Methylmercury Effect and Distribution in Two Extremophile Microalgae Strains Dunaliella salina and Coccomyxa onubensis from Andalusia (Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Strains Used in the Study and Standard Cultivation Conditions
2.2. Measurements of the Optical Density (OD), Biomass Concentration (gDW/L), and Quantum Yield (Qy) of the Cultures
2.3. Experimental Setup and Sample Preparation
2.4. Intracellular Microstructure Examination of the Microalgal Cells by Transmission Electron Microscopy (TEM)
2.5. Superoxide Dismutase Activity
2.6. Sample Preparation for Mercury Determination via Atomic Absorption Spectroscopy (AAS) Technique
2.7. Statistical Treatment of the Data
3. Results and Discussion
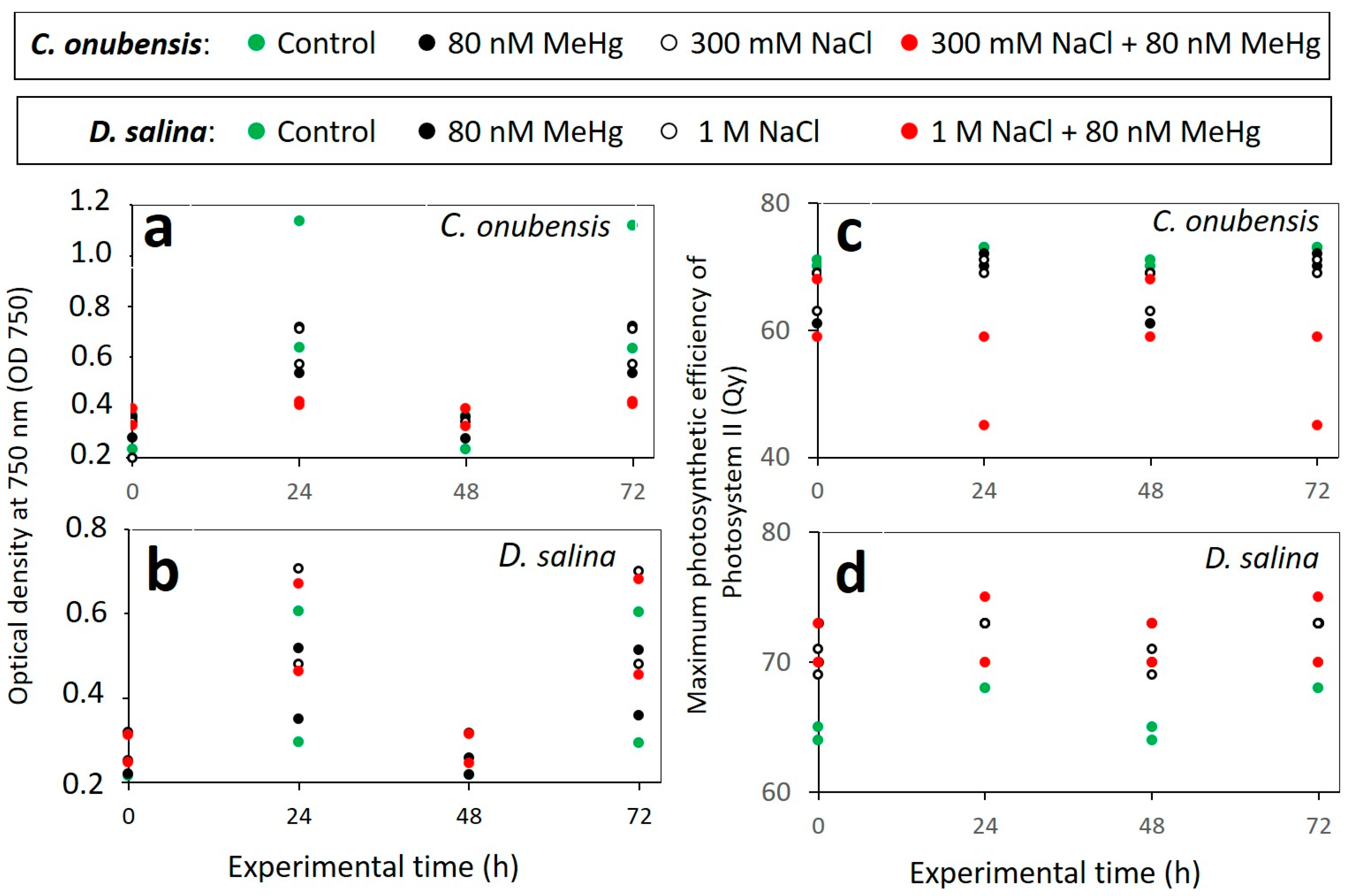
3.1. Growth Performance of Microalgae Exposed to MeHg
3.2. Effect of MeHg on the Culture Health and Photosynthetic Activity
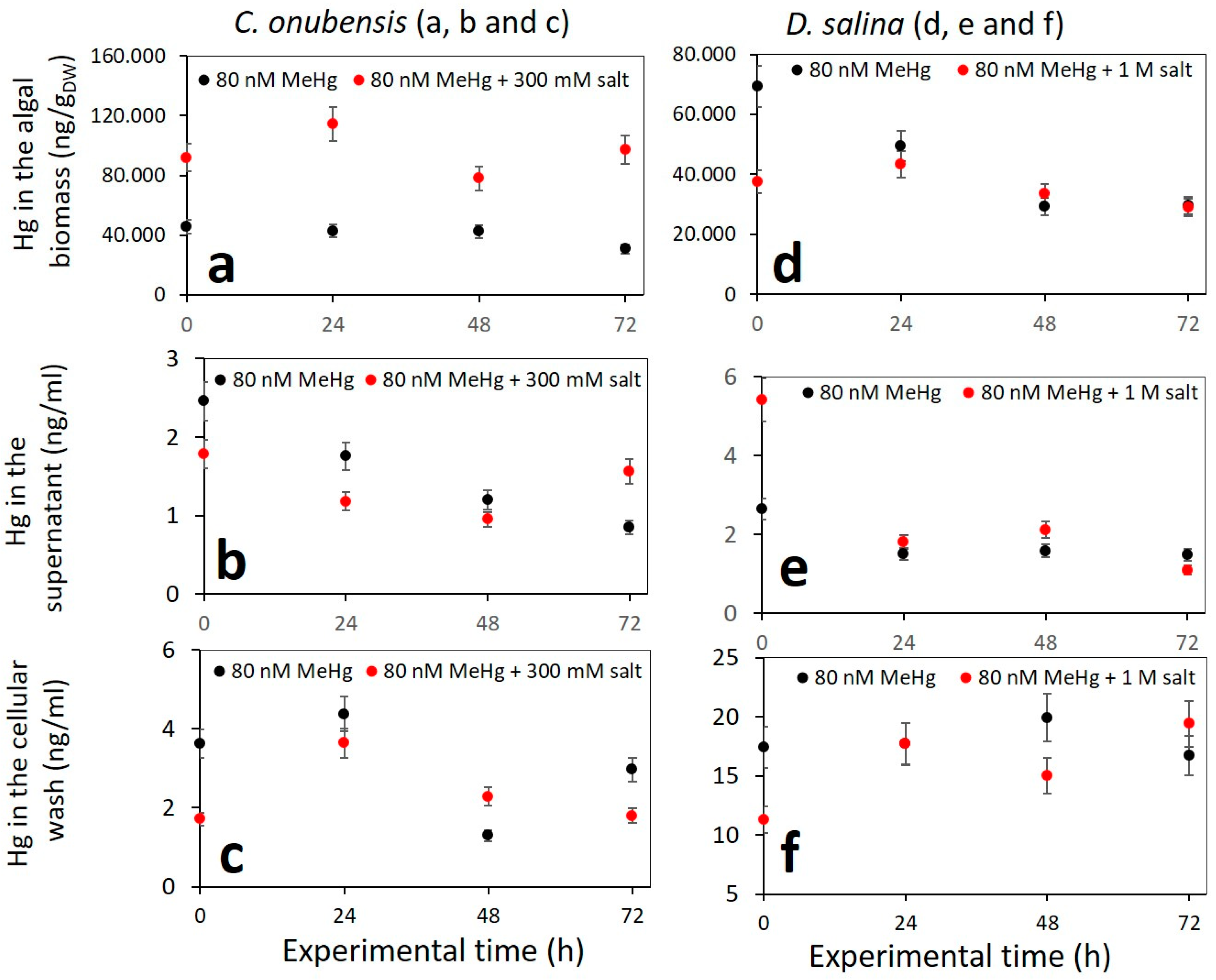
3.3. Culture Growth Performance in Up-Scaled Cultures (V = 1.8 L) Exposed to 80 nM MeHg and Addition of Higher Levels of NaCl (Salt Stress)
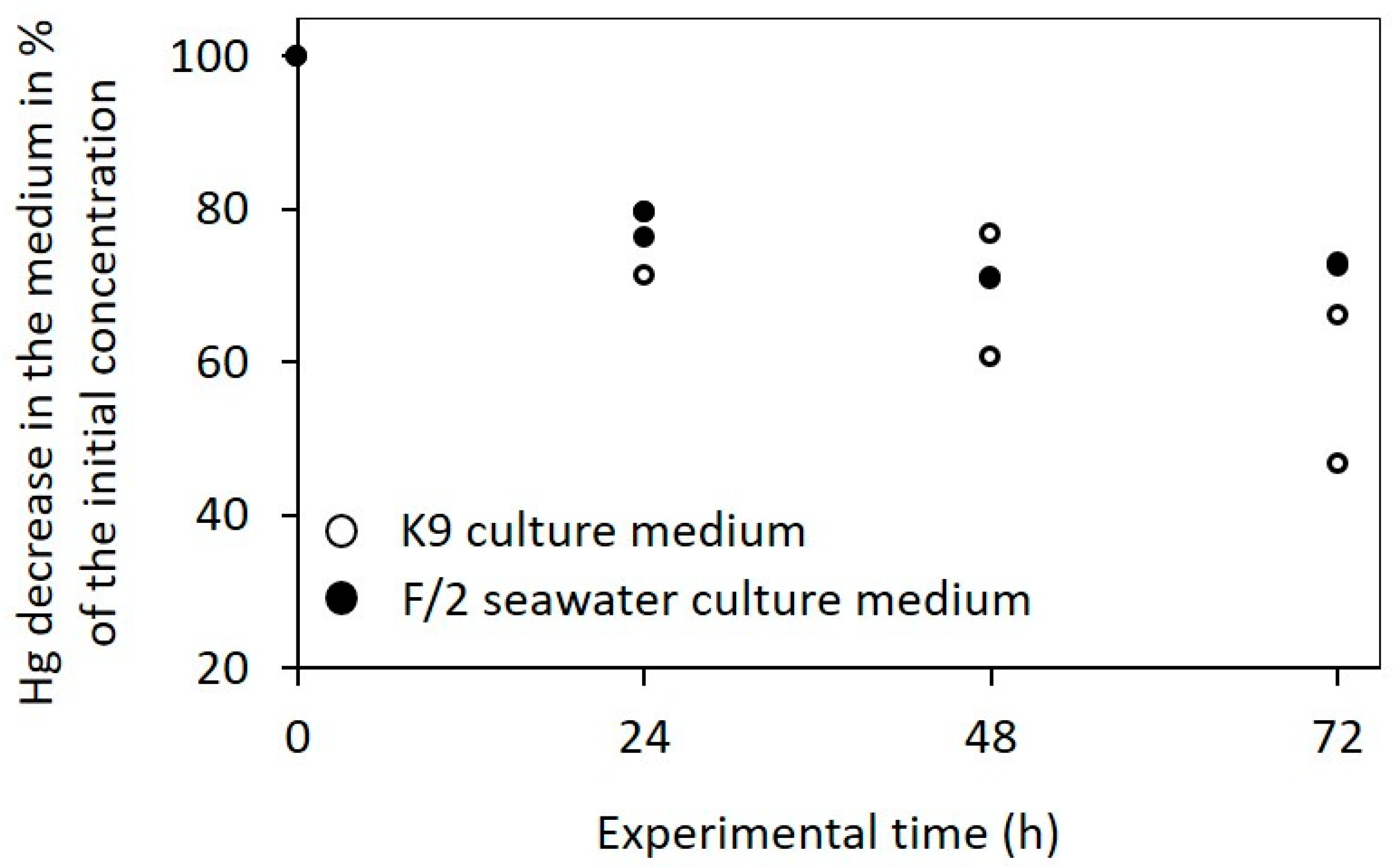
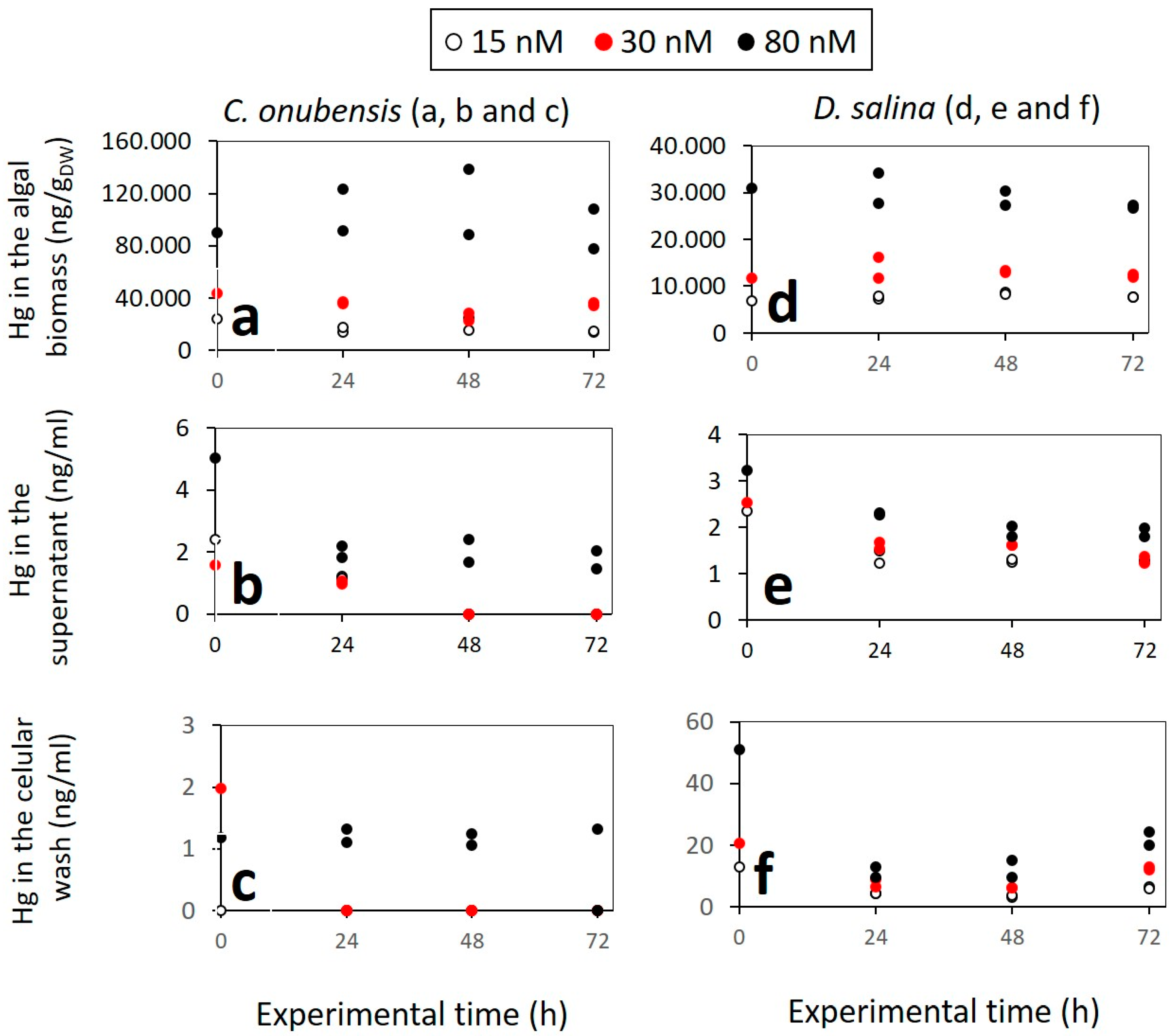
3.4. Mercury Distribution and Content in MeHg Exposed Cultures Measured Using AAS
- -
- Most of the MeHg is imported inside the algal cell: C. onubensis accumulates approx. four times more MeHg than D. salina at all tested concentrations (15, 30, and 80 nM MeHg). The amount of intracellular Hg is proportional to the initial MeHg added and decreases greatly from 80 nM to 15 nM cultures. For example, intracellular Hg in C. onubensis ranged from 77 to 108 µg/gDW in 80 nM culture and 14 to 15 µg/gDW in 15 nM MeHg culture. And intracellular Hg in D. salina ranged from 27 to 34 µg/gDW in 80 nM and 7.6 to 7.7 µg/gDW in 15 nM MeHg culture. The maximum intracellular accumulation at the highest concentration of 80 nM MeHg for both species was not at the end of the experiment (72 h) but earlier—at 24 h—for D. salina (from 27 to 34 µg/gDW) and at 48 h for C. onubensis (up to 138 µg/gDW)—then it partially decreased till the end of the experiment (Figure 3a,b—black dots), which suggests active biotransformation and removal of Hg possibly via transformation to elemental mercury, which is then removed as exudate from the cell [39]. A similar pattern, although less pronounced, can be observed in 30 nM MeHg-exposed cultures (Figure 3—red dots).
- -
- The high internalization of MeHg in C. onubensis was almost doubled by additional exposure to 300 mM NaCl and 80 nM MeHg (Figure 4a), which suggests shared Na and Hg import mechanisms and a common cell defense strategy. However, the addition of salt to the 80 nM MeHg culture in D. salina had no effect on MeHg accumulation in the 72 h time period (Figure 4b). Salt addition also had no obvious effect on MeHg removal from the supernatant nor adsorption to the cell surface in either of the studied microalgae.
- -
- MeHg content in the supernatant was very low throughout the experiment for both species and both culture volumes, and almost all MeHg was removed from the liquid medium at the end of the experiment (c(Hg) ≤ 1.99 ng/mL).
- -
- MeHg that was removed from the cell surface (using 0.1 M Na2EDTA solution) contained a very low amount of Hg. Nevertheless, the Hg amount at 80 nM MeHg was ten times higher for D. salina (20 to 24.2 ng/mL) compared to C. onubensis (≤1.99 ng/mL), which was almost identical to the very low supernatant Hg content.
- -
- The difference in cell surface adhesion and intracellular accumulation of MeHg in the tested species can be attributed to the difference in the cell surface structure of these two species. The microalga C. onubensis has a thin but rigid cell wall similar to other Trebouxiphyceae strains, while D. salina completely lacks a cell wall and has only a thin plasmalemma, enabling it to withstand osmotic pressure in high-salinity environments. The other indication is that MeHg has a greater chemical ability to pass through the cell wall chemical structure of C. onubensis, perhaps due to its inclusion as a component of amino acid-based hydrophilic complex carriers, which eases its transit through cell walls [45]. Besides photodegradation, the abiotic loss can be associated with the presence of naturally accompanying bacteria in the culture medium that can induce MeHg demethylation [46].
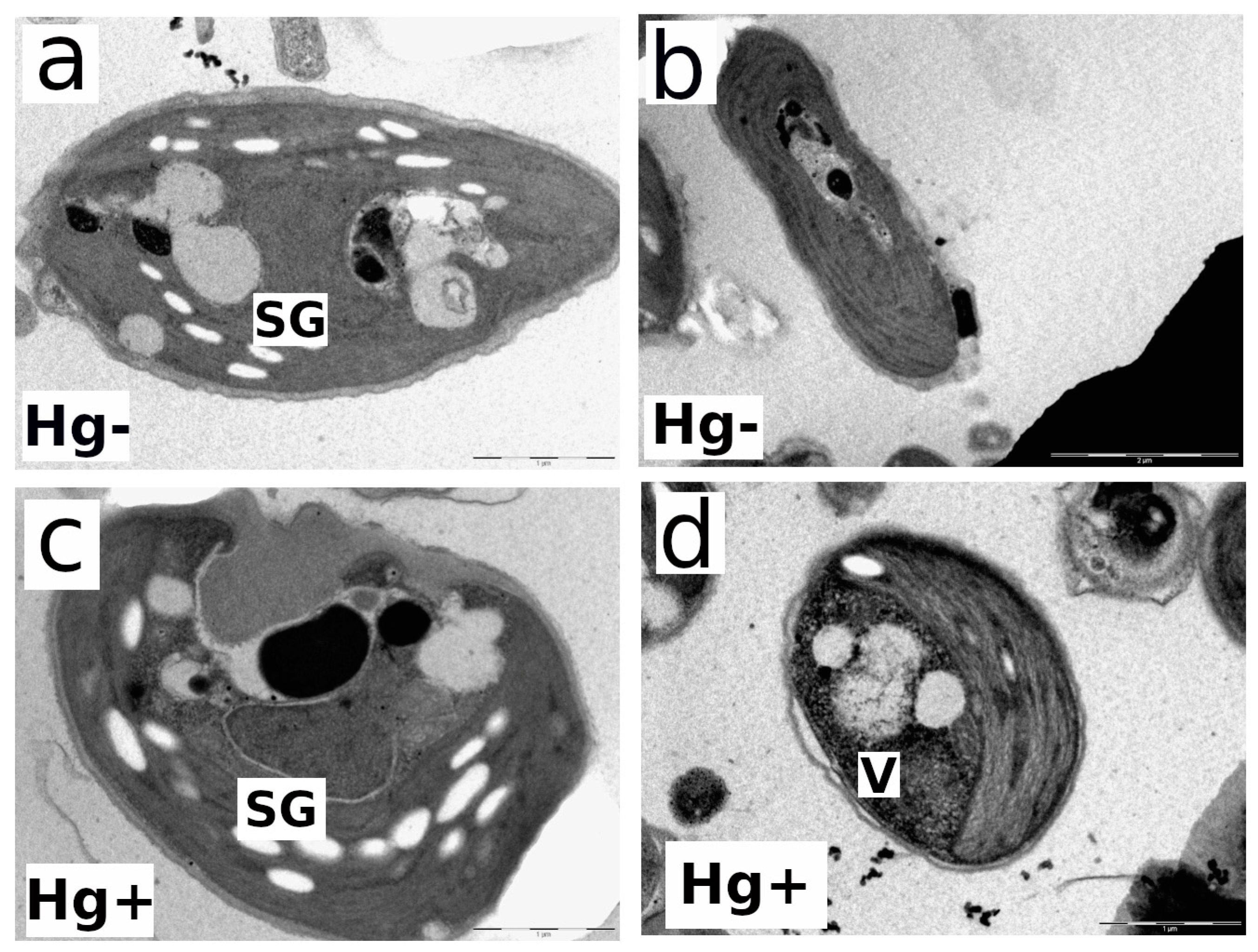
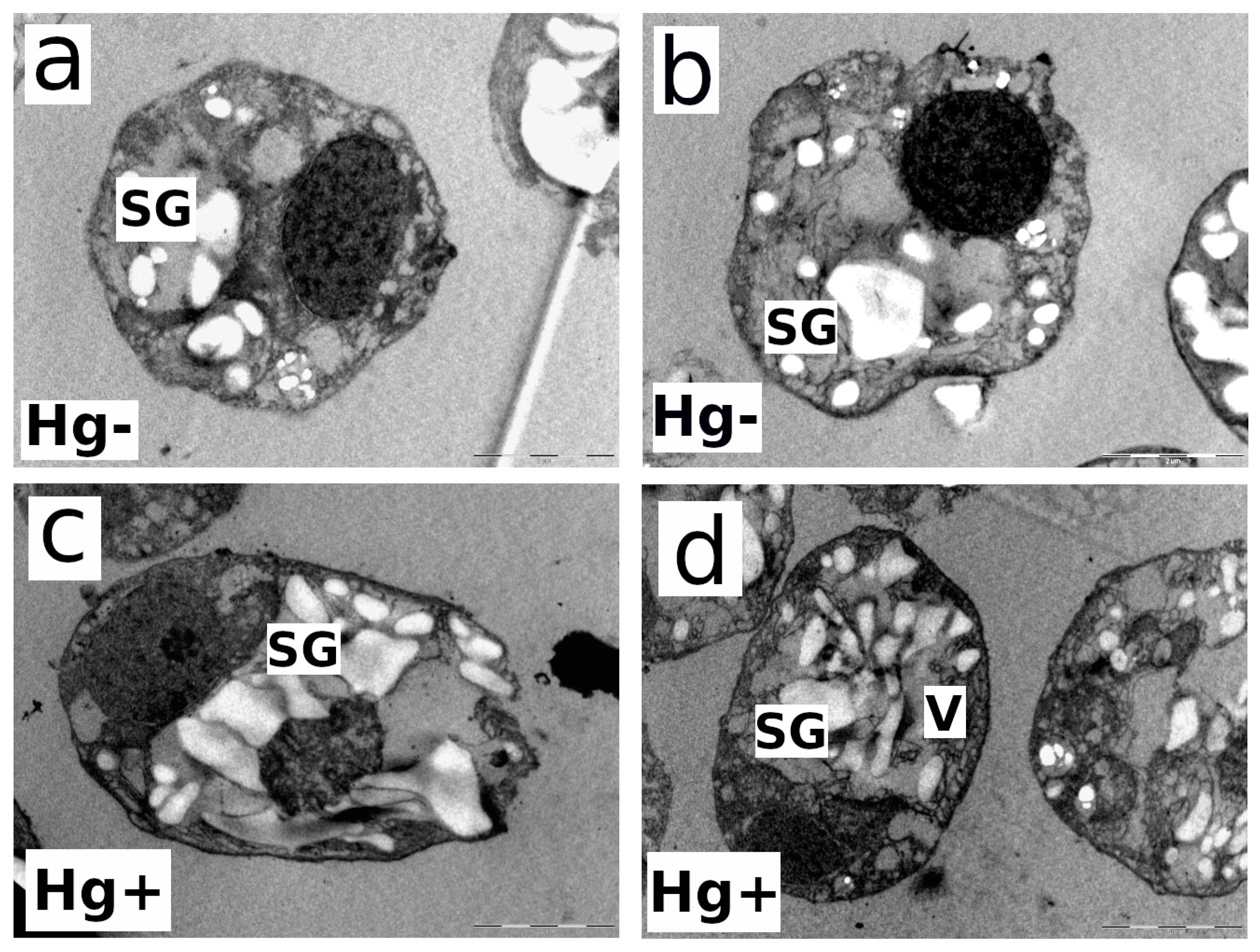
3.5. Impact of MeHg on the Ultrastructure of the Microalgal Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Bravo, A.G.; Cosio, C. Biotic Formation of Methylmercury: A Bio–Physico–Chemical Conundrum. Limnol. Oceanogr. 2020, 65, 1010–1027. [Google Scholar] [CrossRef]
- Karimi, R.; Fitzgerald, T.P.; Fisher, N.S. A Quantitative Synthesis of Mercury in Commercial Seafood and Implications for Exposure in the United States. Environ. Health Perspect. 2012, 120, 1512–1519. [Google Scholar] [CrossRef]
- Beauvais-Flück, R.; Slaveykova, V.I.; Cosio, C. Cellular Toxicity Pathways of Inorganic and Methyl Mercury in the Green Microalga Chlamydomonas reinhardtii. Sci. Rep. 2017, 7, 8034. [Google Scholar] [CrossRef]
- Scheuhammer, A.M.; Meyer, M.W.; Sandheinrich, M.B.; Murray, M.W. Effects of Environmental Methylmercury on the Health of Wild Birds, Mammals, and Fish. Ambio 2007, 36, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.P.; Mehmeti, L.; Slaveykova, V.I. Simple Acid Digestion Procedure for the Determination of Total Mercury in Plankton by Cold Vapor Atomic Fluorescence Spectroscopy. Methods Protoc. 2022, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Fisher, N.S. Bioaccumulation of Methylmercury in a Marine Copepod. Environ. Toxicol. Chem. 2017, 36, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.N.; Karimi, R.; Nyland, J.F.; Harrington, J.; Levine, K.; Meliker, J.R. Mercury Exposure, Serum Antinuclear Antibodies, and Serum Cytokine Levels in the Long Island Study of Seafood Consumption: A Cross-Sectional Study in NY, USA. Environ. Res. 2017, 156, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Nyholt, K.; Jardine, T.D.; Villamarín, F.; Jacobi, C.M.; Hawes, J.E.; Campos-Silva, J.V.; Srayko, S.; Magnusson, W.E. High Rates of Mercury Biomagnification in Fish from Amazonian Floodplain-Lake Food Webs. Sci. Total Environ. 2022, 833, 155161. [Google Scholar] [CrossRef] [PubMed]
- Capo, E.; Broman, E.; Bonaglia, S.; Bravo, A.G.; Bertilsson, S.; Soerensen, A.L.; Pinhassi, J.; Lundin, D.; Buck, M.; Hall, P.O.J.; et al. Oxygen-Deficient Water Zones in the Baltic Sea Promote Uncharacterized Hg Methylating Microorganisms in Underlying Sediments. Limnol. Oceanogr. 2022, 67, 135–146. [Google Scholar] [CrossRef]
- Pinto, E.P.; Paredes, E.; Bellas, J. Influence of Microplastics on the Toxicity of Chlorpyrifos and Mercury on the Marine Microalgae Rhodomonas lens. Sci. Total Environ. 2023, 857, 159605. [Google Scholar] [CrossRef] [PubMed]
- Winder, M.; Sommer, U. Phytoplankton Response to a Changing Climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- B-Béres, V.; Stenger-Kovács, C.; Buczkó, K.; Padisák, J.; Selmeczy, G.B.; Lengyel, E.; Tapolczai, K. Ecosystem Services Provided by Freshwater and Marine Diatoms. Hydrobiologia 2022, 850, 2707. [Google Scholar] [CrossRef]
- Boening, D.W. Ecological Effects, Transport, and Fate of Mercury: A General Review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Thera, J.C.; Kidd, K.A.; Stewart, A.R.; Bertolo, R.F.; O’Driscoll, N.J. Using Tissue Cysteine to Predict the Trophic Transfer of Methylmercury and Selenium in Lake Food Webs. Environ. Pollut. 2022, 311, 119936. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, J.A.; Kirk, J.L.; Muir, D.C.G.; Evans, M.; Yang, F.; Keating, J.; Parsons, M.T. Anthropogenic Mercury Deposition in Flin Flon Manitoba and the Experimental Lakes Area Ontario (Canada): A Multi-Lake Sediment Core Reconstruction. Sci. Total Environ. 2017, 586, 685–695. [Google Scholar] [CrossRef]
- Ullrich, T.W.T.; Abdrashitova, S.A. Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Eklöf, K.; Bishop, K.; Bertilsson, S.; Björn, E.; Buck, M.; Skyllberg, U.; Osman, O.A.; Kronberg, R.-M.; Bravo, A.G. Formation of Mercury Methylation Hotspots as a Consequence of Forestry Operations. Sci. Total Environ. 2018, 613–614, 1069–1078. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the Terrestrial Environment: A Review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Shahid, T.; Sultana, S.; Sultan, T.; Hussain, B.; Ahmed, Z. Impact of Water Pollution on Trophic Transfer of Fatty Acids in Fish, Microalgae, and Zoobenthos in the Food Web of a Freshwater Ecosystem. Biomolecules 2019, 9, 231. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Huss, V.A.R.; Montero, Z.; Torronteras, R.; Cuaresma, M.; Garbayo, I.; Vílchez, C. Phylogenetic Characterization and Morphological and Physiological Aspects of a Novel Acidotolerant and Halotolerant Microalga Coccomyxa onubensis sp. nov. (Chlorophyta, Trebouxiophyceae). J. Appl. Phycol. 2016, 28, 3269–3279. [Google Scholar] [CrossRef]
- Silverman, M.P.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [CrossRef]
- Guillard, R.R.; Ryther, J.H. Studies of Marine Planktonic Diatoms. I. Cyclotella Nana Hustedt, and Detonula Confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Skrobonja, A.; Gojkovic, Z.; Soerensen, A.L.; Westlund, P.-O.; Funk, C.; Björn, E. Uptake Kinetics of Methylmercury in a Freshwater Alga Exposed to Methylmercury Complexes with Environmentally Relevant Thiols. Environ. Sci. Technol. 2019, 53, 13757–13766. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Vilchez, C.; Torronteras, R.; Vigara, J.; Gomez-Jacinto, V.; Janzer, N.; Gomez-Ariza, J.-L.; Marova, I.; Garbayo, I. Effect of Selenate on Viability and Selenomethionine Accumulation of Chlorella sorokiniana Grown in Batch Culture. Sci. World J. 2014, 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.T.Y.; Sivaloganathan, B.; Obbard, J.P. Screening of Marine Microalgae for Biodiesel Feedstock. Biomass Bioenergy 2011, 35, 2534–2544. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Lu, Y.; Ferro, L.; Toffolo, A.; Funk, C. Modeling Biomass Production during Progressive Nitrogen Starvation by North Swedish Green Microalgae. Algal Res. 2020, 47, 101835. [Google Scholar] [CrossRef]
- Moye, H.A.; Miles, C.J.; Phlips, E.J.; Sargent, B.; Merritt, K.K. Kinetics and Uptake Mechanisms for Monomethylmercury between Freshwater Algae and Water. Environ. Sci. Technol. 2002, 36, 3550–3555. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. The Utility of Superoxide Dismutase in Studying Free Radical Reactions: I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 1969, 244, 6056–6063. [Google Scholar] [CrossRef]
- Robles, M.; Torronteras, R.; Ostojic, C.; Oria, C.; Cuaresma, M.; Garbayo, I.; Navarro, F.; Vílchez, C. Fe (III)-Mediated Antioxidant Response of the Acidotolerant Microalga Coccomyxa onubensis. Antioxidants 2023, 12, 610. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.; Ryan, P. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Gorski, P.R.; Armstrong, D.E.; Hurley, J.P.; Krabbenhoft, D.P. Influence of Natural Dissolved Organic Carbon on the Bioavailability of Mercury to a Freshwater Alga. Environ. Pollut. 2008, 154, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Masojídek, J.; Torzillo, G.; Koblížek, M. Photosynthesis in Microalgae. In Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 0-632-05953-2. [Google Scholar]
- Le Faucheur, S.; Campbell, P.G.C.; Fortin, C.; Slaveykova, V.I. Interactions between Mercury and Phytoplankton: Speciation, Bioavailability, and Internal Handling. Environ. Toxicol. Chem. 2014, 33, 1211–1224. [Google Scholar] [CrossRef]
- Beauvais-Flück, R.; Slaveykova, V.I.; Cosio, C. Transcriptomic and Physiological Responses of the Green Microalga Chlamydomonas reinhardtii during Short-Term Exposure to Subnanomolar Methylmercury Concentrations. Environ. Sci. Technol. 2016, 50, 7126–7134. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.M.; Chau, C.W.; Zhang, J.H. Acute Toxicity of Excess Mercury on the Photosynthetic Performance of Cyanobacterium, S. Platensis—Assessment by Chlorophyll Fluorescence Analysis. Chemosphere 2000, 41, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, W.-X. Accumulation, Subcellular Distribution and Toxicity of Inorganic Mercury and Methylmercury in Marine Phytoplankton. Environ. Pollut. 2011, 159, 3097–3105. [Google Scholar] [CrossRef]
- Beauvais-Flück, R.; Slaveykova, V.I.; Cosio, C. Molecular Effects of Inorganic and Methyl Mercury in Aquatic Primary Producers: Comparing Impact to A Macrophyte and A Green Microalga in Controlled Conditions. Geosciences 2018, 8, 393. [Google Scholar] [CrossRef]
- Bravo, A.G.; Faucheur, S.L.; Monperrus, M.; Amouroux, D.; Slaveykova, V.I. Species-Specific Isotope Tracers to Study the Accumulation and Biotransformation of Mixtures of Inorganic and Methyl Mercury by the Microalga Chlamydomonas reinhardtii. Environ. Pollut. 2014, 192, 212–215. [Google Scholar] [CrossRef]
- Oren, A. The Ecology of Dunaliella in High-Salt Environments. J. Biol. Res. 2014, 21, 23. [Google Scholar] [CrossRef]
- Barkay, T.; Gu, B. Demethylation—The Other Side of the Mercury Methylation Coin: A Critical Review. ACS Environ. Au 2022, 2, 77–97. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Skrobonja, A.; Funk, C.; Garbayo, I.; Vílchez, C. The Role of Microalgae in the Biogeochemical Cycling of Methylmercury (MeHg) in Aquatic Environments. Phycology 2022, 2, 344–362. [Google Scholar] [CrossRef]
- Soerensen, A.L.; Schartup, A.T.; Skrobonja, A.; Björn, E. Organic Matter Drives High Interannual Variability in Methylmercury Concentrations in a Subarctic Coastal Sea. Environ. Pollut. 2017, 229, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Quiroga-Flores, R.; Guédron, S.; Achá, D. High Methylmercury Uptake by Green Algae in Lake Titicaca: Potential Implications for Remediation. Ecotoxicol. Environ. Saf. 2021, 207, 111256. [Google Scholar] [CrossRef] [PubMed]
- Simmons-Willis, T.A.; Koh, A.S.; Clarkson, T.W.; Ballatori, N. Transport of a Neurotoxicant by Molecular Mimicry: The Methylmercury-L-Cysteine Complex Is a Substrate for Human L-Type Large Neutral Amino Acid Transporter (LAT) 1 and LAT2. Biochem. J. 2002, 367, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Song, B.; Li, Y. The Potential of Mercury Methylation and Demethylation by 15 Species of Marine Microalgae. Water Res. 2022, 215, 118266. [Google Scholar] [CrossRef] [PubMed]
- Cossart, T.; Garcia-Calleja, J.; Santos, J.P.; Kalahroodi, E.L.; Worms, I.A.M.; Pedrero, Z.; Amouroux, D.; Slaveykova, V.I. Role of Phytoplankton in Aquatic Mercury Speciation and Transformations. Environ. Chem. 2022, 19, 104–115. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Memetshaeva, O.A.; Avsiyan, A.L.; Lelekov, A.S.; Novikova, T.M. Morphological and morphometrical features in Dunaliella salina (Chlamydomonadales, Dunaliellaceae) during the two-phase cultivation mode. Ecol. Monten. 2019, 22, 157–165. [Google Scholar] [CrossRef][Green Version]

| Microalga | SOD Activity Units/mL of Cell Extract | SOD Activity Units/mg of Cell Proteins |
|---|---|---|
| C. onubensis + 80 nM MeHg | 16.3–16.5 | 4.84–7.57 |
| C. onubensis (control—MeHg free) | 16.3–16.9 | 5.50–6.48 |
| D. salina + 80 nM MeHg | 14.5–19.0 | 2.35–2.95 |
| D. salina (control—MeHg free) | 15.5–16.3 | 2.22–2.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simansky, S.; Holub, J.; Márová, I.; Cuaresma, M.; Garbayo, I.; Torronteras, R.; Vílchez, C.; Gojkovic, Z. Methylmercury Effect and Distribution in Two Extremophile Microalgae Strains Dunaliella salina and Coccomyxa onubensis from Andalusia (Spain). Microorganisms 2024, 12, 434. https://doi.org/10.3390/microorganisms12030434
Simansky S, Holub J, Márová I, Cuaresma M, Garbayo I, Torronteras R, Vílchez C, Gojkovic Z. Methylmercury Effect and Distribution in Two Extremophile Microalgae Strains Dunaliella salina and Coccomyxa onubensis from Andalusia (Spain). Microorganisms. 2024; 12(3):434. https://doi.org/10.3390/microorganisms12030434
Chicago/Turabian StyleSimansky, Samuel, Jiří Holub, Ivana Márová, María Cuaresma, Ines Garbayo, Rafael Torronteras, Carlos Vílchez, and Zivan Gojkovic. 2024. "Methylmercury Effect and Distribution in Two Extremophile Microalgae Strains Dunaliella salina and Coccomyxa onubensis from Andalusia (Spain)" Microorganisms 12, no. 3: 434. https://doi.org/10.3390/microorganisms12030434
APA StyleSimansky, S., Holub, J., Márová, I., Cuaresma, M., Garbayo, I., Torronteras, R., Vílchez, C., & Gojkovic, Z. (2024). Methylmercury Effect and Distribution in Two Extremophile Microalgae Strains Dunaliella salina and Coccomyxa onubensis from Andalusia (Spain). Microorganisms, 12(3), 434. https://doi.org/10.3390/microorganisms12030434









