G.T.; Menezes, C.; Pupo, M.T.; Rosa, C.A. Stingless Bees and Microbial Interactions. Curr. Opin. Insect Sci. 2021, 44, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal Insects: Diversity and Function of Resident Microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.; Vollet-Neto, A.; Contrera, F.A.F.L.; Venturieri, G.C.; Imperatriz-Fonseca, V.L. The Role of Useful Microorganisms to Stingless Bees and Stingless Beekeeping. In Pot-Honey; Springer: New York, NY, USA, 2013; pp. 153–171. [Google Scholar]
- Stefanini, I. Yeast-Insect Associations: It Takes Guts. Yeast 2018, 35, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M.; Pozo, M.I.; Medrano, M. Yeasts in Nectar of an Early-Blooming Herb: Sought by Bumble Bees, Detrimental to Plant Fecundity. Ecology 2013, 94, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.A.; Lachance, M.A.; Silva, J.O.C.; Teixeira, A.C.P.; Marini, M.M.; Antonini, Y.; Martins, R.P. Yeast Communities Associated with Stingless Bees. FEMS Yeast Res. 2003, 4, 271–275. [Google Scholar] [CrossRef]
- Viana, R.O.; Magalhães-Guedes, K.T.; Braga, R.A.; Dias, D.R.; Schwan, R.F. Fermentation Process for Production of Apple-Based Kefir Vinegar: Microbiological, Chemical and Sensory Analysis. Braz. J. Microbiol. 2017, 48, 592–601. [Google Scholar] [CrossRef]
- Zhang, J.; Plowman, J.E.; Tian, B.; Clerens, S.; On, S.L.W. Application of MALDI-TOF Analysis to Reveal Diversity and Dynamics of Winemaking Yeast Species in Wild-Fermented, Organically Produced, New Zealand Pinot Noir Wine. Food Microbiol. 2021, 99, 103824. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Jeong, S.H. Maldi-Tof Mass Spectrometry Technology as a Tool for the Rapid Diagnosis of Antimicrobial Resistance in Bacteria. Antibiotics 2021, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Espinosa, R.; Costa, A.; Cejas, D.; Barrios, R.; Vay, C.; Radice, M.; Gutkind, G.; Di Conza, J. MALDI-TOF MS Based Procedure to Detect KPC-2 Directly from Positive Blood Culture Bottles and Colonies. J. Microbiol. Methods 2019, 159, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Quéro, L.; Girard, V.; Pawtowski, A.; Tréguer, S.; Weill, A.; Arend, S.; Cellière, B.; Polsinelli, S.; Monnin, V.; van Belkum, A.; et al. Development and Application of MALDI-TOF MS for Identification of Food Spoilage Fungi. Food Microbiol. 2019, 81, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Quintilla, R.; Kolecka, A.; Casaregola, S.; Daniel, H.M.; Houbraken, J.; Kostrzewa, M.; Boekhout, T.; Groenewald, M. MALDI-TOF MS as a Tool to Identify Foodborne Yeasts and Yeast-like Fungi. Int. J. Food Microbiol. 2018, 266, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.O.; Magalhães-Guedes, K.T.; Dias, D.R.; Schwan, R.F. Use of Maldi-Tof MS Biosensor in Microbial Assessment of Brazilian Kefir Grains. Rev. Ceres 2019, 66, 72–76. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Qiblawey, H.; Rodrigues, D.F.; Hu, Y.; Zouari, N. Isolation, Identification and Biodiversity of Antiscalant Degrading Seawater Bacteria Using MALDI-TOF-MS and Multivariate Analysis. Sci. Total Environ. 2019, 656, 910–920. [Google Scholar] [CrossRef]
- Lappa, I.K.; Gantzias, C.; Manolopoulou, E.; De Brandt, E.; Aerts, M.; Vandamme, P.; Tsakalidou, E.; Georgalaki, M. MALDI-TOF MS Insight into the Biodiversity of Staka, the Artisanal Cretan Soured Cream. Int. Dairy J. 2021, 116, 104969. [Google Scholar] [CrossRef]
- Ogunremi, O.R.; Freimüller Leischtfeld, S.; Miescher Schwenninger, S. MALDI-TOF MS Profiling and Exopolysaccharide Production Properties of Lactic Acid Bacteria from Kunu-Zaki—A Cereal-Based Nigerian Fermented Beverage. Int. J. Food Microbiol. 2022, 366, 109563. [Google Scholar] [CrossRef]
- Ramos, C.L.; Magalhães-Guedes, K.T. Preparing Yeast Suspension through Serial Dilution for Enumeration; Humana: New York, NY, USA, 2021; pp. 77–81. [Google Scholar]
- de Sousa, J.M.B.; de Souza, E.L.; Marques, G.; Benassi, M.d.T.; Gullón, B.; Pintado, M.M.; Magnani, M. Sugar Profile, Physicochemical and Sensory Aspects of Monofloral Honeys Produced by Different Stingless Bee Species in Brazilian Semi-Arid Region. LWT 2016, 65, 645–651. [Google Scholar] [CrossRef]
- Silva, M.S.; Rabadzhiev, Y.; Eller, M.R.; Iliev, I.; Ivanova, I.; Santana, W.C. Microorganisms in Honey. In Honey Analysis; InTech: London, UK, 2017. [Google Scholar]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts, a Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1. [Google Scholar]
- Gantzias, C.; Lappa, I.K.; Aerts, M.; Georgalaki, M.; Manolopoulou, E.; Papadimitriou, K.; De Brandt, E.; Tsakalidou, E.; Vandamme, P. MALDI-TOF MS Profiling of Non-Starter Lactic Acid Bacteria from Artisanal Cheeses of the Greek Island of Naxos. Int. J. Food Microbiol. 2020, 323, 108586. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Oualha, M.; Ashfaq, M.Y.; Suleiman, M.T.; Zouari, N. Isolation, Differentiation and Biodiversity of Ureolytic Bacteria of Qatari Soil and Their Potential in Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. RSC Adv. 2018, 8, 5854–5863. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.M.; de Souza, A.C.; Assis, D.M.; Almeida, A.C.O.; Oliveira, C.d.M.S.; Junior, L.A.A.P.; Carvalho, M.D.G.D.S.; Santos, G.B.; Amaral, P.I.S.; Oliveira, W.R.M.; et al. Zoothentic Evaluation of Tilapian Alevines Feed Fed with Kefir. Res. Soc. Dev. 2021, 10, e10610212209. [Google Scholar] [CrossRef]
- Paludo, C.R.; Menezes, C.; Silva-Junior, E.A.; Vollet-Neto, A.; Andrade-Dominguez, A.; Pishchany, G.; Khadempour, L.; Do Nascimento, F.S.; Currie, C.R.; Kolter, R.; et al. Stingless Bee Larvae Require Fungal Steroid to Pupate. Sci. Rep. 2018, 8, 1122. [Google Scholar] [CrossRef] [PubMed]
- Van Arnam, E.B.; Ruzzini, A.C.; Sit, C.S.; Horn, H.; Pinto-Tomás, A.A.; Currie, C.R.; Clardy, J. Selvamicin, an Atypical Antifungal Polyene from Two Alternative Genomic Contexts. Proc. Natl. Acad. Sci. USA 2016, 113, 12940–12945. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, É.W.; Message, D.; Meira, R.M.S.A.; Salantino, A. Indicadores Da Origem Botânica Da Própolis Importância E Perspectivas. Bol. Indústria Anim. 2003, 60, 83–106. [Google Scholar]
- Daniel, H.-M.; Rosa, C.A.; Thiago-Calaça, P.S.S.; Antonini, Y.; Bastos, E.M.A.F.; Evrard, P.; Huret, S.; Fidalgo-Jiménez, A.; Lachance, M.-A. Starmerella neotropicalis f. a., sp. nov., a Yeast Species Found in Bees and Pollen. Int. J. Syst. Evol. Microbiol. 2013, 63, 3896–3903. [Google Scholar] [CrossRef]
- Estevinho, L.M.; Rodrigues, S.; Pereira, A.P.; Feás, X. Portuguese Bee Pollen: Palynological Study, Nutritional and Microbiological Evaluation. Int. J. Food Sci. Technol. 2012, 47, 429–435. [Google Scholar] [CrossRef]
- Rózańska, H.; Osek, J. Effect of Storage on Microbiological Quality of Honey. Bull. Vet. Inst. Pulawy 2012, 56, 161–163. [Google Scholar] [CrossRef]
- Brasil. Available online: https://Www.Dourados.Ms.Gov.Br/Wp-Content/Uploads/2016/05/RTIQ-Mel-Completo-IN-11_2000.Pdf (accessed on 20 January 2023).
- Brasil. Available online: https://Antigo.Anvisa.Gov.Br/Documents/10181/2718376/IN_161_2022_.Pdf/B08d70cb-Add6-47e3-A5d3-Fa317c2d54b2 (accessed on 20 January 2023).
- Brasil. Available online: https://Antigo.Anvisa.Gov.Br/Documents/10181/2718376/RDC_724_2022_.Pdf/33c61081-4f32-43c2-9105-C318fa6069ce (accessed on 20 January 2023).
- Sinacori, M.; Francesca, N.; Alfonzo, A.; Cruciata, M.; Sannino, C.; Settanni, L.; Moschetti, G. Cultivable Microorganisms Associated with Honeys of Different Geographical and Botanical Origin. Food Microbiol. 2014, 38, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Seijo, M.C.; Escuredo, O.; Fernández-González, M. Fungal Diversity in Honeys from Northwest Spain and Their Relationship to the Ecological Origin of the Product. Grana 2011, 50, 55–62. [Google Scholar] [CrossRef]
- Fernandes, R.T.; Rosa, I.G.; Conti-Silva, A.C. Microbiological and Physical-Chemical Characteristics of Honeys from the Bee Melipona Fasciculata Produced in Two Regions of Brazil. Cienc. Rural 2018, 48, e20180025. [Google Scholar] [CrossRef]
- Barbosa, R.N.; Bezerra, J.; Souza-Motta, C.; Gomes, B.S.; Costa, C.; Melo, H. Prospection on Yeasts from Stingless Bees Honey in BrazilianTropical Dry Forest (Caatinga). Gaia Sci. 2016, 10, 151–159. [Google Scholar] [CrossRef]
- Zhang, J.; Plowman, J.E.; Tian, B.; Clerens, S.; On, S.L.W. An Improved Method for MALDI-TOF Analysis of Wine-Associated Yeasts. J. Microbiol. Methods 2020, 172, 105904. [Google Scholar] [CrossRef] [PubMed]
- Borneman, A.R.; Zeppel, R.; Chambers, P.J.; Curtin, C.D. Insights into the Dekkera bruxellensis Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates. PLoS Genet. 2014, 10, e1004161. [Google Scholar] [CrossRef]
- Crauwels, S.; Van Opstaele, F.; Jaskula-Goiris, B.; Steensels, J.; Verreth, C.; Bosmans, L.; Paulussen, C.; Herrera-Malaver, B.; de Jonge, R.; De Clippeleer, J.; et al. Fermentation Assays Reveal Differences in Sugar and (off-) Flavor Metabolism across Different Brettanomyces bruxellensis Strains. FEMS Yeast Res. 2017, 17, fow105. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces Yeasts—From Spoilage Organisms to Valuable Contributors to Industrial Fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef]
- Spitaels, F.; Wieme, A.D.; Janssens, M.; Aerts, M.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. The Microbial Diversity of an Industrially Produced Lambic Beer Shares Members of a Traditionally Produced One and Reveals a Core Microbiota for Lambic Beer Fermentation. Food Microbiol. 2015, 49, 23–32. [Google Scholar] [CrossRef]
- Landis, E.A.; Fogarty, E.; Edwards, J.C.; Popa, O.; Eren, A.M.; Wolfe, B.E. Microbial Diversity and Interaction Specificity in Kombucha Tea Fermentations. mSystems 2022, 7, e0015722. [Google Scholar] [CrossRef]
- Angela, C.; Young, J.; Kordayanti, S.; Devanthi, P.V.P. Isolation and Screening of Microbial Isolates from Kombucha Culture for Bacterial Cellulose Production in Sugarcane Molasses Medium. KnE Life Sci. 2020, 5, 111–127. [Google Scholar] [CrossRef]
- Schifferdecker, A.J.; Dashko, S.; Ishchuk, O.P.; Piškur, J. The Wine and Beer Yeast Dekkera bruxellensis. Yeast 2014, 31, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling Microbial Ecology of Industrial-Scale Kombucha Fermentations by Metabarcoding and Culture-Based Methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef] [PubMed]
- Longin, C.; Degueurce, C.; Julliat, F.; Guilloux-Benatier, M.; Rousseaux, S.; Alexandre, H. Efficiency of Population-Dependent Sulfite against Brettanomyces bruxellensis in Red Wine. Food Res. Int. 2016, 89, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Jeffares, D.; Palomero, F.; Calderón, F.; Bai, F.Y.; Bähler, J.; Benito, S. Selected Schizosaccharomyces pombe Strains Have Characteristics That Are Beneficial for Winemaking. PLoS ONE 2016, 11, e0151102. [Google Scholar] [CrossRef] [PubMed]
- Brysch-Herzberg, M.; Jia, G.S.; Seidel, M.; Assali, I.; Du, L.L. Insights into the Ecology of Schizosaccharomyces Species in Natural and Artificial Habitats. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2022, 115, 661–695. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.C.O.; Pataro, C.; Guerra, J.B.; Neves, M.J.; Corrêa, S.R.; Moreira, E.S.A.; Rosa, C.A. Physiological Diversity and Trehalose Accumulation in Schizosaccharomyces pombe Strains Isolated from Spontaneous Fermentations during the Production of the Artisanal Brazilian Cachaça. Can. J. Microbiol. 2002, 48, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Morata, A.; Palomero, F.; González, C.; Suárez-Lepe, J.A. Schizosaccharomyces pombe: A Promising Biotechnology for Modulating Wine Composition. Fermentation 2018, 4, 70. [Google Scholar] [CrossRef]
- Minnaar, P.P.; Jolly, N.P.; Paulsen, V.; Du Plessis, H.W.; Van Der Rijst, M. Schizosaccharomyces pombe and Saccharomyces cerevisiae Yeasts in Sequential Fermentations: Effect on Phenolic Acids of Fermented Kei-Apple (Dovyalis caffra L.) Juice. Int. J. Food Microbiol. 2017, 257, 232–237. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, Y.; Zhang, J.; Zeng, W.; Yang, Y.; Guan, J.; Pan, L.; Li, W. Enhanced Deacidification Activity in Schizosaccharomyces pombe by Genome Shuffling. Yeast 2015, 32, 317–325. [Google Scholar] [CrossRef]
- Pereira, E.L.; Ramalhosa, E.; Borges, A.; Pereira, J.A.; Baptista, P. YEAST Dynamics during the Natural Fermentation Process of Table Olives (Negrinha de Freixo cv.). Food Microbiol. 2015, 46, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.; Ramalhosa, E.; Nunes, L.; Pereira, J.A.; Colla, E.; Pereira, E.L. Probiotic Potential of Indigenous Yeasts Isolated during the Fermentation of Table Olives from Northeast of Portugal. Innov. Food Sci. Emerg. Technol. 2017, 44, 167–172. [Google Scholar] [CrossRef]
- Lachance, M.-A.; Starmer, W.T.; Rosa, C.A.; Bowles, J.M.; Barker, J.S.F.; Janzen, D.H. Biogeography of the Yeasts of Ephemeral Flowers and Their Insects. FEMS Yeast Res. 2001, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Úbeda, J.; Maldonado Gil, M.; Chiva, R.; Guillamón, J.M.; Briones, A. Biodiversity of Non-Saccharomyces Yeasts in Distilleries of the La Mancha Region (Spain). FEMS Yeast Res. 2014, 14, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Agnolucci, M.; Tirelli, A.; Cocolin, L.; Toffanin, A. Brettanomyces bruxellensis Yeasts: Impact on Wine and Winemaking. World J. Microbiol. Biotechnol. 2017, 33, 180. [Google Scholar] [CrossRef]
- Csoma, H.; Sipiczki, M. Taxonomic Reclassification of Candida stellata Strains Reveals Frequent Occurrence of Candida Zemplinina in Wine Fermentation. FEMS Yeast Res. 2008, 8, 328–336. [Google Scholar] [CrossRef]
- Thompson, S. Microbiological Spoilage of High-Sugar Products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2009; pp. 301–324. [Google Scholar]
- Csoma, H.; Kállai, Z.; Antunovics, Z.; Czentye, K.; Sipiczki, M. Vinification without Saccharomyces: Interacting Osmotolerant and “Spoilage” Yeast Communities in Fermenting and Ageing Botrytised High-Sugar Wines (Tokaj Essence). Microorganisms 2021, 9, 19. [Google Scholar] [CrossRef]
- Combina, M.; Daguerre, C.; Massera, A.; Mercado, L.; Sturm, M.E.; Ganga, A.; Martinez, C. Yeast Identification in Grape Juice Concentrates from Argentina. Lett. Appl. Microbiol. 2008, 46, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Rojo, M.C.; Arroyo López, F.N.; Lerena, M.C.; Mercado, L.; Torres, A.; Combina, M. Effects of PH and Sugar Concentration in Zygosaccharomyces rouxii Growth and Time for Spoilage in Concentrated Grape Juice at Isothermal and Non-Isothermal Conditions. Food Microbiol. 2014, 38, 143–150. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Z.; Long, F.; Niu, C.; Yuan, Y.; Yue, T. Characterization of Osmotolerant Yeasts and Yeast-Like Molds from Apple Orchards and Apple Juice Processing Plants in China and Investigation of Their Spoilage Potential. J. Food Sci. 2015, 80, M1850–M1860. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage Yeasts in the Wine Industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Solieri, L.; Giudici, P. Yeasts Associated to Traditional Balsamic Vinegar: Ecological and Technological Features. Int. J. Food Microbiol. 2008, 125, 36–45. [Google Scholar] [CrossRef]
- Ece, G.; Samlioglu, P.; Akkoclu, G.; Atalay, S.; Kose, S. The Evaluation of the Distribution of Yeast like Fungi “Candida Species” at a Tertiary Care Center in Western Turkey. Int. J. Med. Sci. 2012, 9, 617–620. [Google Scholar] [CrossRef]
- Guo, N.; Gong, F.; Chi, Z.; Sheng, J.; Li, J. Enhanced Inulinase Production in Solid State Fermentation by a Mutant of the Marine Yeast Pichia guilliermondii Using Surface Response Methodology and Inulin Hydrolysis. J. Ind. Microbiol. Biotechnol. 2009, 36, 499–507. [Google Scholar] [CrossRef]
- Schirmer-Michel, Â.C.; Flôres, S.H.; Hertz, P.F.; Matos, G.S.; Ayub, M.A.Z. Production of Ethanol from Soybean Hull Hydrolysate by Osmotolerant Candida guilliermondii NRRL Y-2075. Bioresour. Technol. 2008, 99, 2898–2904. [Google Scholar] [CrossRef]
- Wah, T.T.; Walaisri, S.; Assavanig, A.; Niamsiri, N.; Lertsiri, S. Co-Culturing of Pichia guilliermondii Enhanced Volatile Flavor Compound Formation by Zygosaccharomyces rouxii in the Model System of Thai Soy Sauce Fermentation. Int. J. Food Microbiol. 2013, 160, 282–289. [Google Scholar] [CrossRef]
- Papon, N.; Savini, V.; Lanoue, A.; Simkin, A.J.; Crèche, J.; Giglioli-Guivarc’H, N.; Clastre, M.; Courdavault, V.; Sibirny, A.A. Candida guilliermondii: Biotechnological Applications, Perspectives for Biological Control, Emerging Clinical Importance and Recent Advances in Genetics. Curr. Genet. 2013, 59, 73–90. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Cadete, R.M.; Gomes, F.C.O.; Lachance, M.-A.; Rosa, C.A. Candida materiae sp. nov., a Yeast Species Isolated from Rotting Wood in the Atlantic Rain Forest. Int. J. Syst. Evol. Microbiol. 2009, 59, 2104–2106. [Google Scholar] [CrossRef] [PubMed]
- Dashtban, M.; Schraft, H.; Qin, W. Fungal Bioconversion of Lignocellulosic Residues; Opportunities & Per-Spectives. Int. J. Biol. Sci. 2009, 5, 578–595. [Google Scholar] [PubMed]
- Awe, S.; Mikolasch, A.; Hammer, E.; Schauer, F. Degradation of Phenylalkanes and Characterization of Aromatic Intermediates Acting as Growth Inhibiting Substances in Hydrocarbon Utilizing Yeast Candida Maltosa. Int. Biodeterior. Biodegrad. 2008, 62, 408–414. [Google Scholar] [CrossRef]
- Dmitriev, V.V.; Crowley, D.; Rogachevsky, V.V.; Negri, C.M.; Rusakova, T.G.; Kolesnikova, S.A.; Akhmetov, L.I. Microorganisms Form Exocellular Structures, Trophosomes, to Facilitate Biodegradation of Oil in Aqueous Media. FEMS Microbiol. Lett. 2011, 315, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Jacques, N.; Sarilar, V.; Urien, C.; Lopes, M.R.; Morais, C.G.; Uetanabaro, A.P.T.; Tinsley, C.R.; Rosa, C.A.; Sicard, D.; Casaregola, S. Three Novel Ascomycetous Yeast Species of the Kazachstania clade, Kazachstania saulgeensis sp. nov., Kazachstania serrabonitensis sp. nov. and Kazachstania australis sp. nov. Reassignment of Candida humilis to Kazachstania humilis f.a. comb. nov. and Candida pseudohumilis to Kazachstania pseudohumilis f.a. comb. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5192–5200. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Barriga, E.J.C.; Barahona, P.P.; Nueno-Palop, C.; Cross, K.; Bond, C.J.; Roberts, I.N. Kazachstania yasuniensis sp. nov., an Ascomycetous Yeast Species Found in Mainland Ecuador and on the Galápagos. Int. J. Syst. Evol. Microbiol. 2015, 65, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, J.; Höning, C.; Böhnlein, C.; Pichner, R.; Gareis, M.; Wenning, M. Kazachstania psychrophila sp. nov., a Novel Psychrophilic Yeast Isolated from Vacuum-Packed Beef. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2013, 104, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J.; Ward, J.M.; Brayton, C.; Gorelick, P.; Walsh, T.J. Multigene Phylogenetic Analysis of Pathogenic Candida Species in the Kazachstania (Arxiozyma) telluris Complex and Description of Their Ascosporic States as Kazachstania bovina sp. nov., K. Heterogenica sp. nov., K. pintolopesii sp. nov., and K. Slooffiae sp. nov. J. Clin. Microbiol. 2005, 43, 101–111. [Google Scholar] [CrossRef]
- Perez, M.F.; Isas, A.S.; Aladdin, A.; El Enshasy, H.A.; Dib, J.R. Killer Yeasts as Biocontrol Agents of Postharvest Fungal Diseases in Lemons. In Sustainable Technologies for the Management of Agricultural Wastes; Springer: Singapore, 2018; pp. 87–98. [Google Scholar]
- Urubschurov, V.; Janczyk, P.; Souffrant, W.-B.; Freyer, G.; Zeyner, A. Establishment of Intestinal Microbiota with Focus on Yeasts of Unweaned and Weaned Piglets Kept under Different Farm Conditions. FEMS Microbiol. Ecol. 2011, 77, 493–502. [Google Scholar] [CrossRef]
- Morio, F.; O’Brien, C.E.; Butler, G. Draft Genome Sequence of the Yeast Kazachstania telluris CBS 16338 Isolated from Forest Soil in Ireland. Mycopathologia 2020, 185, 587–590. [Google Scholar] [CrossRef]
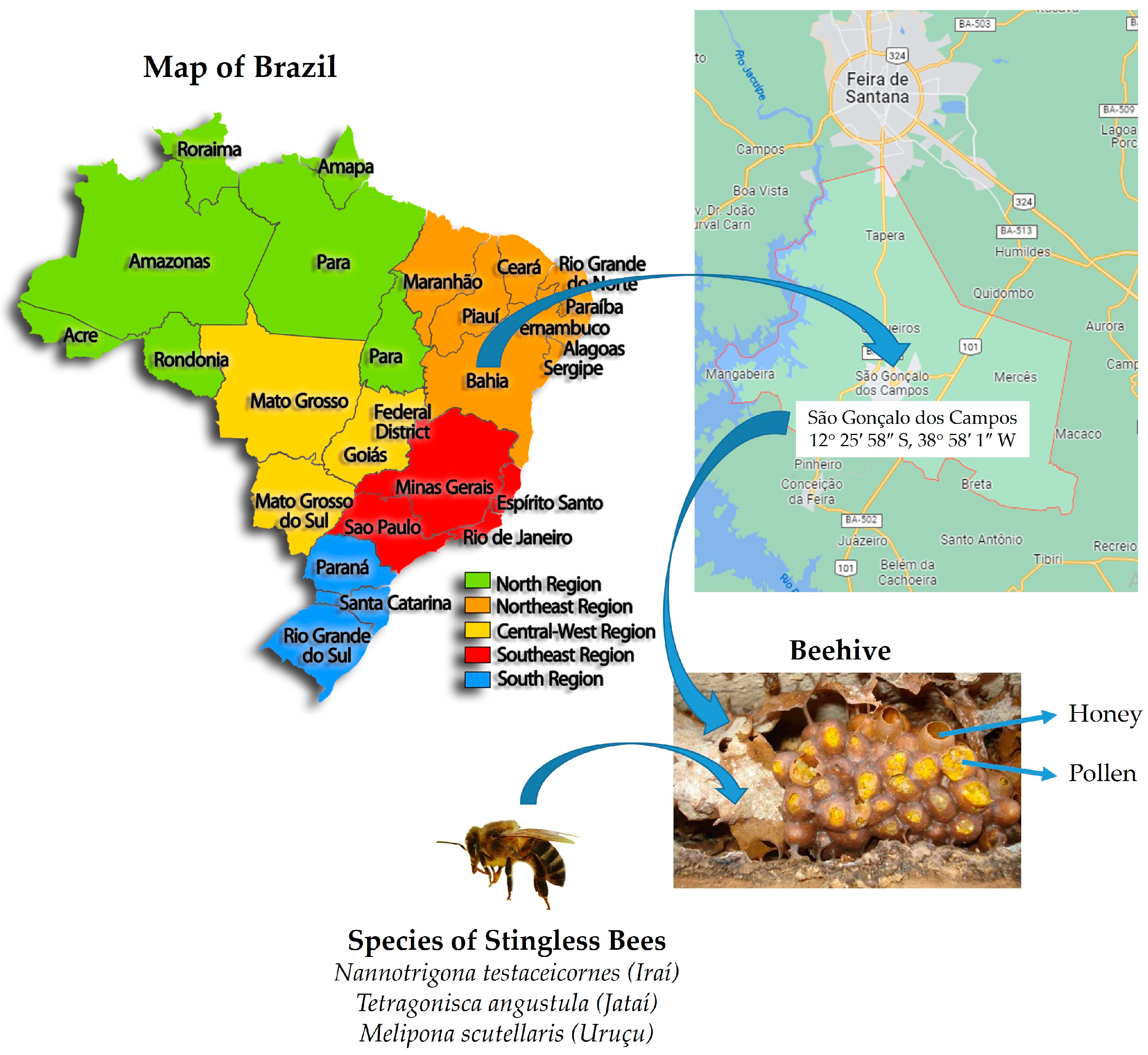
| Morphotype Code (Number of Isolates) | Sample | Stingless Bee Species (“Popular Name” in Brazil) | Culture Medium | Yeast Species | NCBI Identifier | Score Value | Symbol |
|---|---|---|---|---|---|---|---|
| 1 (230) | POLLEN | Nannotrigona testaceicornes (Iraí) | SABOURAUD | Starmerella lactis-condensi DSM 70635T DSM | NCBI:txid 45562 | 2091 | (++) |
| 2 (142) | POLLEN | Nannotrigona testaceicornes (Iraí) | SABOURAUD | Starmerella lactis-condensi DSM 70635T DSM | NCBI:txid 45562 | 2091 | (++) |
| 3 (137) | POLLEN | Nannotrigona testaceicornes (Iraí) | SABOURAUD | Kazachstania exigua CBS 379NT CBS | NCBI:txid 34358 | 2522 | (+++) |
| 4 (122) | POLLEN | Nannotrigona testaceicornes (Iraí) | YEAST EXTRACT | Brettanomyces bruxellensis DSM 70742 DSM | NCBI:txid 5007 | 2546 | (+++) |
| 5 (99) | POLLEN | Nannotrigona testaceicornes (Iraí) | YEAST EXTRACT | Meyerozyma guilliermondii CBS 2082 CBS | NCBI:txid 4929 | 2346 | (+++) |
| 6 (87) | POLLEN | Nannotrigona testaceicornes (Iraí) | YEAST EXTRACT | Brettanomyces bruxellensis DSM 70742 DSM | NCBI:txid 5007 | 2546 | (+++) |
| 7 (230) | POLLEN | Nannotrigona testaceicornes (Iraí) | MALT EXTRACT | Kazachstania exigua CBS 379NT CBS | NCBI:txid 34358 | 2522 | (+++) |
| 8 (205) | HONEY | Nannotrigona testaceicornes (Iraí) | SABOURAUD | Brettanomyces bruxellensis DSM 70742 DSM | NCBI:txid 5007 | 2546 | (+++) |
| 9 (56) | HONEY | Nannotrigona testaceicornes (Iraí) | SABOURAUD | Kazachstania exigua CBS 379NT CBS | NCBI:txid 34358 | 2091 | (++) |
| 10 (73) | HONEY | Nannotrigona testaceicornes (Iraí) | YEAST EXTRACT | Brettanomyces bruxellensis DSM 70742 DSM | NCBI:txid 5007 | 2642 | (+++) |
| 11 (34) | HONEY | Nannotrigona testaceicornes (Iraí) | YEAST EXTRACT | Brettanomyces bruxellensis DSM 70742 DSM | NCBI:txid 5007 | 2702 | (+++) |
| 12 (210) | HONEY | Nannotrigona testaceicornes (Iraí) | YEAST EXTRACT | Starmerella lactis-condensi DSM 70635T DSM | NCBI:txid 45562 | 2057 | (++) |
| 13 (82) | HONEY | Nannotrigona testaceicornes (Iraí) | YEAST EXTRACT | Kazachstania exigua CBS 379NT CBS | NCBI:txid 34358 | 2836 | (+++) |
| 14 (57) | HONEY | Nannotrigona testaceicornes (Iraí) | MALT EXTRACT | Kazachstania exigua CBS 379NT CBS | NCBI:txid 34358 | 2088 | (++) |
| 15 (32) | HONEY | Nannotrigona testaceicornes (Iraí) | MALT EXTRACT | Kazachstania exigua CBS 379NT CBS | NCBI:txid 34358 | 2736 | (+++) |
| 16 (47) | POLLEN | Tetragonisca angustula (Jataí) | SABOURAUD | Scheffersomyces insectosus CBS 4286 CBS | NCBI:txid 45590 | 2342 | (+++) |
| 17 (71) | POLLEN | Tetragonisca angustula (Jataí) | SABOURAUD | Schizosaccharomyces pombe DSM 70577 DSM | NCBI:txid 4896 | 2842 | (+++) |
| 18 (23) | POLLEN | Tetragonisca angustula (Jataí) | SABOURAUD | Kazachstania telluris CBS 6265 CBS | NCBI:txid 36907 | 2892 | (+++) |
| 19 (11) | POLLEN | Tetragonisca angustula (Jataí) | SABOURAUD | Kazachstania telluris CBS 6265 CBS | NCBI:txid 36907 | 2842 | (+++) |
| 20 (103) | POLLEN | Tetragonisca angustula (Jataí) | YEAST EXTRACT | Candida norvegica DSM 70863 DSM | NCBI:txid 49330 | 2301 | (+++) |
| 21 (52) | POLLEN | Tetragonisca angustula (Jataí) | YEAST EXTRACT | Candida maltosa DSM 15531 DSM | NCBI:txid 5479 | 2091 | (++) |
| 22 (23) | POLLEN | Tetragonisca angustula (Jataí) | MALT EXTRACT | Kazachstania telluris CBS 6265 CBS | NCBI:txid 36907 | 2892 | (+++) |
| 23 (28) | HONEY | Tetragonisca angustula (Jataí) | SABOURAUD | Schizosaccharomyces pombe DSM 70577 DSM | NCBI:txid 4896 | 2892 | (+++) |
| 24 (42) | HONEY | Tetragonisca angustula (Jataí) | SABOURAUD | Schizosaccharomyces pombe DSM 70577 DSM | NCBI:txid 4896 | 2441 | (+++) |
| 25 (52) | HONEY | Tetragonisca angustula (Jataí) | SABOURAUD | Scheffersomyces insectosus CBS 4286 CBS | NCBI:txid 45590 | 2053 | (++) |
| 26 (127) | HONEY | Tetragonisca angustula (Jataí) | SABOURAUD | Kazachstania telluris CBS 2685T CBS | NCBI:txid 36907 | 2842 | (+++) |
| 27 (18) | HONEY | Tetragonisca angustula (Jataí) | YEAST EXTRACT | Schizosaccharomyces pombe DSM 70577 DSM | NCBI:txid 4896 | 2892 | (+++) |
| 28 (21) | HONEY | Tetragonisca angustula (Jataí) | MALT EXTRACT | Kazachstania telluris CBS 2685T CBS | NCBI:txid 36907 | 2342 | (+++) |
| 29 (227) | HONEY | Melipona scutellaris (Uruçu) | SABOURAUD | Kazachstania telluris CBS 2685T CBS | NCBI:txid 36907 | 2342 | (+++) |
| 30 (92) | HONEY | Melipona scutellaris (Uruçu) | YEAST EXTRACT | Schizosaccharomyces pombe DSM 70577 DSM | NCBI:txid 4896 | 2892 | (+++) |
| 31 (104) | HONEY | Melipona scutellaris (Uruçu) | MALT EXTRACT | Brettanomyces bruxellensis DSM 70742 DSM | NCBI:txid 5007 | 2653 | (+++) |
| Total number of microbial isolates—2837 No colonies were found for Melipona scutellaris (Uruçu) pollen (+++)—highly probable species identification (++)—secure genus identification, probable species identification https://www.ncbi.nlm.nih.gov/taxonomy and https://www.mycobank.org/ | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, R.N.A.; Magalhães-Guedes, K.T.; de Oliveira Alves, R.M.; Souza, A.C.; Schwan, R.F.; Umsza-Guez, M.A. Yeast Diversity in Honey and Pollen Samples from Stingless Bees in the State of Bahia, Brazil: Use of the MALDI-TOF MS/Genbank Proteomic Technique. Microorganisms 2024, 12, 678. https://doi.org/10.3390/microorganisms12040678
da Silva RNA, Magalhães-Guedes KT, de Oliveira Alves RM, Souza AC, Schwan RF, Umsza-Guez MA. Yeast Diversity in Honey and Pollen Samples from Stingless Bees in the State of Bahia, Brazil: Use of the MALDI-TOF MS/Genbank Proteomic Technique. Microorganisms. 2024; 12(4):678. https://doi.org/10.3390/microorganisms12040678
Chicago/Turabian Styleda Silva, Raquel Nunes Almeida, Karina Teixeira Magalhães-Guedes, Rogério Marcos de Oliveira Alves, Angélica Cristina Souza, Rosane Freitas Schwan, and Marcelo Andrés Umsza-Guez. 2024. "Yeast Diversity in Honey and Pollen Samples from Stingless Bees in the State of Bahia, Brazil: Use of the MALDI-TOF MS/Genbank Proteomic Technique" Microorganisms 12, no. 4: 678. https://doi.org/10.3390/microorganisms12040678
APA Styleda Silva, R. N. A., Magalhães-Guedes, K. T., de Oliveira Alves, R. M., Souza, A. C., Schwan, R. F., & Umsza-Guez, M. A. (2024). Yeast Diversity in Honey and Pollen Samples from Stingless Bees in the State of Bahia, Brazil: Use of the MALDI-TOF MS/Genbank Proteomic Technique. Microorganisms, 12(4), 678. https://doi.org/10.3390/microorganisms12040678








