Harmful and Harmless Soil-Dwelling Fungi Indicate Microhabitat Suitability for Off-Host Ixodid Ticks
Abstract
1. Introduction
2. Methods
2.1. Tick Maintenance
2.2. Propagation of Fungi and Collection of Conidia
2.3. Preparation of Experimental Substrates
2.4. General Experimental Design
2.5. Specific Experiments
2.5.1. Effect of Bb-Inoculated Substrate on Behavioral Responses of Diverse Tick Taxa
2.5.2. Effect of Bb-Incubation Period on Behavioral Responses of Ticks
2.5.3. Olfactory or Contact-Chemoreceptive Recognition of Bb (or Its Metabolites) by Ticks
2.5.4. Effect of Cellulose or Its Fungal Metabolites on Behavioral Responses of Ticks
2.5.5. Effect of Various Soil-Dwelling Fungi on Behavioral Responses of Ticks
2.5.6. Effect of Fungus-Derived Volatiles on Attraction of Ticks
2.6. Statistical Analysis
3. Results
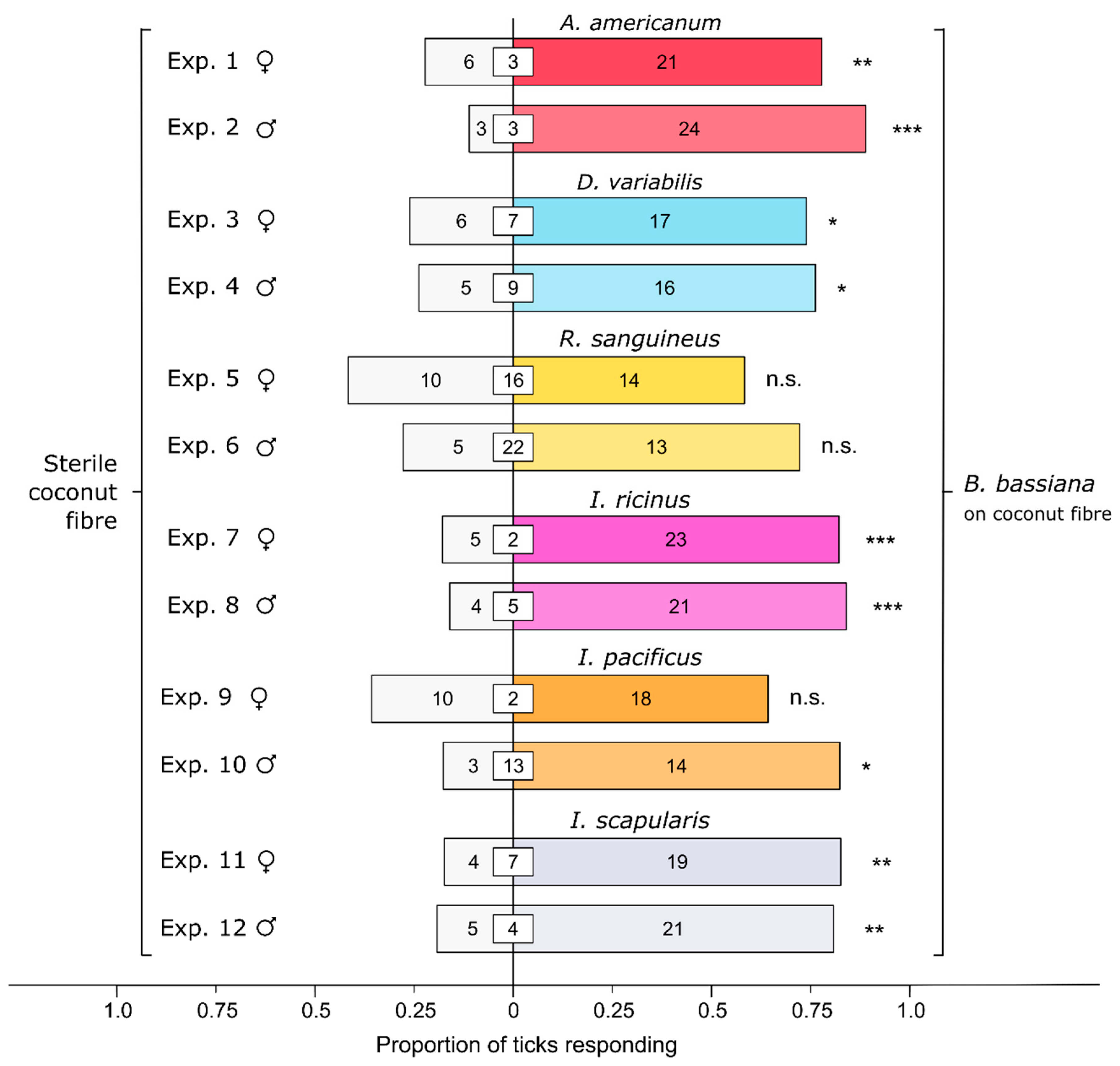
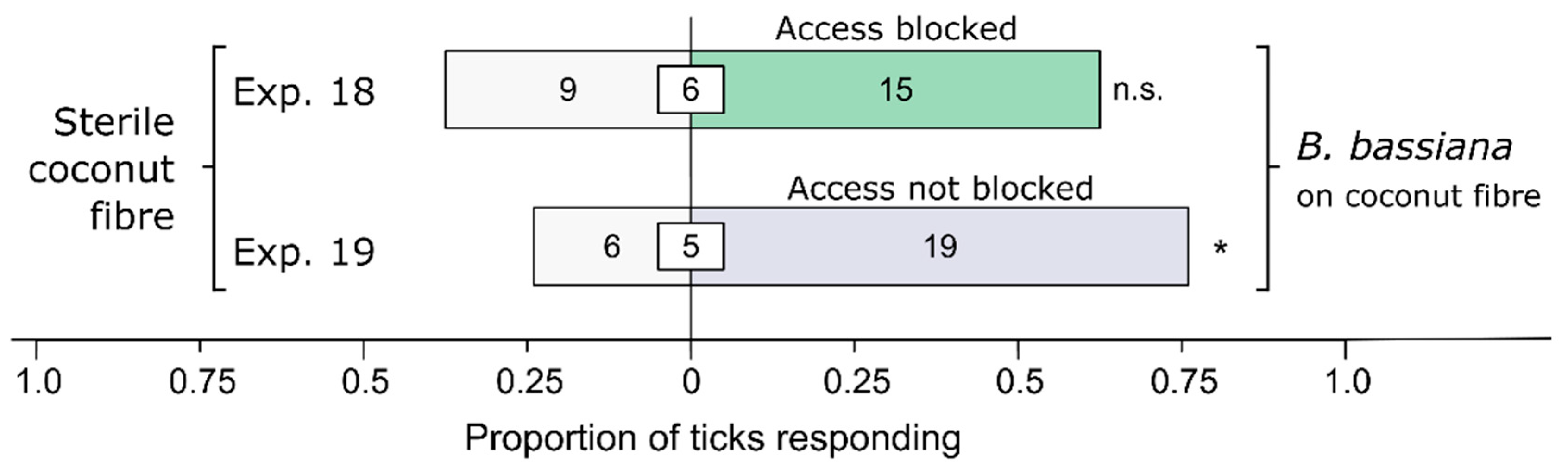
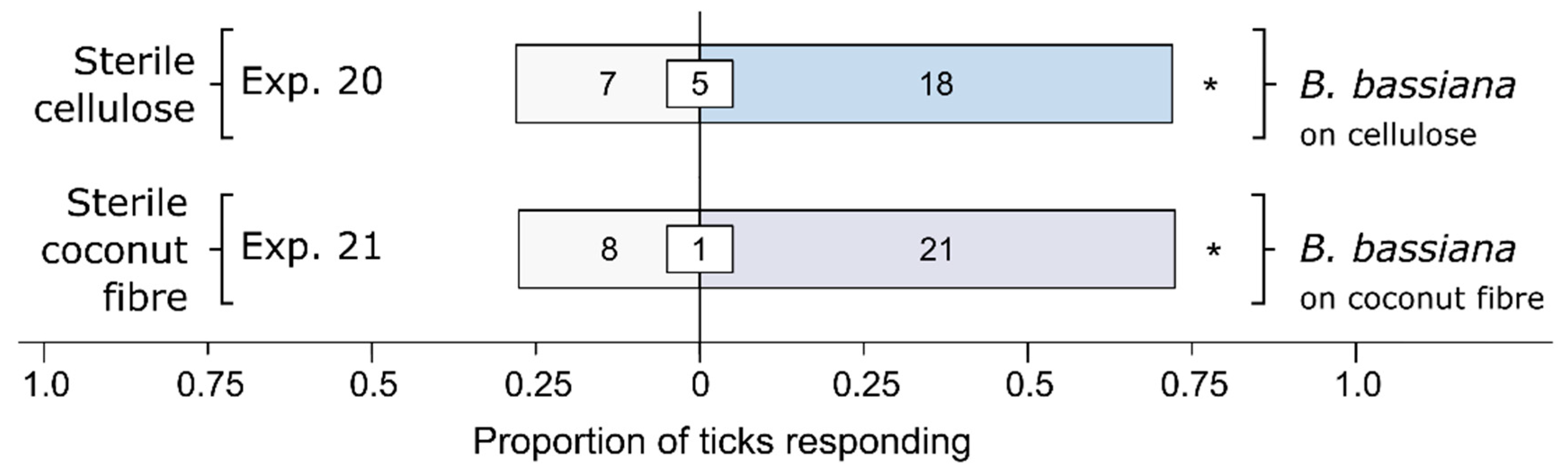
3.1. Effect of Bb-Inoculated Substrate on Behavioral Responses of Ticks
3.2. Effect of Bb-Incubation Period on Behavioral Responses of Ticks
3.3. Olfactory or Contact-Chemoreceptive Recognition of Bb (or Its Metabolites) by Ticks
3.4. Effect of Cellulose or Its Fungal Metabolites on Behavioral Responses of Ticks
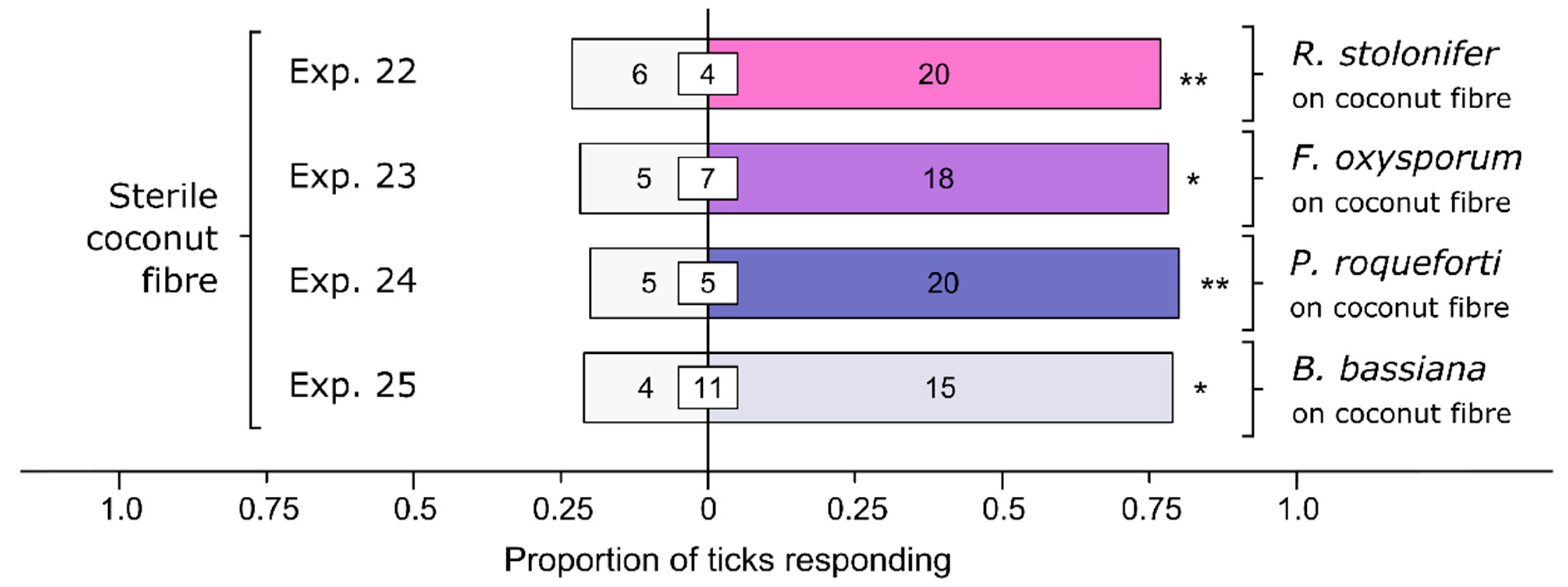
3.5. Effect of Various Soil-Dwelling Fungi on Behavioral Responses of Ticks
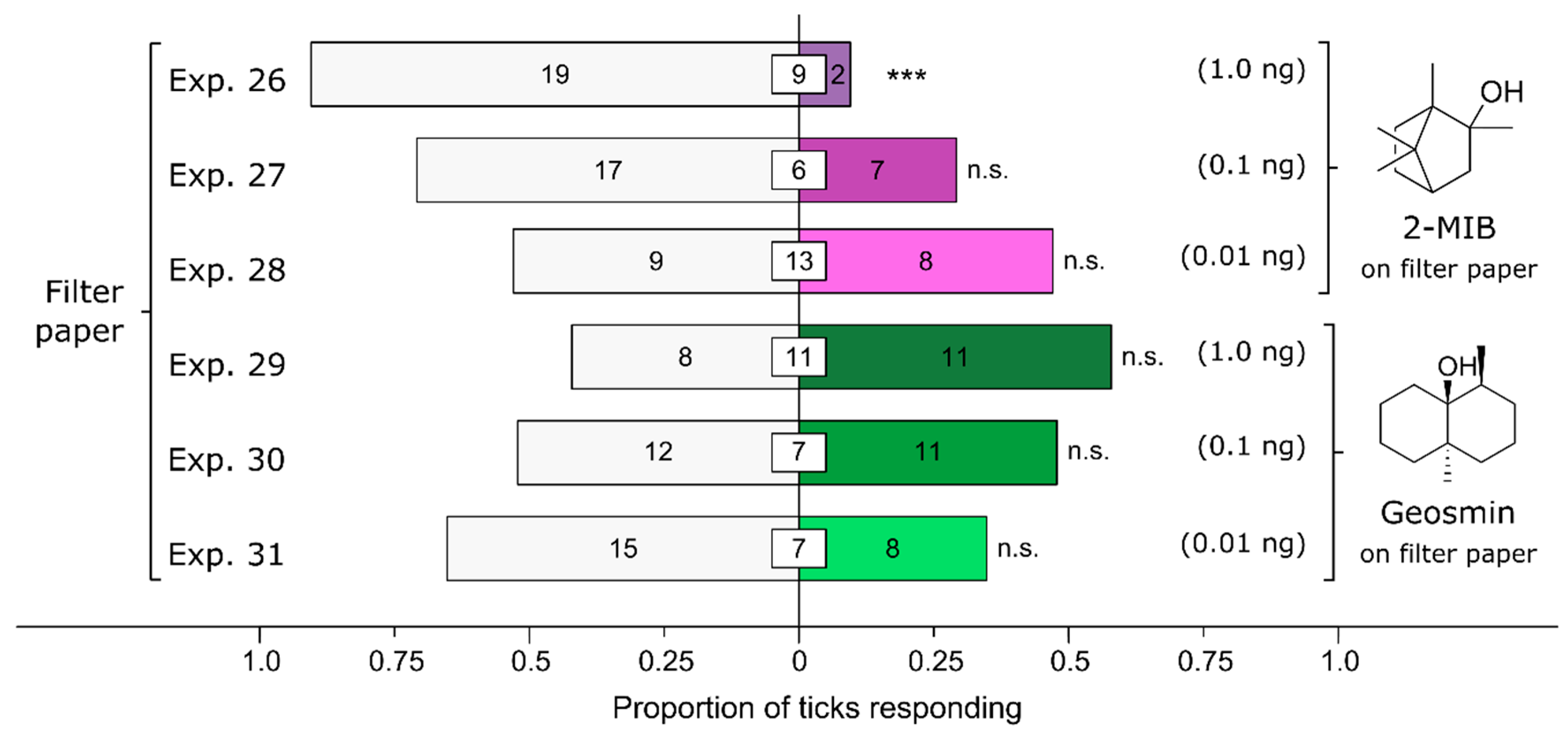
3.6. Effect of Fungus-Derived Volatiles on Attraction of Ticks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wikel, S.K. Tick-Host Interactions. In Biology of Ticks; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 2, pp. 88–128. [Google Scholar]
- Gray, J.S.; Estrada-Peña, A.; Vial, L. Ecology of Nidicolous Ticks. In Biology of Ticks; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 2, pp. 3–38. [Google Scholar]
- Zając, Z.; Bartosik, K.; Woźniak, A. Monitoring Dermacentor reticulatus host-seeking activity in natural conditions. Insects 2020, 11, 264. [Google Scholar] [CrossRef]
- Suzin, A.; Vogliotti, A.; Nunes, P.H.; Barbieri, A.R.M.; Labruna, M.B.; Szabó, M.P.J. Free-living ticks (Acari: Ixodidae) in the Iguaçu National Park, Brazil: Temporal dynamics and questing behavior on vegetation. Ticks Tick. Borne. Dis. 2020, 11, 101471. [Google Scholar] [CrossRef]
- Loye, J.E.; Lane, R.S. Questing behavior of Ixodes pacificus (Acari: Ixodidae) in relation to meteorological and seasonal factors. J. Med. Entomol. 1988, 25, 391–398. [Google Scholar] [CrossRef]
- Arsnoe, I.M.; Hickling, G.J.; Ginsberg, H.S.; McElreath, R.; Tsao, J.I. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PloS ONE 2015, 10, e0127450. [Google Scholar] [CrossRef]
- Mejlon, H.A.; Jaenson, T.G.T. Questing behaviour of Ixodes ricinus ticks (Acari: Ixodidae). Exp. Appl. Acarol. 1997, 21, 747–754. [Google Scholar] [CrossRef]
- Waladde, S.M.; Rice, M.J. The sensory basis of tick feeding behaviour. In Physiology of Ticks; Obenchain, F.D., Galun, R., Eds.; Pergamon Press: Oxford, UK, 1982; pp. 71–118. [Google Scholar]
- Crooks, E.; Randolph, S.E. Walking by Ixodes ricinus ticks: Intrinsic and extrinsic factors determine the attraction of moisture or host odour. J. Exp. Biol. 2006, 209, 2138–2142. [Google Scholar] [CrossRef]
- Sonenshine, D.E. Tick pheromones and their use in tick control. Annu. Rev. Entomol. 2006, 51, 557–580. [Google Scholar] [CrossRef]
- Randolph, S.E. Ecology of Non-Nidicolous Ticks. In Biology of Ticks; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 2. [Google Scholar]
- Vail, S.G.; Smith, G. Vertical movement and posture of blacklegged tick (Acari: Ixodidae) nymphs as a function of temperature and relative humidity in laboratory experiments. J. Med. Entomol. 2002, 39, 842–846. [Google Scholar] [CrossRef]
- Perret, J.L.; Guerin, P.M.; Diehl, P.A.; Vlimant, M.; Gern, L. Darkness induces mobility, and saturation deficit limits questing duration, in the tick Ixodes ricinus. J. Exp. Biol. 2003, 206, 1809–1815. [Google Scholar] [CrossRef]
- Lubelczyk, C.B.; Elias, S.P.; Rand, P.W.; Holman, M.S.; Lacombe, E.H.; Smith, R.P. Habitat associations of Ixodes scapularis (Acari: Ixodidae) in Maine. Community Ecosyst. Ecol. 2004, 33, 900–906. [Google Scholar] [CrossRef]
- Bertrand, M.R.; Wilson, M.L. Microclimate-dependent survival of unfed adult Ixodes scapularis (Acari: Ixodidae) in nature: Life cycle and study design implications. J. Med. Entomol. 1996, 33, 619–627. [Google Scholar] [CrossRef]
- Goddard, J. Ticks and tick ecology in Mississippi: Implications for human disease transmission. J. Mississippi Acad. Sci. 2001, 46, 100–101. [Google Scholar]
- Burtis, J.C.; Yavitt, J.B.; Fahey, T.J.; Ostfeld, R.S. Ticks as soil-dwelling arthropods: An intersection between disease and soil ecology. J. Med. Entomol. 2019, 56, 1555–1564. [Google Scholar] [CrossRef]
- Linske, M.A.; Stafford, K.C.; Williams, S.C.; Lubelczyk, C.B.; Welch, M.; Henderson, E.F. Impacts of deciduous leaf litter and snow presence on nymphal Ixodes scapularis (Acari: Ixodidae) overwintering survival in coastal New England, USA. Insects 2019, 10, 227. [Google Scholar] [CrossRef]
- Leal, B.; Zamora, E.; Fuentes, A.; Thomas, D.B.; Dearth, R.K. Questing by tick larvae (Acari: Ixodidae): A review of the influences that affect off-host survival. Ann. Entomol. Soc. Am. 2020, 113, 425–438. [Google Scholar] [CrossRef]
- Cusumano, A.; Lievens, B. Microbe-mediated alterations in floral nectar: Consequences for insect parasitoids. Curr. Opin. Insect Sci. 2023, 60, 101116. [Google Scholar] [CrossRef]
- Peach, D.A.H.; Carroll, C.; Meraj, S.; Gomes, S.; Galloway, E.; Balcita, A.; Coatsworth, H.; Young, N.; Uriel, Y.; Gries, R.; et al. Nectar-dwelling microbes of common tansy are attractive to its mosquito pollinator, Culex pipiens L. BMC Ecol. Evol. 2021, 21, 29. [Google Scholar] [CrossRef]
- Hubbard, C.J.; Li, B.; McMinn, R.; Brock, M.T.; Maignien, L.; Ewers, B.E.; Kliebenstein, D.; Weinig, C. The effect of rhizosphere microbes outweighs host plant genetics in reducing insect herbivory. Mol. Ecol. 2019, 28, 1801–1811. [Google Scholar] [CrossRef]
- Tao, L.; Hunter, M.D.; de Roode, J.C. Microbial root mutualists affect the predators and pathogens of herbivores above ground: Mechanisms, magnitudes, and missing links. Front. Ecol. Evol. 2017, 5, 160. [Google Scholar] [CrossRef]
- Romero, A.; Broce, A.; Zurek, L. Role of bacteria in the oviposition behaviour and larval development of stable flies. Med. Vet. Entomol. 2006, 20, 115–121. [Google Scholar] [CrossRef]
- Melo, N.; Wolff, G.H.; Costa-da-Silva, A.L.; Arribas, R.; Triana, M.F.; Gugger, M.; Riffell, J.A.; DeGennaro, M.; Stensmyr, M.C. Geosmin attracts Aedes aegypti mosquitoes to oviposition sites. Curr. Biol. 2020, 30, 127–134.e5. [Google Scholar] [CrossRef]
- Girard, M.; Martin, E.; Vallon, L.; Raquin, V.; Bellet, C.; Rozier, Y.; Desouhant, E.; Hay, A.E.; Luis, P.; Moro, C.V.; et al. Microorganisms associated with mosquito oviposition sites: Implications for habitat selection and insect life histories. Microorganisms 2021, 9, 1589. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Crippen, T.L.; Holmes, L.; Singh, B.; Pimsler, M.L.; Benbow, M.E.; Tarone, A.M.; Dowd, S.; Yu, Z.; Vanlaerhoven, S.L.; et al. Bacteria mediate oviposition by the black soldier fly, Hermetia illucens (L.), (Diptera: Stratiomyidae). Sci. Rep. 2013, 3, srep02563. [Google Scholar] [CrossRef]
- Long, J.; Maskell, K.; Gries, R.; Nayani, S.; Gooding, C.; Gries, G. Synergistic attraction of western black-legged ticks, Ixodes pacificus, to CO2 and odorant emissions from deer-associated microbes. R. Soc. Open Sci. 2023, 10, 230084. [Google Scholar] [CrossRef]
- Becher, P.G.; Verschut, V.; Bibb, M.J.; Bush, M.J.; Molnár, B.P.; Barane, E.; Al-Bassam, M.M.; Chandra, G.; Song, L.; Challis, G.L.; et al. Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat. Microbiol. 2020, 5, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ren, L.; Li, H.; Schmidt, A.; Gershenzon, J.; Lu, Y.; Cheng, D. The nesting preference of an invasive ant is associated with the cues produced by actinobacteria in soil. PloS Pathog. 2020, 16, e1008800. [Google Scholar] [CrossRef] [PubMed]
- Tabachek, J.-A.L.; Yurkowski, M. Isolation and identification of blue-green algae producing muddy odor metabolites, geosmin, and 2-methylisoborneol, in saline lakes in Manitoba. J. Fish. Res. Board Canada 1976, 33, 25–35. [Google Scholar] [CrossRef]
- Safferman, R.S.; Rosen, A.A.; Mashni, C.I.; Morris, M.E. Earthy-smelling substance from a blue-green alga. Environ. Sci. Technol. 1967, 1, 429–430. [Google Scholar] [CrossRef]
- Klausen, C.; Nicolaisen, M.H.; Strobel, B.W.; Warnecke, F.; Nielsen, J.L.; Jørgensen, N.O.G. Abundance of actinobacteria and production of geosmin and 2-methylisoborneol in Danish streams and fish ponds. FEMS Microbiol. Ecol. 2005, 52, 265–278. [Google Scholar] [CrossRef]
- La Guerche, S.; Dauphin, B.; Pons, M.; Blancard, D.; Darriet, P. Characterization of some mushroom and earthy off-odors microbially induced by the development of rot on grapes. J. Agric. Food Chem. 2006, 54, 9193–9200. [Google Scholar] [CrossRef]
- Fravel, D.R.; Connick, W.J.; Grimm, C.C.; Lloyd, S.W. Volatile compounds emitted by sclerotia of Sclerotinia minor, Sclerotinia sclerotiorum, and Sclerotium rolfsii. J. Agric. Food Chem. 2002, 50, 3761–3764. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Roberts, R.G. Identification of geosmin as a volatile metabolite of Penicillium expansum. Appl. Environ. Microbiol. 1992, 58, 3170–3172. [Google Scholar] [CrossRef]
- Gooding, C.E.; Pinard, C.; Gries, R.; Devireddy, A.; Gries, G. Blacklegged ticks, Ixodes scapularis, reduce predation risk by eavesdropping on communication signals of Formica oreas thatching ants. R. Soc. Open Sci. 2024, 11, 231355. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Tan, K.; Qu, Y.; Nieh, J.C. Giant Asian honeybees use olfactory eavesdropping to detect and avoid ant predators. Anim. Behav. 2014, 97, 69–76. [Google Scholar] [CrossRef]
- Storm, J.J.; Lima, S.L. Predator-naïve fall field crickets respond to the chemical cues of wolf spiders. Can. J. Zool. 2008, 86, 1259–1263. [Google Scholar] [CrossRef]
- Sidhu, C.S.; Wilson Rankin, E.E. Honey bees avoiding ant harassment at flowers using scent cues. Environ. Entomol. 2016, 45, 420–426. [Google Scholar] [CrossRef]
- Kempraj, V.; Park, S.J.; Taylor, P.W. Forewarned is forearmed: Queensland fruit flies detect olfactory cues from predators and respond with predator-specific behaviour. Sci. Rep. 2020, 10, 7297. [Google Scholar] [CrossRef]
- Mestre, L.; Bucher, R.; Entling, M.H. Trait-mediated effects between predators: Ant chemical cues induce spider dispersal. J. Zool. 2014, 293, 119–125. [Google Scholar] [CrossRef]
- Bucher, R.; Binz, H.; Menzel, F.; Entling, M.H. Effects of spider chemotactile cues on arthropod behavior. J. Insect Behav. 2014, 27, 567–580. [Google Scholar] [CrossRef]
- Bucher, R.; Menzel, F.; Entling, M.H. Risk of spider predation alters food web structure and reduces local herbivory in the field. Oecologia 2015, 178, 571–577. [Google Scholar] [CrossRef]
- Ormond, E.L.; Thomas, A.P.M.; Pugh, P.J.A.; Pell, J.K.; Roy, H.E. A fungal pathogen in time and space: The population dynamics of Beauveria bassiana in a conifer forest. FEMS Microbiol. Ecol. 2010, 74, 146–154. [Google Scholar] [CrossRef][Green Version]
- Shimazu, M.; Sato, H.; Maehara, N. Density of the entomopathogenic fungus, Beauveria bassiana Vuillemin (Deuteromycotina: Hyphomycetes) in forest air and soil. J. Appl. Entomol. Zool. 2002, 37, 19–26. [Google Scholar] [CrossRef][Green Version]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Kirkland, B.H.; Westwood, G.S.; Keyhani, N.O. Pathogenicity of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae to Ixodidae tick species Dermacentor variabilis, Rhipicephalus sanguineus, and Ixodes scapularis. J. Med. Entomol. 2004, 41, 705–711. [Google Scholar] [CrossRef]
- Sullivan, C.F.; Parker, B.L.; Skinner, M. A review of commercial Metarhizium and Beauveria based biopesticides for the biological control of ticks in the USA. Insects 2022, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Pell, J.K. Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecol. Entomol. 2004, 31, 162–171. [Google Scholar] [CrossRef]
- Ormond, E.L.; Thomas, A.P.M.; Pell, J.K.; Freeman, S.N.; Roy, H.E. Avoidance of a generalist entomopathogenic fungus by the ladybird, Coccinella septempunctata. FEMS Microbiol. Ecol. 2011, 77, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Mburu, D.M.; Ochola, L.; Maniania, N.K.; Njagi, P.G.N.; Gitonga, L.M.; Ndung’u, M.W.; Wanjoya, A.K.; Hassanali, A. Relationship between virulence and repellency of entomopathogenic isolates of Metarhizium anisopliae and Beauveria bassiana to the termite Macrotermes michaelseni. J. Insect Physiol. 2009, 55, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Alma, C.R.; Gillespie, D.R.; Roitberg, B.D.; Goettel, M.S. Threat of infection and threat-avoidance behavior in the predator Dicyphus esperus feeding on whitefly nymphs infected with an entomopathogen. J. Insect Behav. 2010, 23, 90–99. [Google Scholar] [CrossRef]
- Wu, S.; Xing, Z.; Sun, W.; Xu, X.; Meng, R.; Lei, Z. Effects of Beauveria bassiana on predation and behavior of the predatory mite Phytoseiulus persimilis. J. Invertebr. Pathol. 2018, 153, 51–56. [Google Scholar] [CrossRef]
- Thompson, S.R.; Brandenburg, R.L.; Roberson, G.T. Entomopathogenic fungi detection and avoidance by mole crickets (Orthoptera: Gryllotalpidae). Environ. Entomol. 2007, 36, 165–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- George, J.; Jenkins, N.E.; Blanford, S.; Thomas, M.B.; Baker, T.C. Malaria mosquitoes attracted by fatal fungus. PloS ONE 2013, 8, e62632. [Google Scholar] [CrossRef] [PubMed]
- Geedi, R.; Canas, L.; Reding, M.E.; Ranger, C.M. Attraction of Myzus persicae (Hemiptera: Aphididae) to volatiles emitted from the entomopathogenic fungus Beauveria bassiana. Environ. Entomol. 2023, 52, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, B.W.; Roe, R.M. Tick Repellent Research, Methods, and Development. In Biology of Ticks; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 2, pp. 382–408. [Google Scholar]
- Ritz, K.; Young, I.M. Interactions between soil structure and fungi. Mycologist 2004, 18, 52–59. [Google Scholar] [CrossRef]
- Albariño, R.; Villanueva, V.D.; Canhoto, C. The effect of sunlight on leaf litter quality reduces growth of the shredder Klapopteryx kuscheli. Freshw. Biol. 2008, 53, 1881–1889. [Google Scholar] [CrossRef]
- Pieristè, M.; Forey, E.; Lounès-Hadj Sahraoui, A.; Meglouli, H.; Laruelle, F.; Delporte, P.; Robson, T.M.; Chauvat, M. Spectral composition of sunlight affects the microbial functional structure of beech leaf litter during the initial phase of decomposition. Plant Soil 2020, 451, 515–530. [Google Scholar] [CrossRef]
- Baldrian, P.; Merhautová, V.; Petránková, M.; Cajthaml, T.; Šnajdr, J. Distribution of microbial biomass and activity of extracellular enzymes in a hardwood forest soil reflect soil moisture content. Appl. Soil Ecol. 2010, 46, 177–182. [Google Scholar] [CrossRef]
- Samanta, A.K.; Basu, G.; Mishra, L. Role of major constituents of coconut fibres on absorption of ionic dyes. Ind. Crops Prod. 2018, 117, 20–27. [Google Scholar] [CrossRef]
- Eggins, H.O.W.; Pugh, G.J.F. Isolation of cellulose-decomposing fungi from the soil. Nature 1962, 193, 94–95. [Google Scholar] [CrossRef]
- Conrady, M.W.; Bauer, M.; Jo, K.D.; Cropek, D.M.; Busby, R.R. Solid-phase microextraction (SPME) for determination of geosmin and 2-methylisoborneol in volatile emissions from soil disturbance. Chemosphere 2021, 284, 131333. [Google Scholar] [CrossRef]
- Wickham, H.; Seidel, D. Scales: Scale Functions for Visualization 2022. Available online: https://cran.r-project.org/package=scales (accessed on 11 December 2023).
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit. Vectors 2010, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Knülle, W.; Rudolph, D. Humidity relationships and water balance of ticks. In Physiology of Ticks; Obenchain, F.D., Galun, R., Eds.; Pergamon Press: Oxford, UK, 1982; pp. 43–70. [Google Scholar]
- Baldrian, P.; Valášková, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Carlile, M.J.; Watkinson, S.C.; Gooday, G.W. Fungal Cells and Vegetative Growth. In The Fungi; Carlile, M.J., Watkinson, S.C., Gooday, G.W., Eds.; Academic Press: London, UK, 2001; pp. 85–184. [Google Scholar]
- Trabalon, M. Chemical Communication and Contact Cuticular Compounds in Spiders. In Spider Ecophysiology; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 125–140. [Google Scholar]
- Stensmyr, M.C.; Dweck, H.K.M.; Farhan, A.; Ibba, I.; Strutz, A.; Mukunda, L.; Linz, J.; Grabe, V.; Steck, K.; Lavista-Llanos, S.; et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 2012, 151, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Zaroubi, L.; Ozugergin, I.; Mastronardi, K.; Imfeld, A.; Law, C.; Gélinas, Y.; Piekny, A.; Findlay, B.L. The ubiquitous soil terpene geosmin acts as a warning chemical. Appl. Environ. Microbiol. 2022, 88, e0009322. [Google Scholar] [CrossRef] [PubMed]
- Busby, R.R.; Conrady, M.W.; Jo, K.D.; Cropek, D.M. Characterising earth scent. Environ. Chem. 2023, 20, 226–234. [Google Scholar] [CrossRef]
- Carroll, J.F.; Benante, J.P.; Kramer, M.; Lohmeyer, K.H.; Lawrence, K. Formulations of DEET, picaridin, and IR3535 applied to skin repel nymphs of the lone star tick (Acari: Ixodidae) for 12 hours. J. Med. Entomol. 2010, 47, 699–704. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gooding, C.E.; Gould, L.; Gries, G. Harmful and Harmless Soil-Dwelling Fungi Indicate Microhabitat Suitability for Off-Host Ixodid Ticks. Microorganisms 2024, 12, 609. https://doi.org/10.3390/microorganisms12030609
Gooding CE, Gould L, Gries G. Harmful and Harmless Soil-Dwelling Fungi Indicate Microhabitat Suitability for Off-Host Ixodid Ticks. Microorganisms. 2024; 12(3):609. https://doi.org/10.3390/microorganisms12030609
Chicago/Turabian StyleGooding, Claire E., Layla Gould, and Gerhard Gries. 2024. "Harmful and Harmless Soil-Dwelling Fungi Indicate Microhabitat Suitability for Off-Host Ixodid Ticks" Microorganisms 12, no. 3: 609. https://doi.org/10.3390/microorganisms12030609
APA StyleGooding, C. E., Gould, L., & Gries, G. (2024). Harmful and Harmless Soil-Dwelling Fungi Indicate Microhabitat Suitability for Off-Host Ixodid Ticks. Microorganisms, 12(3), 609. https://doi.org/10.3390/microorganisms12030609





