Comparison of Microbial Diversity of Two Typical Volcanic Soils in Wudalianchi, China
Abstract
1. Introduction
2. Materials and Methods
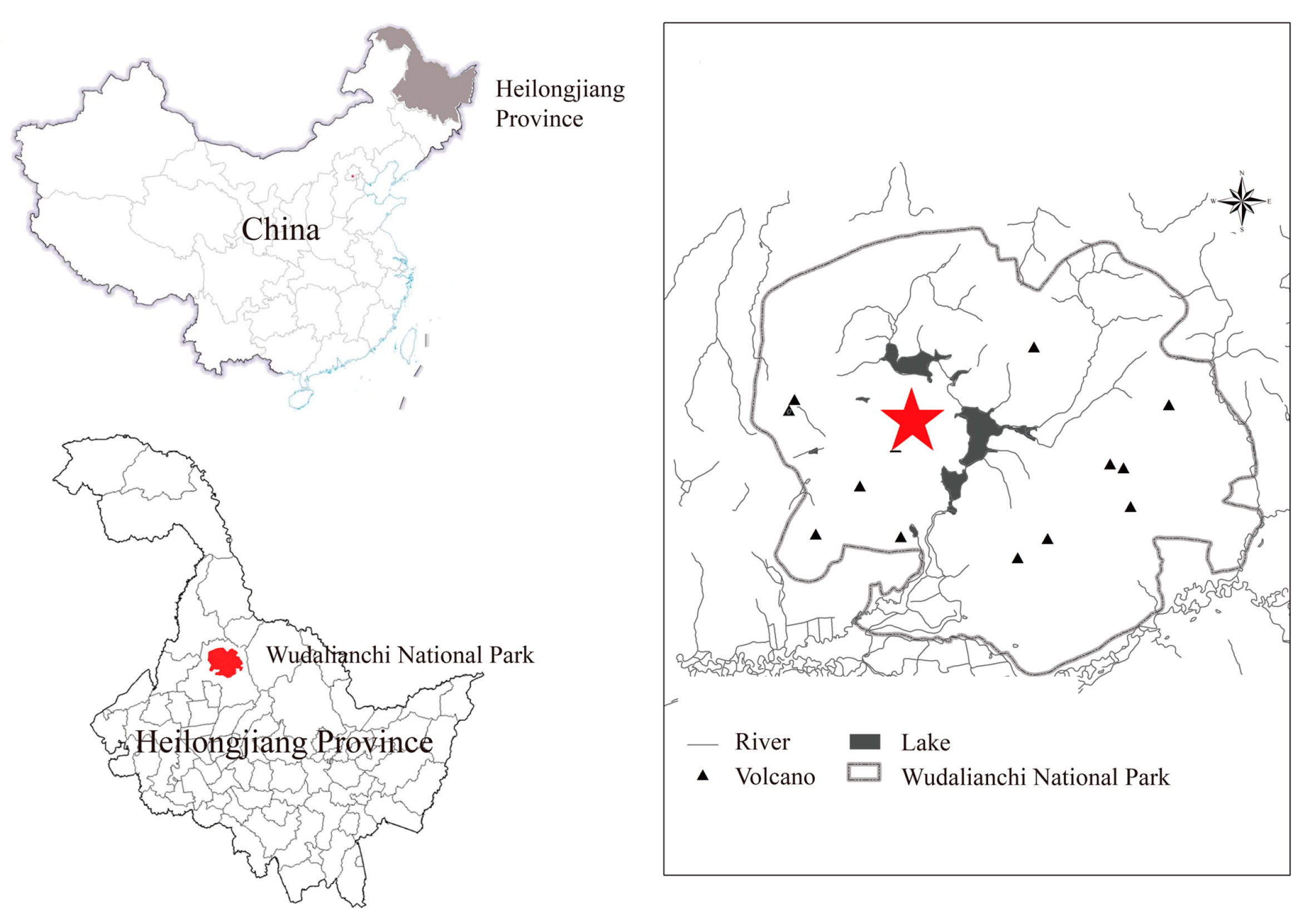
2.1. Site Description and Soil Sampling
2.2. Chemical and Microbiological Analyses
2.3. Statistical Analysis
3. Results
3.1. Soil Physico-Chemical Properties
3.2. Soil Microbial Diversity
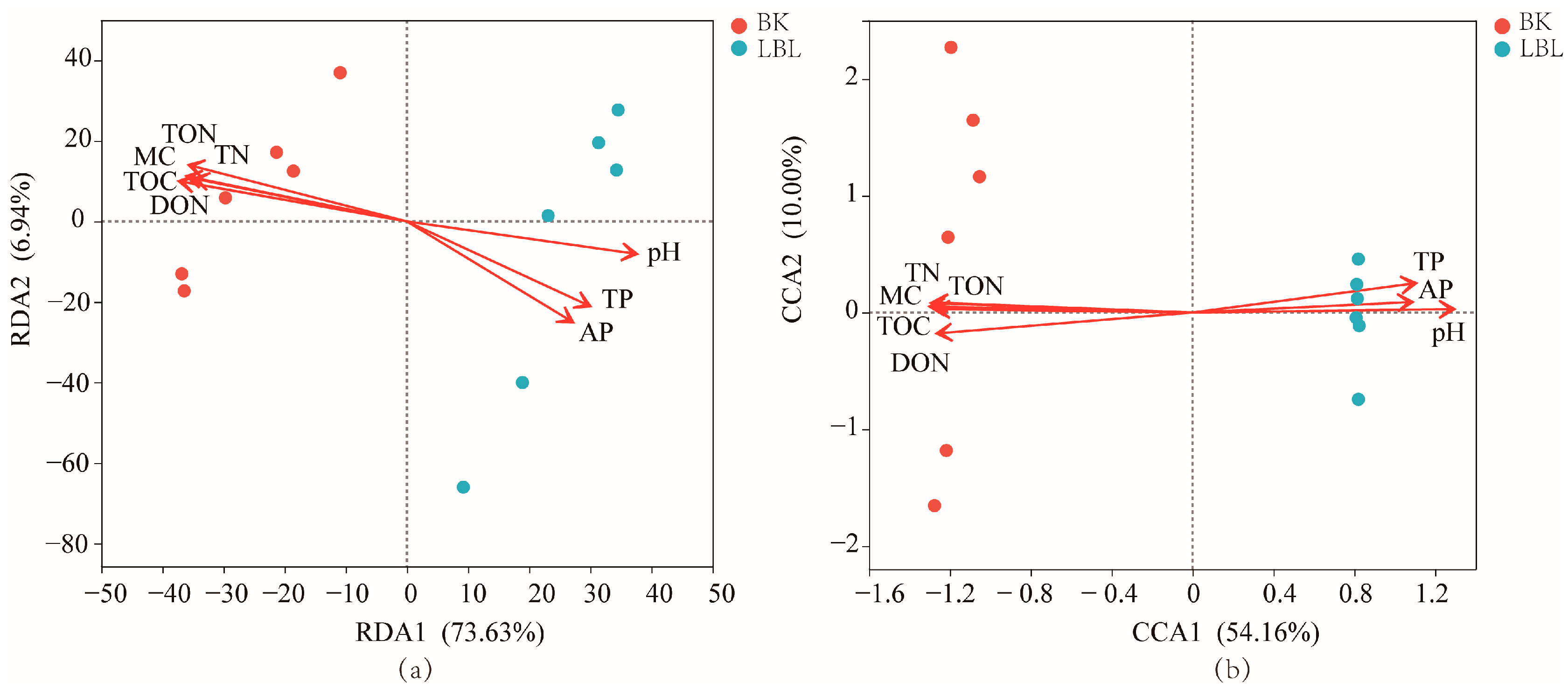
3.3. Diversity and Community Composition
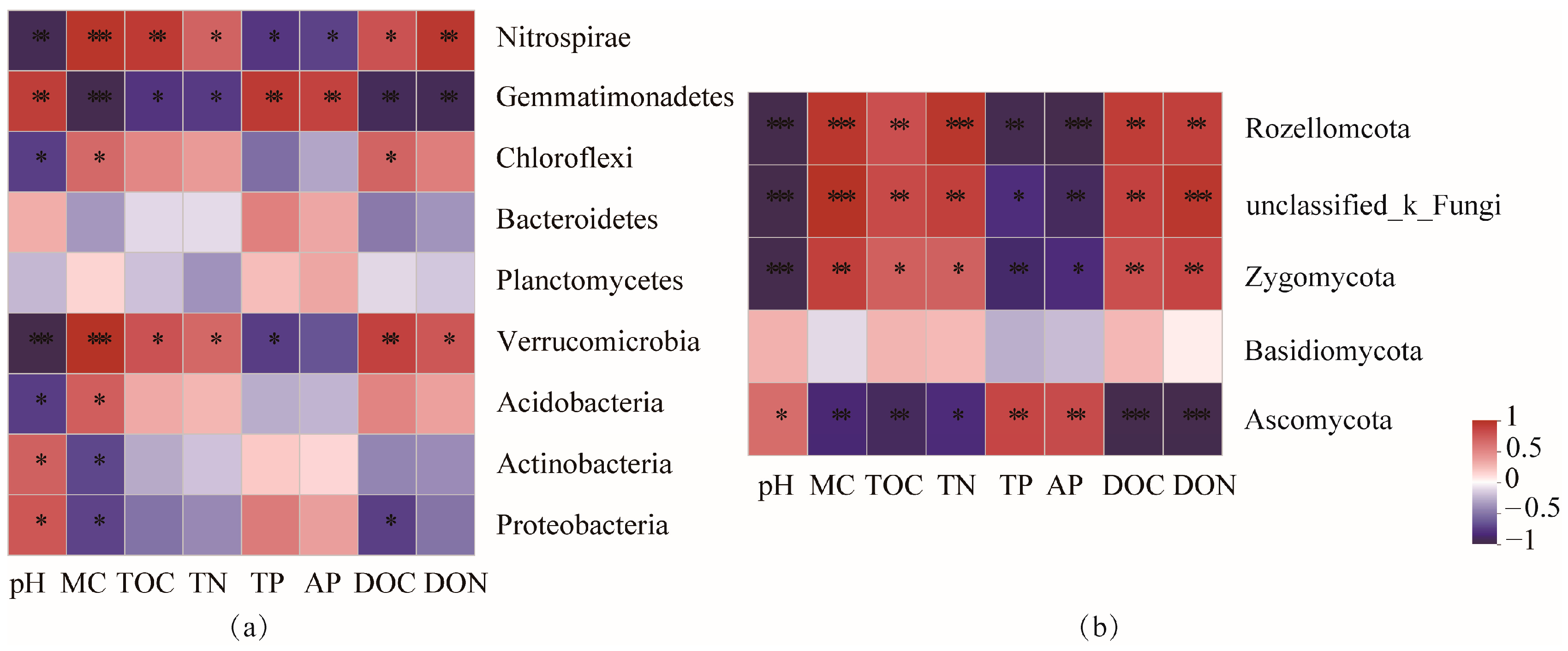
3.4. Correlations among Soil Nutrients, Microbial Community Composition
3.5. Function Prediction
4. Discussion
4.1. Soil Properties in LBL vs. BK
4.2. Diversity and Structures of Microbial Communities in LBL vs. BK
4.3. Relationships between Soil Microbial and Soil Properties
4.4. Potential Functional Groups of Soil Microbial
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.G.; Shen, R.F.; He, J.Z.; Wang, Y.F.; Han, X.G.; Jia, Z.J. China Soil Microbiome Initiative: Progress and Perspective. Bull. Chin. Acade. Sci. 2017, 32, 554–565. [Google Scholar]
- Valle, S.R.; Carrasco, J. Soil quality indicator selection in Chilean volcanic soils formed under temperate and humid conditions. Catena 2018, 162, 386–395. [Google Scholar] [CrossRef]
- Valle, S.R.; Dörner, J.; Zúñiga, F.; Dec, D. Seasonal dynamics of the physical quality of volcanic ash soils under different land uses in southern Chile. Soil Tillage Res. 2018, 182, 25–34. [Google Scholar] [CrossRef]
- Garrido Benavent, I.; Pérez Ortega, S.; Durán, J.; Ascaso, C.; Pointing, S.B.; Rodríguez-Cielos, R.; Navarro, F.; de Los Ríos, A. Differential Colonization and Succession of Microbial Communities in Rock and Soil Substrates on a Maritime Antarctic Glacier Forefield. Front. Microbiol. 2020, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Molina, C.I.; Martínez-Romero, E.; Ruíz-Valdiviezo, V.M.; Velázquez, E.; Ruíz-Lau, N.; Rogel-Hernández, M.A.; Villalobos-Maldonado, J.J.; Rincón-Rosales, R. Plant growth-promoting potential of bacteria associated to pioneer plants from an active volcanic site of Chiapas (Mexico). Appl. Soil Ecol. 2020, 146, 103390. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Q.C.; Xu, D.L.; Li, Z.S.; Chao, L.M.; Li, X.Y.; Liu, H.J.; Wang, P.F.; Zheng, Y.X.; Liu, X.Y.; et al. Soil microbial community composition and co-occurrence network responses to mild and severe disturbances in volcanic areas. Sci. Total Environ. 2023, 901, 165889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Q.; Xu, L.J.; Zhang, Y.H.; Xia, C.M.; Li, H.G.; Liu, T. An analysis of the ecological value of Wudalianchi, Heilongjiang Province, China. Biodiver. Sci. 2011, 19, 63–70. [Google Scholar]
- Saputra, D.D.; Sari, R.R.; Hairiah, K.; Widianto; Suprayogo, D.; van Noordwijk, M. Recovery after volcanic ash deposition: Vegetation effects on soil organic carbon, soil structure and infiltration rates. Plant Soil 2022, 474, 163–179. [Google Scholar] [CrossRef]
- Fiantis, D.; Ginting, F.I.; Seprianto; Halfero, F.; Saputra, A.P.; Nelson, M.; Van Ranst, E.; Minasny, B. Geochemical and mineralogical composition of the 2018 volcanic deposits of Mt. Anak Krakatau. Geodermal Reg. 2021, 25, e00393. [Google Scholar] [CrossRef]
- Anda, M.; Suparto; Sukarman. Characteristics of pristine volcanic materials: Beneficial and harmful effects and their management for restoration of agroecosystem. Sci. Total Environ. 2016, 543, 480–492. [Google Scholar] [CrossRef]
- Tateno, R.; Tatsumi, C.; Nakayama, M.; Takahashi, K.; Kerfahi, D.; Adams, J. Temperature effects on the first three years of soil ecosystem development on volcanic ash. Catena 2019, 172, 1–10. [Google Scholar] [CrossRef]
- Shillam, L.; Hopkins, D.W.; Badalucco, L.; Laudicina, V.A. Structural diversity and enzyme activity of volcanic soils at different stages of development and response to experimental disturbance. Soil Biol. Biochem. 2008, 40, 2182–2185. [Google Scholar] [CrossRef]
- Xu, S.Q.; Zhang, J.F.; Luo, S.S.; Zhou, X.; Shi, S.H.; Tian, C.J. Similar soil microbial community structure across different environments after long-term succession: Evidence from volcanoes of different ages. J. Basic Microbiol. 2018, 58, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Byloos, B.; Monsieurs, P.; Mysara, M.; Leys, N.; Houdt, R.V. Characterization of the bacterial communities on recent Icelandic volcanic deposits of different ages. BMC Microbiol. 2018, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.J.; Wang, L.M.; Xu, M.Y.; Huang, Q.Y.; Luo, C.Y.; Xie, L.H.; Ni, H.W. Variation of soil microbial biomass and enzyme activities under different vegetation types in the neogene volcanic lava platform of Wudalianchi. J. Cent. South Univ. 2019, 39, 88–97. [Google Scholar]
- Chen, J.; Zheng, Y.X.; Guo, Y.Q.; Li, F.S.; Xu, D.L.; Chao, L.M.; Qu, H.T.; Wang, B.J.; Ma, X.D.; Wang, S.Y.; et al. Differences in microbial communities from Quaternary volcanic soils at different stages of development: Evidence from Late Pleistocene and Holocene volcanoes. Catena 2021, 201, 105211. [Google Scholar] [CrossRef]
- Negi, A.; Sarethy, I.P. Microbial Biodeterioration of Cultural Heritage: Events, Colonization, and Analyses. Microb. Ecol. 2019, 78, 1014–1029. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, T.; Zhao, M.; Hu, Z.; Ni, Y.; Jiang, J.; Cheng, B.; Li, X.; Chen, J. Comparison of soil microbial abundances and co-occurrence networks in the volcanic soil of the cone and crater. Catena 2024, 236, 107734. [Google Scholar] [CrossRef]
- Vavourakis, C.D.; Andrei, A.-S.; Mehrshad, M.; Ghai, R.; Sorokin, D.Y.; Muyzer, G. A metagenomics roadmap to the uncultured genome diversity in hypersaline soda lake sediments. Microbiome 2018, 6, 168–179. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Yang, F.; Xie, L.H.; Cao, H.J.; Luo, C.Y.; Wang, J.F.; Yan, Z.Y.; Ni, H.W. Diversity and communlty structure of soil bacteria in different volcanoes, Wudalianchi. Acta Ecol. Sin. 2021, 41, 8276–8284. [Google Scholar]
- Gao, W.Y.; Li, J.H.; Mao, X.; Li, H.G. Geological and Geomorphological Value of the Monogenetic Volcanoes in;Wudalianchi National Park, NE China. Geoheritage 2013, 5, 73–85. [Google Scholar] [CrossRef]
- Hargitai, H. Kipuka. In Encyclopedia of Planetary Landforms; Springer: New York, NY, USA, 2014. [Google Scholar]
- Wang, X.J.; Zhang, Z.C.; Yu, Z.Q.; Shen, G.F.; Cheng, H.G.; Tao, S. Composition and diversity of soil microbial communities in the alpine wetland and alpine forest ecosystems on the Tibetan Plateau. Sci. Total Environ. 2020, 747, 41358. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Wu, X.D.; Zhao, L.; Yue, G.Y.; Zhao, Y.H.; Qiao, Y.P.; Li, Y.Q. Seasonal variations in labile soil organic matter fractions in permafrost soils with different vegetation types in the central Qinghai–Tibet Plateau. Catena 2016, 137, 670–678. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Lu, M.; Sun, X.Y.; Tian, K.; Ren, Y.L.; Wang, S.J.; Wang, H.; Peng, S.X. Characteristics of soil fungal community structure at different degraded stages in Napahai Plateau Wetland of northwestern China. J. Beijing For. Univ. 2018, 40, 55–65. [Google Scholar]
- Kuritani, T.; Kimura, J.I.; Ohtani, E.; Miyamoto, H.; Furuyama, K. Transition zone origin of potassic basalts from Wudalianchi volcano, northeast China. Lithos 2013, 156–159, 1–12. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Cao, H.J.; Wang, L.M.; Xie, L.H.; Ni, H.W. Species diversity and soil nutrients in lava platforms of Wudalianchi. J. Z. Agri. For. Univ. 2019, 36, 80–87. [Google Scholar]
- Cui, N.J.; Zhang, D.J.; Liu, Y.; Zhang, J.; Jiang, O.U.; Zhang, J.; Deng, C.; Ji, T.W. Plant diversity and soil physicochemical properties under different aged Pinus massoniana plantations. J. Ecol. 2014, 33, 2610–2617. [Google Scholar]
- James, P.; Chester, D.K.; Duncan, A.M. Development and spatial distribution of soils on an active volcano: Mt Etna, Sicily. Catena 2016, 137, 277–297. [Google Scholar] [CrossRef]
- Tsozué, D.; Nghonda, J.; Tematio, P.; Basga, S. Changes in soil properties and soil organic carbon stocks along an elevation gradient at Mount Bambouto, Central Africa. Catena 2019, 175, 251–262. [Google Scholar] [CrossRef]
- Mcgee, L.E.; Mcleod, C.; Davidson, J.P. A spectrum of disequilibrium melting preserved in lava-hosted, partially melted crustal xenoliths from the Wudalianchi volcanic field, NE China. Chem. Geol. 2015, 417, 184–199. [Google Scholar] [CrossRef]
- Fu, Y.; Bai, X.L.; Zhang, L.; Bi, G.C.; Feng, C.; Kou, J.; Sarula. The effect of bryophytes on nutrient accumulation in surface soil in the Wudalianchi volcanic area. Acta Genet. Sin. 2015, 35, 3288–3297. [Google Scholar]
- Odum, E.P. The Strategy of Ecosystem Development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Liu, S.; Shi, Z.M.; Feng, Q.H.; Liu, Q.L.; Zhang, L.; Huang, Q.; He, J.S. Soil microbial community structure in Picea asperata plantations with different ages in subalpine of western Sichuan, Southwest China. Chin. J. Plant Ecol. 2017, 28, 519–527. [Google Scholar]
- Tebo, B.M.; Davis, R.E.; Anitori, R.P.; Connell, L.B.; Schiffman, P.; Staudigel, H. Microbial communities in dark oligotrophic volcanic ice cave ecosystems of Mt. Erebus, Antarctica. Front. Microbiol. 2015, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Yuan, G.; Yang, T.; Chu, H.Y. Verrucomicrobial elevational distribution was strongly influenced by soil pH and carbon/nitrogen ratio. J. Soil. Sendiment. 2017, 17, 2449–2456. [Google Scholar] [CrossRef]
- Mohammadi, S.S.; Pol, A.; Alen, T.V.; Jetten, M.S.M.; Camp, H.J.M.O.D. Ammonia Oxidation and Nitrite Reduction in the Verrucomicrobial MethanotrophMethylacidiphilum fumariolicumSolV. Front. Microbiol. 2017, 8, 1901. [Google Scholar] [CrossRef] [PubMed]
- Erlacher, A.; Cernava, T.; Cardinale, M.; Soh, J.; Sensen, C.W.; Grube, M.; Berg, G. Rhizobiales as functional and endosymbiontic members in the lichen symbiosis of Lobaria pulmonaria L. Front. Microbiol. 2015, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Olsson-Francis, K.; Boardman, C.P.; Pearson, V.K.; Schofield, P.F.; Oliver, A.; Summers, S. A Culture-Independent and Culture-Dependent Study of the Bacterial Community from the Bedrock Soil Interface. Adv. Microbiol. 2015, 5, 842–857. [Google Scholar] [CrossRef][Green Version]
- Yuan, B.; Ju, Y.W.; Li, B.i.; Sun, Y.M. Migration of trace elements from basalt substrate to co-located vegetation (lichens and mosses) at the Wudalianchi volcanos, Northeast China. J. Asian Earth Sci. 2016, 118, 95–100. [Google Scholar]
- Beckett, R.P.; Zavarzina, A.G.; Liers, C. Oxidoreductases and cellulases in lichens: Possible roles in lichen biology and soil organic matter turnover. Fungal Biol. 2013, 117, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, D.G.; Jiang, Z.H.; Sun, P.; Chen, J.G. Changes in the soil microbial communities of alpine steppe at Qinghai-Tibetan Plateau under different degradation levels. Sci. Total Environ. 2018, 651, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Xu, S.J.; Li, C.M.; Zhao, L.; Feng, H.Y.; Yue, G.Y.; Ren, Z.W.; Cheng, G.D. The soil carbon/nitrogen ratio and moisture affect microbial community structures in alkaline permafrost-affected soils with different vegetation types on the Tibetan plateau. Res. Microbiol. 2014, 165, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Ingwersen, J.; Poll, C.; Streck, T.; Kandeler, E. Micro-scale modelling of carbon turnover driven by microbial succession at a biogeochemical interface. Soil Biol. Biochem. 2008, 40, 864–878. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wang, C.; Xu, W.W.; Huang, X.R.; Zhang, Z.D. Changes in soil nutrients and bacterial communities of Larix principis-rupprechtii plantations with different generations. J. Beijing For. Univ. 2020, 42, 36–45. [Google Scholar]
- Xiang, X.; Man, B.Y.; Zhang, J.Z.; Luo, Y.; Mao, X.T.; Zhang, C.; Sun, B.H.; Wang, X. Vertical Distribution of Bacterial Community and Functional Groups Mediating Nitrogen Cycling in Mount Huangshan, Anhui, China. Ecol. Environ. 2023, 32, 56–69. [Google Scholar]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Yang, C.; Yu, S.; Liu, C.Y.; Chi, N.Y. Bacterial diversity of marine sediments in the Yellow Sea. Microbial 2020, 47, 370–378. [Google Scholar]
- Wang, Z.; Liu, Y.; Wang, F. Spatial Patterns of Soil Bacterial Communities and N-cycling Functional Groups along an Elevation Gradient in Datong River Basin. Environ. Sci. 2023, 9, 1–13. [Google Scholar]
- Cao, T.T.; Fang, Y.; Chen, Y.R.; Kong, X.S.; Yang, J.B.; Alharbi, H.; Kuzyakov, Y.; Tian, X.J. Synergy of saprotrophs with mycorrhiza for litter decomposition and hotspot formation depends on nutrient availability in the rhizosphere. Geoderma 2022, 410, 115662. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, F.B.; Wang, Y.; Wang, J.M.; Li, J.W.; Zhang, Z.X. Variation and drivers of soil fungal and functional groups among different forest types in warm temperate secondary forests. Global Ecol. Conserv. 2023, 45, e02523. [Google Scholar] [CrossRef]
- Yang, H.Q.; Xiang, Y.Q.; Li, Q.; Yin, B.R.; Tang, Z.R.; Zhang, Y.; Chen, G.; Lai, J.M.; Fan, C.; Li, X.W. A comparative study on the soil fungal community structure across three mixed forests at the initial stage of afforestation. Acta Ecol. Sin. 2024, 44, 1–12. [Google Scholar]
- Nguyen, D.; Castagneyrol, B.; Bruelheide, H.; Bussotti, F.; Guyot, V.; Jactel, H.; Jaroszewicz, B.; Valladares, F.; Stenlid, J.; Boberg, J. Fungal disease incidence along tree diversity gradients depends on latitude in European forests. Ecol. Evol. 2016, 6, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Hönig, L.; Braun, U.; Haase, J.; Purschke, O.; Scherer-Lorenzen, M.; Bruelheide, H. No plant functional diversity effects on foliar fungal pathogens in experimental tree communities. Fungal Divers. 2014, 66, 139–151. [Google Scholar]


| Sample | pH | MC (%) | TOC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | AP (mg·kg−1) | DOC (g·kg−1) | DON (g·kg−1) |
|---|---|---|---|---|---|---|---|---|
| LBL | 6.40 ± 0.01 a | 16.69 ± 0.94 b | 36.62 ± 1.40 b | 4.69 ± 0.31 b | 2.24 ± 0.03 a | 17.32 ± 0.45 a | 3.40 ± 0.02 b | 0.37 ± 0.01 b |
| BK | 5.88 ± 0.01 b | 53.12 ± 1.63 a | 212.43 ± 1.30 a | 16.90 ± 0.07 a | 1.68 ± 0.03 b | 11.23 ± 0.25 b | 6.96 ± 0.19 a | 2.72 ± 0.03 a |
| Sample/Index | Bacterial | Fungal | ||
|---|---|---|---|---|
| LBL | BK | LBL | BK | |
| Sobs | 2319.33 ± 43.94 a | 2360.83 ± 36.41 a | 205.00 ± 9.31 b | 281.00 ± 10.05 a |
| Shannon | 6.35 ± 0.11 a | 6.44 ± 0.02 a | 3.39 ± 0.14 a | 3.21 ± 0.34 a |
| Ace | 2413.91 ± 23.60 a | 2478.56 ± 32.03 a | 218.92 ± 9.05 b | 294.04 ± 11.51 a |
| Pd | 111.65 ± 1.82 a | 108.53 ± 2.26 a | 42.10 ± 2.22 b | 65.45 ± 2.88 a |
| Coverage | 0.9967 ± 0.0004 a | 0.9962 ± 0.0005 a | 0.9995 ± 0.0001 a | 0.9994 ± 0.0002 a |
| Soil Factors | Bacterial | Fungal | ||
|---|---|---|---|---|
| R2 | p-Value | R2 | p-Value | |
| pH | 0.8558 | 0.001 * | 0.9840 | 0.004 * |
| MC | 0.8773 | 0.002 * | 0.9951 | 0.006 * |
| TOC | 0.8056 | 0.002 * | 0.9746 | 0.006 * |
| TN | 0.8548 | 0.001 * | 0.9544 | 0.004 * |
| TP | 0.7823 | 0.002 * | 0.7482 | 0.008 * |
| AP | 0.7986 | 0.002 * | 0.6946 | 0.012 * |
| DOC | 0.7310 | 0.002 * | 0.9568 | 0.003 * |
| DON | 0.8623 | 0.002 * | 0.9864 | 0.006 * |
| Types | Functional Groups | LBL-Mean (%) | BK-Mean (%) |
|---|---|---|---|
| Bacterial | Chemoheterotrophy | 34.32 ± 0.72 a | 28.67 ± 1.28 b |
| Aerobic chemoheterotrophy | 33.92 ± 0.72 a | 28.28 ± 1.27 b | |
| Nitrogen fixation | 7.65 ± 1.09 a | 6.1 ± 0.5 a | |
| Nitrification | 1.39 ± 0.41 b | 4.12 ± 0.58 a | |
| Aerobic ammonia oxidation | 1.12 ± 0.3 b | 3.03 ± 0.37 a | |
| Nitrate reduction | 2.11 ± 0.19 a | 1.96 ± 0.14 a | |
| Animal parasites or symbionts | 0.63 ± 0.21 b | 3.13 ± 1.41 a | |
| Ureolysis | 1.93 ± 0.24 a | 1.43 ± 0.2 b | |
| Aromatic compound degradation | 1.11 ± 0.22 b | 1.85 ± 0.32 a | |
| Nitrate respiration | 1.19 ± 0.18 b | 1.42 ± 0.17 a | |
| Nitrogen respiration | 1.19 ± 0.18 b | 1.42 ± 0.17 a | |
| Fungal | Undefined Saprotroph | 20.67 ± 5.3 a | 6.18 ± 1.5 b |
| Ectomycorrhizal | 18.87 ± 4.5 a | 7.82 ± 2.24 b | |
| Plant Saprotroph-Wood Saprotroph | 25.47 ± 4.42 a | 0.01 ± 0.01 b | |
| Endophyte-Litter Saprotroph-Soil SaproTroph-Undefined Saprotroph | 2.54 ± 0.55 b | 15.96 ± 3.9 a | |
| Endophyte | 9.46 ± 2.52 a | 0.57 ± 0.09 b | |
| Ericoid Mycorrhizal | 4.45 ± 1.49 a | 5 ± 0.86 a | |
| Ectomycorrhizal-Orchid Mycorrhizal- Root Associated Biotroph | 1.19 ± 0.22 b | 4.77 ± 1.43 a | |
| Animal Pathogen | 0.14 ± 0.06 b | 5.18 ± 0.75 a | |
| Animal Pathogen-Undefined Saprotroph | 1.07 ± 0.19 b | 3.9 ± 0.91 a | |
| Fungal Parasite-Undefined Saprotroph | 1.26 ± 0.24 a | 0.66 ± 0.19 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Yang, F.; Cao, H.; Cheng, J.; Jiang, M.; Li, M.; Ni, H.; Xie, L. Comparison of Microbial Diversity of Two Typical Volcanic Soils in Wudalianchi, China. Microorganisms 2024, 12, 656. https://doi.org/10.3390/microorganisms12040656
Huang Q, Yang F, Cao H, Cheng J, Jiang M, Li M, Ni H, Xie L. Comparison of Microbial Diversity of Two Typical Volcanic Soils in Wudalianchi, China. Microorganisms. 2024; 12(4):656. https://doi.org/10.3390/microorganisms12040656
Chicago/Turabian StyleHuang, Qingyang, Fan Yang, Hongjie Cao, Jiahui Cheng, Mingyue Jiang, Maihe Li, Hongwei Ni, and Lihong Xie. 2024. "Comparison of Microbial Diversity of Two Typical Volcanic Soils in Wudalianchi, China" Microorganisms 12, no. 4: 656. https://doi.org/10.3390/microorganisms12040656
APA StyleHuang, Q., Yang, F., Cao, H., Cheng, J., Jiang, M., Li, M., Ni, H., & Xie, L. (2024). Comparison of Microbial Diversity of Two Typical Volcanic Soils in Wudalianchi, China. Microorganisms, 12(4), 656. https://doi.org/10.3390/microorganisms12040656







