Phycoremediation Potential of Salt-Tolerant Microalgal Species: Motion, Metabolic Characteristics, and Their Application for Saline–Alkali Soil Improvement in Eco-Farms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of soil and Soil Extract
2.2. Microalgal Species and Monosodium Glutamate Residue
2.3. Experimental Design
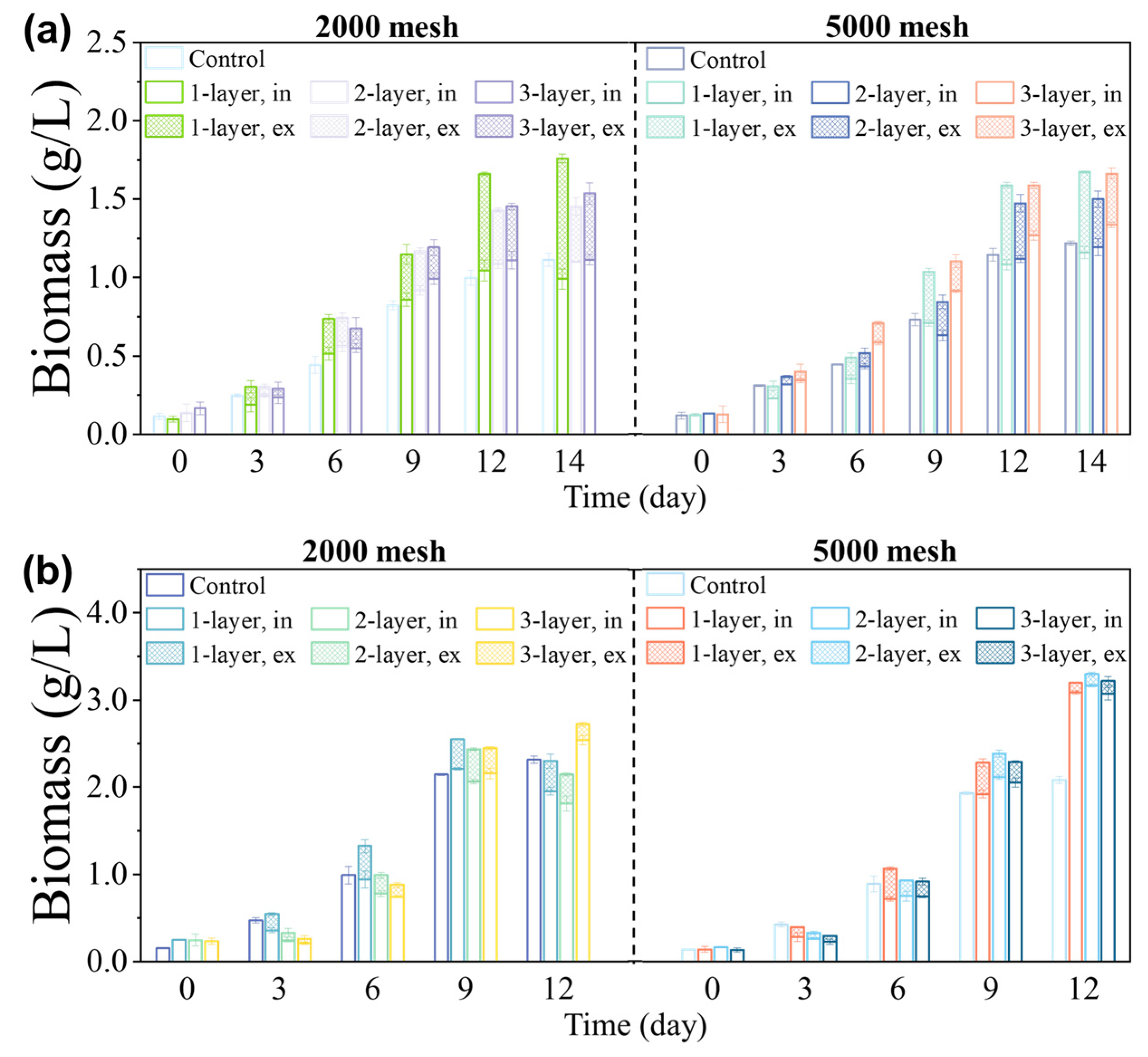
2.3.1. Experiment 1—Choice of Screen Aperture and Number of Layers
2.3.2. Experiment 2—Choice of Wastewater Addition Ratio
2.4. Analysis of Algal Growth
2.5. Analysis of the Water Quality Index
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of Acreen Aperture Size and Number of Layers on Growth of Microalgae
3.2. Relationship between the Microalgae’s Characteristics and Screen Properties
3.3. Effects of MSGR Diluted by Soil Extract on Microalgae
3.3.1. Biomass Concentration and Photosynthetic Pigments
3.3.2. Microalgae Cultivation Effects on Physical and Chemical Indicators
3.4. Nutrient Assimilation and Economic Benefits in Microalgal Eco-Farms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for feeding the world more sustainably with organic agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Ronga, D.; Rizza, F.; Badeck, F.W.; Milc, J.; Laviano, L.; Montevecchi, G.; Pecchioni, N.; Francia, E. Physiological responses to chilling in cultivars of processing tomato released and cultivated over the past decades in Southern Europe. Sci. Hortic. 2018, 231, 118–125. [Google Scholar] [CrossRef]
- Bouras, H.; Choukr-Allah, R.; Amouaouch, Y.; Bouaziz, A.; Devkota, K.P.; El Mouttaqi, A.; Bouazzama, B.; Hirich, A. How does quinoa (Chenopodium quinoa Willd.) respond to phosphorus fertilization and irrigation water salinity? Plants 2022, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Yu, Z. Microalgae: A revolution for salt-affected soil remediation. Trends Biotechnol. 2023, 41, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Truman, C.; Nuti, R.; Truman, L.; Dean, J. Feasibility of using FGD gypsum to conserve water and reduce erosion from an agricultural soil in Georgia. CATENA 2010, 81, 234–239. [Google Scholar] [CrossRef]
- Duarte, I.; Hernández, S.; Cantó, A. Macroalgae as soil conditioners or growth promoters of Pisum sativum (L.). Ann. Res. Rev. Biol. 2018, 27, 1–8. [Google Scholar] [CrossRef]
- Phillips, M.L.; McNellis, B.E.; Howell, A.; Lauria, C.M.; Belnap, J.; Reed, S.C. Biocrusts mediate a new mechanism for land degradation under a changing climate. Nat. Clim. Chang. 2022, 12, 71–76. [Google Scholar] [CrossRef]
- De, P.K. The role of blue-green algae in nitrogen fixation in rice-fields. Proc. R. Soc. Lond. B 1939, 127, 121–139. [Google Scholar]
- Cao, T.N.D.; Mukhtar, H.; Le, L.T.; Tran, D.P.H.; Ngo, M.T.T.; Pham, M.D.T.; Nguyen, T.B.; Vo, T.K.Q.; Bui, X.T. Roles of microalgae-based biofertilizer in sustainability of green agriculture and food-water-energy security nexus. Sci. Total Environ. 2023, 870, 161927. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T. A study for the biological CO2 fixation and utilization system. Sci. Total Environ. 2001, 277, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Burlacot, A.; Dao, O.; Auroy, P.; Cuiné, S.; Li-Beisson, Y.; Peltier, G. Alternative photosynthesis pathways drive the algal CO2-concentrating mechanism. Nature 2022, 605, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Song, W.; Yang, J.; Ren, C.; Du, H.; Tang, T.; Qin, S.; Liu, Z.; Cui, H. The role and mechanism of commercial macroalgae for soil conditioner and nutrient uptake catalyzer. Plant Growth Regul. 2022, 97, 455–476. [Google Scholar] [CrossRef]
- Rao, D.L.N.; Burns, R.G. The influence of blue-green algae on the biological amelioration of alkali soils. Biol. Fertil. Soils. 1991, 11, 306–312. [Google Scholar] [CrossRef]
- Wang, R.; Peng, B.; Huang, K. The research progress of CO2 sequestration by algal bio-fertilizer in China. J. CO2 Util. 2015, 11, 67–70. [Google Scholar] [CrossRef]
- Nisha, R.; Kiran, B.; Kaushik, A.; Kaushik, C.P. Bioremediation of salt affected soils using cyanobacteria in terms of physical structure, nutrient status and microbial activity. Int. J. Environ. Sci. Technol. 2018, 15, 571–580. [Google Scholar] [CrossRef]
- Singh, R. Reclamation of ‘Usar’ Lands in India through blue-green algae. Nature 1950, 165, 325–326. [Google Scholar] [CrossRef]
- Zhao, Z.R.; Liu, L.; Sun, Y.; Xie, L.L.; Liu, S.; Li, M.C.; Yu, Q.L. Combined microbe-plant remediation of cadmium in saline–alkali soil assisted by fungal mycelium-derived biochar. Environ. Res. 2024, 240, 117424. [Google Scholar] [CrossRef]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae cultivation and harvesting: Growth performance and use of flocculants—A review. Renew. Sust. Energ. Rev. 2019, 115, 109364. [Google Scholar] [CrossRef]
- Bahadar, A.; Khan, M.B. Progress in energy from microalgae: A review. Renew. Sust. Energ. Rev. 2013, 27, 128–148. [Google Scholar] [CrossRef]
- Hooda, S.; Malik, G.; Saini, P.; Grewall, A.; Pandey, V.C. Cyanobacteria as a potential bioasset for restoring degraded land. Land Degrad. Dev. 2023, 34, 3435–3450. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, J.; Nie, C.; Li, Y.; Jenkins, J.; Pei, H. Filamentous cyanobacteria triples oil production in seawater-based medium supplemented with industrial waste: Monosodium glutamate residue. Biotechnol. Biofuels. 2019, 12, 53. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Z.; Jiang, L.; Hou, Q.; Xie, Z.; Ma, M.; Yu, S.; Pei, H. Monosodium glutamate wastewater assisted seawater to increase lipid productivity in single-celled algae. Renew. Energ. 2021, 179, 1793–1802. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Chen, Z.; Chen, Y.; Wen, Y.; Chen, B. Removal of nutrients from undiluted anaerobically treated piggery wastewater by improved microalgae. Bioresour. Technol. 2016, 222, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lavrinovics, A.; Murby, F.; Ziverte, E.; Mezule, L.; Juhna, T. Increasing phosphorus uptake efficiency by phosphorus-starved microalgae for municipal wastewater post-treatment. Microorganisms 2021, 9, 1598. [Google Scholar] [CrossRef]
- Ritchie, R.J.; Sma-Air, S.; Phongphattarawat, S. Using DMSO for chlorophyll spectroscopy. J. Appl. Phycol. 2021, 33, 2047–2055. [Google Scholar] [CrossRef]
- State Environmental Protection, Administration. Monitoring Method of Water and Wastewater; China Environmental Science Press: Beijing, China, 2002; pp. 246–248. [Google Scholar]
- Fasaei, F.J.; Bitter, H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Marbelia, L.; Mulier, M.; Vandamme, D.; Muylaert, K.; Szymczyk, A.; Vankelecom, I.F. Polyacrylonitrile membranes for microalgae filtration: Influence of porosity, surface charge and microalgae species on membrane fouling. Algal Res. 2016, 19, 128–137. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Roth, R.; Ben-Amotz, A.; Goodenough, U. Ultrastructure of the green alga Dunaliella salina strain CCAP19/18 (Chlorophyta) as investigated by quick-freeze deep-etch electron microscopy. Algal Res. 2020, 49, 101953. [Google Scholar] [CrossRef]
- Abbasi, A.; Amiri, S. Emulsifying behavior of an exopolysaccharide produced by Enterobacter cloacae. Afr. J. Biomed. Res. 2008, 7, 1574–1576. [Google Scholar]
- Mishra, A.; Kavita, K.; Jha, B. Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr. Polym. 2011, 83, 852–857. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, H.; Liao, Q.; Huang, Y.; Xia, A.; Zhu, X.; Xiao, C.; Reungsang, A.; Liu, Z. Drag reduction and shear-induced cells migration behavior of microalgae slurry in tube flow. Bioresour. Technol. 2018, 270, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xu, J.; Huang, Y.; Li, Y.; Zhou, J.; Cen, K. Growth optimisation of microalga mutant at high CO2 concentration to purify undiluted anaerobic digestion effluent of swine manure. Bioresour. Technol. 2015, 177, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Nishchal; Goud, V.V. Salinity induced lipid production in microalgae and cluster analysis (ICCB 16-BR_047). Bioresour. Technol. 2017, 242, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Pei, H.; Zhang, L.; Yang, Z.; Nie, C.; Hou, Q.; Yu, Z. Accelerating lipid production in freshwater alga Chlorella sorokiniana SDEC-18 by seawater and ultrasound during the stationary phase. Renew. Energ. 2020, 161, 448–456. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Yang, J. Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol. 2010, 27, 59–63. [Google Scholar]
- Talmy, D.; Blackford, J.; Hardman-Mountford, N.; Dumbrell, A.; Geider, R. An optimality model of photoadaptation in contrasting aquatic light regimes. Limnol. Oceanogr. 2013, 58, 1802–1818. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.J.; Ren, Y.Y.; Zhang, H.Y.; Mao, X.M.; Lao, Y.M.; Wang, X.; Chen, F. Boosting carbon availability and value in algal cell for economic deployment of biomass. Bioresour. Technol. 2020, 300, 122640. [Google Scholar] [CrossRef]
- Bélanger-Lépine, F.; Tremblay, A.; Huot, Y.; Barnabé, S. Cultivation of an algae–bacteria consortium in wastewater from an industrial park: Effect of environmental stress and nutrient deficiency on lipid production. Bioresour. Technol. 2018, 267, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, C.; Burnap, R.L.; Peng, L. Carbon/Nitrogen metabolic balance: Lessons from cyanobacteria. Trends Plant Sci. 2018, 23, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Morales-Amaral, M.D.M.; Gómez-Serrano, C.; Acién, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Production of microalgae using centrate from anaerobic digestion as the nutrient source. Algal Res. 2015, 9, 297–305. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhang, Y.; Yang, L.; Chu, H.; Guo, J. Outdoor cultures of Chlorella pyrenoidosa in the effluent of anaerobically digested activated sludge: The effects of pH and free ammonia. Bioresour. Technol. 2016, 200, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Triantaphyllou, E.; Mann, S.H. An examination of the effectiveness of multi-dimensional decision-making methods: A decision-making paradox. Decis. Support Syst. 1989, 5, 303–312. [Google Scholar] [CrossRef]
- Satty, T.L. How to make a decision: The analytic hierarchy process. Eur. J. Oper. Res. 1990, 48, 9–26. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Yu, S.; Yu, Z.; Ma, M.; Liu, M.; Pei, H. Phycoremediation Potential of Salt-Tolerant Microalgal Species: Motion, Metabolic Characteristics, and Their Application for Saline–Alkali Soil Improvement in Eco-Farms. Microorganisms 2024, 12, 676. https://doi.org/10.3390/microorganisms12040676
Chen H, Yu S, Yu Z, Ma M, Liu M, Pei H. Phycoremediation Potential of Salt-Tolerant Microalgal Species: Motion, Metabolic Characteristics, and Their Application for Saline–Alkali Soil Improvement in Eco-Farms. Microorganisms. 2024; 12(4):676. https://doi.org/10.3390/microorganisms12040676
Chicago/Turabian StyleChen, Huiying, Siteng Yu, Ze Yu, Meng Ma, Mingyan Liu, and Haiyan Pei. 2024. "Phycoremediation Potential of Salt-Tolerant Microalgal Species: Motion, Metabolic Characteristics, and Their Application for Saline–Alkali Soil Improvement in Eco-Farms" Microorganisms 12, no. 4: 676. https://doi.org/10.3390/microorganisms12040676




