Abstract
Addressing pressing health concerns, modern medical research seeks to identify new antimicrobials to combat drug resistance, novel molecules for cancer treatment, and antioxidants for inflammation-related diseases. Pisolithus (Basidiomycota) is a ubiquitous and widely distributed fungal genus in forest ecosystems, known for establishing ectomycorrhizal associations with a range of host plants, enhancing their growth, and conferring protection against biotic and abiotic stresses. Beyond ecological applications, Pisolithus yields bioactive compounds with medicinal potential. This comprehensive review explores the transversal biological activity of Pisolithus fungi, aiming to provide a thorough overview of their antimicrobial, anticancer, and antioxidant potential. The focus is on elucidating bioactive compounds within Pisolithus to trigger further research for innovative applications. Compounds from Pisolithus displayed antimicrobial activity against a broad spectrum of microorganisms, including antibiotic-resistant bacteria. The efficacy of Pisolithus-derived compounds matched established medications, emphasizing their therapeutic potential. In anticancer research, the triterpene pisosterol stood out with documented cytotoxicity against various cancer cell lines, showcasing promise for novel anticancer therapies. Pisolithus was also recognized as a potential source of antioxidants, with basidiocarps exhibiting high antioxidant activity. In vivo validation and comprehensive studies on a broader range of compounds, together with mechanistic insights into the mode of action of Pisolithus-derived compounds, are compelling areas for future research.
1. Introduction
Nature stands as a highly promising reservoir of compounds with potential applications in human health. Some groups of organisms are widely known for their bioactive potential, namely plants, macroalgae, microalgae, bacteria, invertebrates, and fungi, particularly members of the Basidiomycota [1,2,3,4].
Basidiomycota is a diverse and ecologically significant phylum of fungi that encompasses over 40,000 species. They exhibit diverse lifestyles, from decomposers breaking down organic matter to pathogens affecting plant health. Additionally, some species function as mycorrhizal symbionts, forming vital partnerships with plants in various ecosystems [5,6]. Several species of this phylum reproduce sexually through basidiospores stored in the basidium, a club-shaped structure that gives these fungi the common name club fungi [7]. Basidiomycota, with their diverse properties, hold crucial applications in biotechnology and industry, contributing to areas such as enzyme production, bioremediation, and pharmaceutical development. Some well-known species of this phylum have been used for hundreds of years as food, such as Lentinula edodes (shiitake) and Agaricus bisporus, and as medicines (e.g., Ganoderma lucidum and Wolfiporia extensa) [8,9,10]. Others are known for their poisonous nature, like the death cap (Amanita phalloides) [11,12]. The number of bioactive natural products from Basidiomycota is considerable, as recently reviewed [8,13,14,15,16,17], and includes compounds with antimicrobial, anticancer, antioxidant, anti-inflammatory, and nutraceutical properties, among others.
Pisolithus, a fascinating genus of fungi within the Basidiomycota, exemplifies the diverse ecological roles and bioactive potential found within this group of fungi. Pisolithus species are widely distributed across various ecosystems on six continents and are recognized for their distinctive fruiting bodies (basidiocarps). Characterized by large, round structures resembling puffballs, Pisolithus basidiocarps are often found partially buried in the soil. Besides the basidiocarps, Pisolithus are composed of hyphae, thread-like structures that make up the mycelium of the fungus, and spores that are produced in the basidiocarps [18,19,20]. One of the notable features of Pisolithus lies in the fact that its species form ectomycorrhizal associations with plants, particularly trees. The Pisolithus genus contains 19 species that colonize the roots of more than 50 host plants. The mycorrhizal partnership involves a mutually beneficial relationship where the fungus assists the plant in nutrient uptake, especially phosphorus, in exchange for carbohydrates produced by the plant through photosynthesis. This symbiotic association enhances the growth and protects the plant against biotic and abiotic stresses [19,21,22]. The beneficial effects of Pisolithus species lead to their incorporation into forestry management plans for promoting healthier ecosystems and enhancing the long-term sustainable productivity of forestry plantations [23,24,25,26]. The most studied Pisolithus species is Pisolithus arhizus (syn. = Pisolithus tinctorius).
Beyond their ecological role and forestry applications, researchers have explored bioactive compounds produced by Pisolithus species, discovering substances with antimicrobial, anticancer, and antioxidant properties. These findings open avenues for further exploration into the medicinal potential of Pisolithus-derived compounds.
In contemporary medical research, critical challenges abound, propelling the field toward innovative solutions. The exploration and identification of novel antimicrobial products are imperative in combatting rising drug resistance [27,28]. Moreover, the ongoing search for new natural molecules in cancer treatment reflects a pressing need for transformative approaches to enhance therapeutic outcomes in the relentless battle against cancer [29,30]. Simultaneously, the quest for antioxidant molecules effective against inflammation-related diseases addresses widespread health concerns [31,32].
In this context, and considering the transversal biological activity of Pisolithus, the objective of this review is to provide a comprehensive overview of the (i) antimicrobial, (ii) anticancer, and (iii) antioxidant potential of these fungi. The focus extends to highlighting the bioactive compounds isolated from Pisolithus with the aim of catalyzing further research for exploring innovative applications of these ubiquitous and widely distributed fungi (Figure 1).
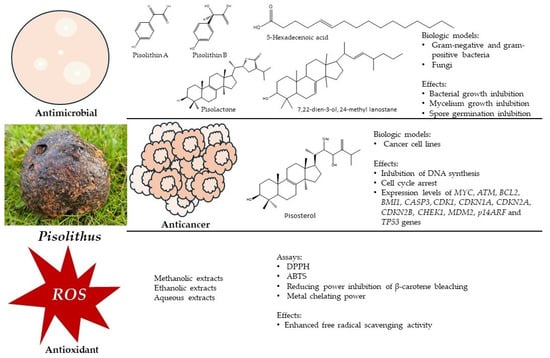
Figure 1.
A schematic representation of the bioactivities of Pisolithus, as described in this review.
2. The Bioactive Potential of Pisolithus
2.1. Antimicrobial Activity
Each year, millions of people worldwide succumb to microbial infections [33,34]. Microbial resistance to antimicrobial drugs is a growing global public health concern. Overuse and misuse of antibiotics have led to the development of resistant strains, rendering once-effective treatments ineffective [27,35]. Pathogenic microorganisms are a challenging threat to both human and animal health, underscoring the critical importance of addressing microbial infections in the context of public health [36,37]. Phytopathogens are harmful organisms that cause plant diseases, posing a significant threat to agriculture. They can devastate crops, leading to yield losses, economic challenges, and food security concerns [38,39]. Consequently, also in this field, an immediate and imperative need arises for the exploration and identification of novel antimicrobial agents. Recently, two new phenols with activity against several plant pathogenic fungi and clinically important bacterial strains were obtained from fungi [40].
Antimicrobial compounds exert their effects through various mechanisms, such as inhibition of the synthesis of the cell wall, proteins, nucleic acids, and cell membrane disruption of target microorganisms [41]. Several studies documented in the literature highlight Pisolithus as a potential genus with antimicrobial activity (Table 1). Kope and Fortin [42] demonstrated that the mycelium of P. tinctorius has antifungal activity by inhibiting spore germination, provoking hyphal lysis, and inhibiting chitin synthesis and consequently disrupting the cell wall in a range of phytopathogenic fungi, including Rhizoctonia praticola, Truncatella hartigii, Sphaerosporella brunnea, Fusarium solani, Brunchorstia pinea, and Cochliobolus sativus. Subsequently, the compounds pisolithin A and pisolithin B (Figure 2) were isolated from P. arhizus mycelium [43]. These two phenolic compounds showed a considerable capacity for reducing the mycelial growth of several phytopathogenic fungi, such as Rhizoctonia solani, Verticillium dahlia, Pyrenochaeta terrestris, Cochliobolus sativus and Septoria musiva, phytopathogenic oomycetes, such as Pythium debaryanum and Pythium ultimum and dermopathogenic fungi, such as Microsporum gypseum and Trichophyton equinum. In some cases, the inhibition was higher than that obtained with other antifungal agents such as nystatin and polyoxin D. With the compound pisolithin A, it was also demonstrated a spore germination inhibition of the plant pathogen T. hartigii, in concentrations ranging from 50 to 150 µg/mL in 24 h [44]. Shrestha et al. [45] showed that Pisolithus sp. extracts were able to inhibit the growth of the bacterial pathogens Klebsiella sp. and Escherichia coli. Another example that reinforces the antimicrobial proprieties of Pisolithus is the work of Ameri et al. [46], who conducted bioassays with Pisolithus albus crude extracts and isolated fractions against strains of methicillin-resistant Staphylococcus aureus. In their study, the authors observed a strong antibiotic action, especially in the fraction that contained sesquiterpenes. Pisolithus microcarpus crude extracts have also shown antibacterial potential against pathogens such as Pseudomonas aeruginosa and S. aureus [47].
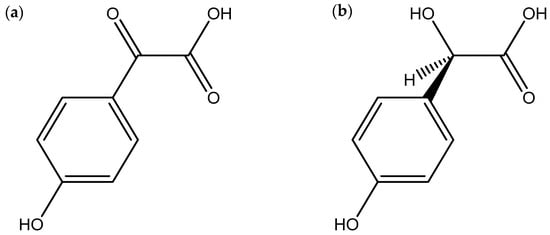
Figure 2.
Chemical structures of pisolithin A (a) and pisolithin B (b), two Pisolithus-derived phenolic compounds with antimicrobial activity.
In another study, ethanolic extract of P. albus displayed antibacterial activity against Gram-positive (Bacillus subtilis, Enterococcus faecalis, Listeria monocytogenes, S. aureus) and Gram-negative bacteria (Aeromonas hydrophila, E. coli, P. aeruginosa, S. typhimurium) [48].
In a recent investigation involving multidrug-resistant Gram-positive and Gram-negative bacteria isolated from wound exudates of hospitalized human patients, Martins et al. [49] found that hydroethanolic extracts of P. tinctorius had minimum inhibitory concentrations (MICs) of 5, <0.156, 10, 5, 5, 5 mg/mL for Enterococcus faecium, S. aureus, Acinetobacter baumannii, Enterobacter aerogenes, Klebsiella pneumoniae, and P. aeruginosa, respectively.
Carmo [50] prepared hexane and ethyl acetate fractions from P. tinctorius basidiocarps. Both fractions inhibited the growth of the Gram-positive bacterium Enterococcus sp., with MICs of 125 and 62.5 μg/mL for hexane and ethyl acetate fractions, respectively. The hexane fraction also inhibited the growth of Bacillus cereus and S. aureus, both with a MIC of 125 μg/mL. The human pathogenic Gram-negative bacteria, Shigella sonnei and Shigella flexneri, were also inhibited by these fractions. The antifungal potential of both fractions was demonstrated against human pathogenic fungi, with MICs of 125 μg/mL for Cryptococcus neoformans and Cryptococcus gattii and 62.5 μg/mL for Candida krusei. Following these promising results, Carmo [50] isolated and identified four secondary metabolites from the P. tinctorius extracts and tested the antimicrobial activity of these compounds separately (Table 1). The compounds were a ceramide P56, 5-hexadecenoic acid, and two triterpenoids: pisolactone and the new metabolite 7,22-dien-3-ol, 24-methyl lanostane. Although all four compounds displayed a certain degree of antibacterial activity, 5-hexadecenoic acid (Figure 3) was notable for inhibiting the pathogenic Gram-negative bacteria S. sonnei and E. aerogenes (MIC 25 μg/mL), P. aeruginosa, Morganella morgani, K. pneumoniae and S. flexneri (MIC 50 μg/mL), and the Gram-positive bacteria B. subtilis (MIC 50 μg/mL) and S. aureus (MIC 12.5 μg/mL).
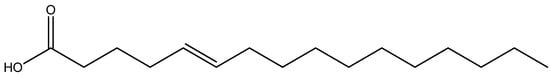
Figure 3.
Chemical structure of 5-hexadecenoic acid, a secondary metabolite with antibacterial activity isolated from Pisolithus tinctorius.
The antimycobacterial capacity was also revealed by pisolactone and ceramide P56, which were effective against the pathogenic Mycobacterium abcessus, exhibiting MIC values of 31.25 and 15.62 μg/mL, respectively. Moreover, the MIC displayed by ceramide P56 against M. abcessus was equivalent to the widely used antibiotic clarithromycin (MIC 16 μg/mL). Ceramide P56 was also effective against Mycobacterium fortuitum, which is commonly associated with infections affecting the skin, soft tissues, and bones. The obtained MIC of 31.25 μg/mL was equivalent to that of sulfametoxazol (MIC 32 μg/mL), a commonly prescribed antibiotic [50].
All four isolated compounds of P. tinctorius presented antifungal activity, with the most significant being pisolactone (Figure 4a) and 7,22-dien-3-ol, 24-methyl lanostane (Figure 4b). The MICs displayed by these two compounds against Candida tropicalis, C. krusei, C. neoformans, C. gattii, and Candida glabrata were 6.25, 1.56, 50, 50, and 1.56 μg/mL, respectively. The MICs of pisolactone and 7,22-dien-3-ol, 24-methyl lanostane against the three Candida species were lower than that of fluconazole, an antifungal medication commonly used to treat fungal infections such as vaginal yeast infections (candidiasis), oral and esophageal thrush, cryptococcal meningitis, and other systemic fungal infections. Additionally, the MICs of pisolactone and 7,22-dien-3-ol, 24-methyl lanostane against C. krusei and C. glabrata were equal to those of nystatin, another widely used antifungal medication to treat fungal infections, particularly those caused by the yeast Candida [50]. These findings indicate the potential of the two Pisolithus-derived compounds to be used as novel antifungal drugs.
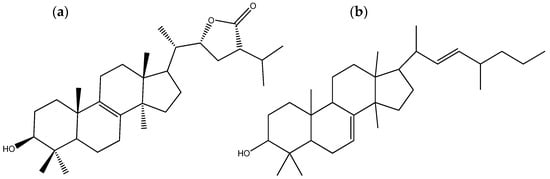
Figure 4.
Chemical structures of the triterpenoids pisolactone (a) and 7,22-dien-3-ol, 24-methyl lanostane (b), two secondary metabolites with antifungal and antibacterial activity isolated from Pisolithus tinctorius.

Table 1.
Pisolithus extracts, fractions, and isolated compounds and their antimicrobial activities.
Table 1.
Pisolithus extracts, fractions, and isolated compounds and their antimicrobial activities.
| Extract/Compound | Species | Fungal Structure | Assay | Antimicrobial Activity | Reference |
|---|---|---|---|---|---|
| Pisolithin A Pisolithin B (phenolic compounds) | P. arhizus | Mycelium | Spore germination Hyphal growth measured by protein estimation | Activity against phytopathogenic fungi, phytopathogenic oomycetes, and dermopathogenic fungi | [43,44] |
| Pisolactone (triterpenoid) | P. tinctorius | Basidiocarp | Broth microdilution | Activity against Gram-negative and Gram-positive bacteria Antimycobacterial Antifungal | [50] |
| 7,22-dien-3-ol, 24-methyl lanostane (triterpenoid) | P. tinctorius | Basidiocarp | Broth microdilution | Activity against Gram-negative and Gram-positive bacteria Antifungal | [50] |
| 5-hexadecenoic acid (unsaturated fatty acid) | P. tinctorius | Basidiocarp | Broth microdilution | Activity against Gram-negative and Gram-positive bacteria Antifungal | [50] |
| Ceramide P56 (ceramide) | P. tinctorius | Basidiocarp | Broth microdilution | Activity against Gram-negative and Gram-positive bacteria Antimycobacterial Antifungal | [50] |
| Crude extracts/fractions | P. albus | Basidiocarp | Agar well diffusion | Activity against methicillin-resistant Staphylococcus aureus | [46] |
| Methanolic extracts/fractions | P. microcarpus | Mycelium | Disk diffusion | Activity against Pseudomonas aeruginosa and S. aureus | [47] |
| Ethanolic extracts | P. albus | Basidiocarp | Disk diffusion | Activity against Gram-negative and Gram-positive bacteria | [48] |
| Ethyl acetate extracts | P. tinctorius | Basidiocarp and spores | Agar well diffusion | Activity against phytopathogenic fungi | [51] |
| Ethyl acetate extracts | P. tinctorius | Mycelium filtrate | Mycelium growth in solid medium | Activity against phytopathogenic fungi | [52] |
| Hydroethanolic extracts | P. tinctorius | Basidiocarp | Broth microdilution | Activity against multidrug-resistant Gram-negative and Gram-positive bacteria | [49] |
2.2. Anticancer Activity
According to the World Health Organization global cancer statistics (GLOBOCAN), in 2020, around 19 million new cancer cases were diagnosed, and over 9.9 million deaths were registered [53]. Although research into cancer treatment has been insistent, the number of diagnosed cases is expected to keep increasing, adding up to 29.4 million cases by 2040 [54]. The conventional approach to cancer treatment involves chemotherapy, which is highly associated with aggressive and prolonged collateral effects, mainly due to the very toxic nature of the involved compounds. Accordingly, the search for alternative and less toxic approaches has gained prominence, encouraging increased research into the discovery and development of new products, namely from natural origin [55,56].
Anticancer compounds exert their effects through various mechanisms to target cancer cells and inhibit tumor growth. Some common mechanisms of action include inhibition of DNA synthesis, cell division and angiogenesis, induction of apoptosis, and disruption of tumor metabolism [57].
Also, in the field of natural compounds for cancer treatment, the genus Pisolithus has aroused interest, with a focus on the triterpene pisosterol. Pisosterol was first isolated from the basidiocarps of P. tinctorius and chemically elucidated by Gill et al. [58] (Figure 5). Over the years, its cytotoxicity and mechanisms of action have been elucidated, mainly in cellular models, with relevant results (Table 2).
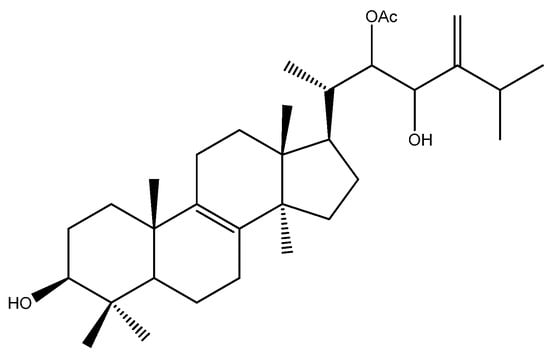
Figure 5.
Chemical structure of pisosterol, a Pisolithus-derived triterpene with anticancer activity.
One of the first reports on the anticarcinogenic action of pisosterol was ascribed to Montenegro and co-workers in 2004 [59]. The effect of the compound was tested in vitro, in mouse erythrocytes to infer its effects on membrane disruption, sea urchin developing embryos, and in the tumor cell lines CEM (human leukemia), HL-60 (human leukemia), B16 (murine melanoma), HCT-8 (human colon cancer), MCF-7 (human breast cancer), PC-3 (human prostate cancer) and SF-268 (human neuroblastoma) for cytotoxicity. The cytotoxicity of the compounds inferred using the viability assay based on the reduction of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide salt (MTT) by viable cells was compared to doxorubicin and etoposide, which are clinically used drugs in cancer chemotherapy. The results showed no activity in erythrocytes or even in the development of the embryos of sea urchins. However, there was a strong growth inhibition in all tumor cell lines, notably in leukemia CEM, HL-60 cells, and B16 melanoma cells with IC50 of 1.55, 1.84, and 1.65 μg/mL, respectively. Sea urchins have been used as model species to infer mechanisms of cell cycle control, cell adhesion, fertilization, cell differentiation, gene expression regulation, and death [60], and in general, cytotoxic substances tested in the sea urchin eggs and tumor cells are active in both assays [61]. This last assumption was not observed by Montenegro et al. [59] in their work with pisosterol. Since phase G1 is arrested in sea urchin cells and G2 is briefer than in mammals, the authors suggested an action of pisosterol on these cell cycle phases.
Given the cytotoxic results induced by pisosterol and described in the previous research, Montenegro et al. [62] conducted a study to determine if pisosterol was able to induce cell differentiation using the leukemia cell line HL-60 as a cancer cell model and peripheral blood mononuclear cells (PBMCs) as non-cancer cells. The data showed that HL-60 cells treated with pisosterol tend to differentiate into monocytic cells, and apoptosis was detected. No cytotoxicity was registered in PBMCs, even in the highest concentration (5 μg/mL), suggesting that pisosterol can be selective to cancer cell lines. In addition, Burbano et al. [63] conducted a study to elucidate the mechanisms of action of pisosterol in HL-60 leukemia cells by analyzing the homogeneously staining region (HSR) 8q24 aberration. Chromosome 8 rearrangements showing HSRs are recurrent karyotype abnormalities predominantly shown by HL-60 cells. The authors found that 99% of cells showed HSRs before pisosterol treatment, while 90% of the analyzed cells lacked this HSR region when treated with pisosterol. Although HL-60 cells resumed their growth after washing and re-incubation in a pisosterol-free culture medium and cells with HSRs did not suffer significant apoptosis or necrosis in the presence of pisosterol at a concentration of 1.8 μg/mL, the results revealed pisosterol as a putative drug to be used in combination with conventional anticancer therapy.
Pereira and co-authors [64] described the effect of pisosterol in the glioblastoma multiform (GBM) cell lines U343 and AHOL1. The treatment with three concentrations of pisosterol (0.5, 1.0, and 1.8 μg/mL) did not alter the cell morphology of the two cell lines, which is an indication that pisosterol does not induce cell differentiation in these GBM cells. Results showed a significant decrease in mitotic index only at 1.8 mg/mL. These results corroborate the findings of Montenegro et al. [59], in which the pisosterol IC50 for HL-60 cell was 1.84 μg/mL as previously described. Cells were also treated for abnormalities involving chromosome 8 or 8q24, the location where the C-MYC gene is mapped. The results indicated that no new chromosomal abnormalities emerged after treatment, suggesting that pisosterol has no clastogenic and/or aneugenic effect. This finding corroborates the earlier work of Burbano et al. [63], which was also conducted on HL-60 cells. Still, in the work of Pereira et al. [64], the interphase nuclei of the U343 and AHOL1 cells were analyzed by fluorescence in situ hybridization (FISH) for C-MYC before and after treatment with pisosterol. Results showed that after treatment with 1.8 μg/mL pisosterol, only 33% of U343 cells and 15% of AHOL1 cells had more than two C-MYC alleles when compared to the 72% of U343 and 65% of AHOL1 cells before the treatment. This result might be of particular importance since the C-MYC protein is known to be involved in cell cycle progression from G1 to S phase.
The potential of pisosterol in cancer therapy was also described on a panel of glioma cell lines by Ferreira et al. [65]. Cellular viability and proliferation of U343, AHOL1, U-87MG, and 1321N1 cells were significantly decreased in a dose-dependent manner (concentrations of 0.97, 1.94, and 3.50 μM), with inhibition of cell proliferation via the G2/M phase arrest and cell death by apoptosis. Treatment with pisosterol also revealed a dose-dependent downregulation of the expression of MYC, BCL2, BMI1, and MDM2 genes and a significant dose-dependent upregulation of gene expression levels of CASP3, TP53, ATM, CDK1, CDKN1A, CDKN2A, CDKN2B, CHK1 and p14ARF, which corroborate the inhibition of cell cycle progression and both the caspase-independent and caspase-dependent apoptotic pathways (Table 2).
While the previously described studies on the anticancer potential of pisosterol were based on cancer cell assays, in 2008 Montenegro and colleagues [66] conducted a study directed to an in vivo evaluation. In the study, sarcoma 180 tumor cells were subcutaneously transplanted into Swiss female mice and treated with 50 and 100 mg/m2 of pisosterol for 7 days. The results showed a tumor growth inhibition ratio of 43.0 and 38.7% for mice treated with pisosterol at 50 and 100 mg/m2, respectively, and 54.9% for mice treated with 5-fluorouracil at 50 mg/m2 as positive control. In order to evaluate the toxicological impact of pisosterol in in vivo models, morphological analyses were made. The treatment had an impact on the liver, showing Kupffer cells hyperplasia, focal infiltrate of inflammatory cells, and centrilobular venous congestion, demonstrating that the liver is a target organ of pisosterol. On the other hand, the authors concluded that the damages can be reversible since no stromal fibrosis was detected and conjunctive tissue was preserved.
Although the most relevant anticancer studies on Pisolithus were assigned to pisosterol, several other compounds have been described. Different medium and long-chain saturated fatty acids that have been isolated from methanolic extracts of P. tinctorius [67] were indicated to have anticancer activity, the most relevant being capric and lauric acids. These compounds were shown to induce apoptosis in colorectal, skin, and breast cancer cell lines [68,69,70]. Lastly, the ergosterol derivate, ergosterol peroxide that had been isolated from basidiocarps of P. tinctorius among other fungi [71], showed promising results against different cancer cell lines such as HT29 colon adenocarcinoma cells [72]. More recently, Parisi and co-authors [73] reported the isolation and structure elucidation of thirteen new and two already known triterpenoids from chloroform and methanolic extracts of basiodiocarps of P. arhizus. From the panel of isolated compounds, 24-methyllanosta-8,24 (31)-diene-3β,22ε-diol previously described by Baumert et al. [74] and the newly isolated 24 (31)-epoxylanost-8-ene-3β, 22S-diol were found to induce moderate cytotoxicity in a dose-dependent manner on the cancer cell lines U-87MG and Jurkat, with no cytotoxicity in the normal keratinocytes cell line HaCaT.
Most of the anticancer studies directed to Pisolithus have been based on compounds isolated from the basiodiocarp. In a pioneering work, Alves et al. [75] evaluated the anticancer potential of DCM/MeOH and EtOAc/MeOH crude extracts of P. tinctorius spores against the human osteosarcoma cell line MG63, the human breast carcinoma cell line T47D, the human colon adenocarcinoma cell line RKO, and the normal human brain capillary endothelial cell line hCMEC/D3. The cytotoxicity results based on the MTT assays showed a decrease in cancer cell line viability of 92% and 88% for DCM/MeOH and EtOAc/MeOH crude extracts, respectively, without a significant reduction in the viability of the normal cells. The most notable results were obtained with the DCM/MeOH extract with cell viability of 12% in RKO and MG63 cells and 6% in T47D cells after 48 h of exposure. These results extend the anticancer activity of Pisolithus to structures other than the basiodiocarp, highlighting the potential of this fungal genus.

Table 2.
Anticancer activity of the triterpene pisosterol isolated from Pisolithus tinctorius.
Table 2.
Anticancer activity of the triterpene pisosterol isolated from Pisolithus tinctorius.
| Biological Model | Assay | Mechanism/Effect | Reference |
|---|---|---|---|
| CEM, HL-60, B16, HCT-8, MCF-7, PC-3, SF-268 cell lines | MTT | Cytotoxicity | [59] |
| HL-60 cell line | MTT | Cytotoxicity | [62] |
| Trypan blue exclusion | Viability | ||
| α-Naphthyl acetate esterase activity | Cell differentiation | ||
| NBT | |||
| BrdU incorporation | Inhibition of DNA synthesis | ||
| Differential fluorescent staining with acridine/orange ethidium bromide | Apoptosis | ||
| Mitotic index | Cell cycle arrest | [63] | |
| Chromosome analysis | Homogeneously staining region (HSR) 8q24 aberration | ||
| U343, AHOL1 cell lines | Cytogenetic characterization: metaphases stained with Giemsa solution and banded with trypsin-Giemsa Mitotic index FISH analysis | 8q24.12–q24.13 chromosome aberrations Cytotoxicity Expression of C-MYC gene | [76] |
| U343, AHOL1, U-87 MG, 1321N1 cell lines | MTT | Cytotoxicity | [65] |
| Trypan blue exclusion | Viability | ||
| Flow cytometry | Cell cycle arrest | ||
| Staining with Annexin V-FITC/PI | Apoptosis | ||
| qPCR and western blotting | Expression levels of MYC, ATM, BCL2, BMI1, CASP3, CDK1, CDKN1A, CDKN2A, CDKN2B, CHEK1, MDM2, p14ARF and TP53 genes | ||
| Swiss female mice | Histopathology and morphologic observations | Induction of cellular and nuclear pleomorphism; coagulative type necrosis | [66] |
| MG63, T47D, RKO cell lines | MTT | Cytotoxicity | [75] |
MTT—3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide salt; NBT—nitro blue tetrazolium salt; CEM—T lymphoblast cell line; B16—murine melanoma cell line; HCT-8—human colon adenocarcinoma cell line; MCF-7—breast cancer cell line; PC-3—prostate cancer cell line; SF-268—human neuroblastoma cell line; HL-60—human leukemia cell line; U343—human glioblastoma cell line; AHOL1—human glioblastoma cell line; U-87 MG—human glioblastoma cell line; 1321N1—human astrocytoma cell line; MG63—human osteosarcoma cell line; T47D—human breast carcinoma cell line; RKO—human colon adenocarcinoma cell line; FISH—fluorescence in situ hybridization; FITC/PI—fluorescein isothiocyanate/propidium iodide; qPCR—quantitative polymerase chain reaction; DNA—deoxyribonucleic acid.
2.3. Antioxidant Activity
More than 50% of global deaths are linked to inflammation-related diseases, such as stroke, diabetes, cancer, and neurodegenerative and autoimmune conditions [31,77].
Inflammation is a natural defense mechanism against endogenous or exogenous antigens and involves enhanced or exacerbated production of reactive nitrogen species (RNS) and reactive oxygen species (ROS). However, ROS and RNS are generated in regular metabolic pathways by most cells, as well as in inflammatory processes to eliminate pathogens when, in excess, these oxidative species can promote oxidative stress associated with chronic inflammation [78]. Antioxidants play a pivotal role in maintaining cellular homeostasis and preventing oxidative stress-induced damage. They are compounds that neutralize or inhibit the detrimental effects of ROS and free radicals, thereby reducing inflammation and preventing cellular damage [79]. Both oxidative and inflammatory processes are thought to be involved in several different pathogenesis mechanisms [77,80,81,82,83,84]. Antioxidants exert their protective effects through a combination of direct scavenging of free radicals, modulation of enzyme activity, induction of antioxidant defenses, and modulation of signaling pathways. These mechanisms collectively contribute to the ability of antioxidants to mitigate oxidative stress and prevent cellular damage associated with aging, inflammation, and various diseases [85,86].
It is crucial to explore and develop new medicines to address these health challenges. The discovery of novel molecules exhibiting antioxidant activity is essential for advancing innovative medical solutions that contribute to global well-being.
Recently, Martins et al. [49] assessed the antioxidant activity of hydroethanolic extracts from eight macrofungi. P. tinctorius stood out as one of the fungi with the highest values of antioxidant activity, evaluated by three different methods: 2,2-di(4-tertoctylphenyl)-1-picrylhydrazyl (DPPH), 2,20-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and ferric reducing antioxidant power (FRAP), with 1291.00, 519.10 and 128.30 µM Trolox (6-hydroxy-2,5,7,8-tetramethlychroman-2-carboxylic acid)/g, respectively (Table 3).

Table 3.
Extracts of Pisolithus species with antioxidant activity.
Similarly, Pringle et al. [87] showed that P. tinctorius ethanolic and aqueous extracts had the highest antioxidant activity among four tested fungi. It is interesting to note that the P. tinctorius ethanolic extract, at 200 µg/mL, had a similar antioxidant activity, assessed by DPPH, as catechin, a reference compound with high antioxidant capacity.
The high antioxidant activity of P. arhizus in comparison with other macrofungi was also demonstrated in the study of Campi et al. [88]. The antioxidant concentration determined by DPPH was 36.66 mg/g of ascorbic acid equivalent (AAE), the second highest among nine tested fungi.
The collective findings from these recent studies show high antioxidant activity across diverse assessments, including DPPH, ABTS, and FRAP methods, positioning Pisolithus as a promising source of antioxidant compounds.
Reis et al. [89] evaluated the antioxidant activity of methanolic extracts of P. arhizus using three different methods. Interestingly, all methods revealed that the antioxidant activity of P. arhizus varied among its different fungal components. In basidiocarps, the antioxidant activity assessed by DPPH radical scavenging activity, reducing power, and inhibition of β-carotene bleaching was EC50 0.56, 0.37, and 0.24 mg/mL, respectively. In mycelium, it was EC50 > 20.00, 7.29, and 2.49 mg/mL, respectively, showing that basidiocarps have higher antioxidant activity than mycelium.
3. Conclusions
In the exploration of Pisolithus, this comprehensive review enlightens the bioactive potential harbored within the genus, offering insights into its antimicrobial, anticancer, and antioxidant properties. Although bioactive compounds isolated from Pisolithus have been understudied, the bioactive potential of the genus is promising and deserves to be compiled to stimulate further investigations.
Three species of Pisolithus that exhibited bioactive potential have been reported, namely P. arhizus/P. tinctorius, P. albus and P. microcarpus. The diverse bioactive potential of Pisolithus is underscored by the discovery of compounds with markedly distinct chemical structures, extracted from different fungal structures—be it the basidiocarp or the mycelium, and the potential hidden in other structures such as the spores. This not only highlights the rich reservoir of bioactive compounds within Pisolithus but also accentuates the importance of exploring the multifaceted contributions of different fungal components in unlocking innovative applications across diverse therapeutic landscapes.
Compounds derived from Pisolithus exhibited antimicrobial activity against a spectrum of microorganisms, showcasing efficacy against both Gram-negative and Gram-positive bacteria, mycobacteria, dermopathogenic fungi, phytopathogenic fungi, and phytopathogenic oomycetes. Particularly noteworthy is the effectiveness of Pisolithus against antibiotic-resistant bacteria, positioning it as a valuable contender in the battle against antibiotic resistance. The bioactive compounds from Pisolithus demonstrated antimicrobial performance comparable to established antibacterial and antifungal medications, accentuating the therapeutic potential of these natural products. Their ability to match the efficacy of current pharmaceuticals is a testament to the capacity of Pisolithus-derived compounds.
In the realm of anticancer activity, the triterpene pisosterol emerges as a standout compound from P. tinctorius. With documented cytotoxicity against various cancer cell lines, pisosterol holds promise as a novel candidate for anticancer therapies. Despite significant strides in understanding its effects in vitro and in vivo, further investigations are warranted to unravel the mechanisms underlying its anticancer properties.
Furthermore, Pisolithus has been unveiled as a potential source of antioxidants, with studies on extracts and fractions showcasing promising results. Notably, basidiocarps exhibited superior antioxidant activity compared to mycelium, offering avenues for targeted extraction and isolation strategies.
While in vitro studies presented compelling evidence of the Pisolithus bioactive prowess, it is imperative to acknowledge the necessity for in vivo validation. Limited information on isolated compounds and a selective focus on certain activities underscore the need for expansive studies encompassing a broader spectrum of compounds and activities. Mechanistic insights into the mode of action of Pisolithus-derived compounds remain a compelling area for future research.
Most of the compounds isolated from Pisolithus belong to chemical groups well known for their bioactive potential. Pisolithin A and B are phenolic compounds. Phenolics exhibit a range of bioactivities, including antimicrobial, anticancer, and antioxidant effects [93]. Pisolactone and 7,22-dien-3-ol, 24-methyl lanostane are two triterpenoids, and this class of organic compounds has been described as potential antimicrobial and anticancer drugs [94,95]. Pisosterol is a triterpene. Triterpenes were found to induce antimicrobial, antiviral, anticancer, and antioxidant properties, among others [96,97,98]. The chemical nature of the isolated compounds thus reflects what is already known regarding their therapeutic potential and elevates Pisolithus to another level on the scale of pharmacological interest. While producing biomass for compound isolation at an industrial scale may pose challenges, natural models can serve as valuable tools for designing novel agents with potent bioactivities. Therefore, compounds from Pisolithus may continue to represent promising leads for the development of new drugs in the future [95].
As our understanding of the multifaceted nature of Pisolithus continues to evolve, the wealth of bioactive potential residing in Pisolithus signifies a promising frontier in the development of innovative antimicrobial, anticancer, and antioxidant applications for addressing contemporary health challenges.
Author Contributions
Conceptualization, R.S.O. and R.M.; data curation, G.S. and A.M.S.; writing—original draft preparation, R.S.O., A.M.S. and R.M.; writing—review and editing, R.S.O., M.P., V.V. and R.M.; supervision, R.S.O., M.P. and R.M.; funding acquisition R.S.O., V.V. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by FCT—Fundação para a Ciência e Tecnologia, I.P. by project reference UIDB/04004/2020 and DOI identifier 10.54499/UIDB/04004/2020 (https://doi.org/10.54499/UIDB/04004/2020, accessed on 17 February 2024), to the Centre for Functional Ecology—Science for People & the Planet, and by the European Regional Development Fund (ERDF) through the Regional Operational Program North 2020, within the scope of Project GreenHealth—Digital strategies in biological assets to improve well-being and promote green health, Norte-01-0145-FEDER-000042. The authors are also grateful to FCT financial support to CIIMAR (UIDB/04423/2020 and UIDP/04423/2020).
Data Availability Statement
Not applicable.
Acknowledgments
A.M.S. acknowledges the GreenHealth project through the POR-TIC/HealthTech/BI/2021/01 grant.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Painuli, S.; Semwal, P.; Egbuna, C. Mushroom: Nutraceutical, mineral, proximate constituents and bioactive component. In Functional Foods and Nutraceuticals; Egbuna, C., Dable Tupas, G., Eds.; Springer: Cham, Switzerland, 2020; pp. 307–336. [Google Scholar]
- Gupta, A.; Meshram, V.; Gupta, M.; Goyal, S.; Qureshi, K.A.; Jaremko, M.; Shukla, K.K. Fungal endophytes: Microfactories of novel bioactive compounds with therapeutic interventions; A comprehensive review on the biotechnological developments in the field of fungal endophytic biology over the last decade. Biomolecules 2023, 13, 1038. [Google Scholar] [CrossRef] [PubMed]
- Ghobad-Nejhad, M.; Dima, B.; Cui, B.-K.; Si, J. Editorial: Basidiomycete fungi: From biosystematics and biodiversity to biotechnology. Front. Microbiol. 2023, 14, 1128319. [Google Scholar] [CrossRef]
- He, M.-Q.; Zhao, R.-L.; Liu, D.-M.; Denchev, T.T.; Begerow, D.; Yurkov, A.; Kemler, M.; Millanes, A.M.; Wedin, M.; McTaggart, A.R.; et al. Species diversity of Basidiomycota. Fungal Divers. 2022, 114, 281–325. [Google Scholar] [CrossRef]
- Taylor, T.N.; Krings, M.; Taylor, E.L. 9–Basidiomycota. In Fossil Fungi; Taylor, T.N., Krings, M., Taylor, E.L., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 173–199. [Google Scholar]
- Sande, D.; Oliveira, G.P.; Moura, M.A.F.E.; Martins, B.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Hobbs, C. The health and clinical benefits of medicinal fungi. In Biochemical Engineering and Biotechnology of Medicinal Mushrooms. Advances in Biochemical Engineering/Biotechnology; Berovic, M., Zhong, J.J., Eds.; Springer: Cham, Switzerland, 2023; Volume 184, pp. 285–356. [Google Scholar]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production–A review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Garcia, J.; Costa, V.M.; Carvalho, A.; Baptista, P.; de Pinho, P.G.; de Lourdes Bastos, M.; Carvalho, F. Amanita phalloides poisoning: Mechanisms of toxicity and treatment. Food Chem. Toxicol. 2015, 86, 41–55. [Google Scholar] [CrossRef]
- Wennig, R.; Eyer, F.; Schaper, A.; Zilker, T.; Andresen-Streichert, H. Mushroom poisoning. Dtsch. Arztebl. Int. 2020, 117, 701–708. [Google Scholar] [CrossRef]
- Krishnakumar, N.M.; Ceasar, S.A. Wild edible and medicinal mushrooms used by the tribes in the State of Kerala, India: A review. Int. J. Med. Mushrooms 2022, 24, 63–72. [Google Scholar] [CrossRef]
- Gomes, D.C.V.; de Alencar, M.V.O.B.; dos Reis, A.C.; de Lima, R.M.T.; Santos, J.V.O.; da Mata, A.M.O.F.; Dias, A.C.S.; Junior, J.S.C.; de Medeiros, M.D.G.F.; Paz, M.F.C.J.; et al. Antioxidant, anti-inflammatory and cytotoxic/antitumoral bioactives from the phylum Basidiomycota and their possible mechanisms of action. Biomed. Pharmacother. 2019, 112, 108643. [Google Scholar]
- Varghese, R.; Dalvi, Y.B.; Lamrood, P.Y.; Shinde, B.P.; Nair, C.K.K. Historical and current perspectives on therapeutic potential of higher basidiomycetes: An overview. 3 Biotech 2019, 9, 362. [Google Scholar] [CrossRef]
- Chopra, H.; Mishra, A.K.; Baig, A.A.; Mohanta, T.K.; Mohanta, Y.K.; Baek, K.-H. Narrative review: Bioactive potential of various mushrooms as the treasure of versatile therapeutic natural product. J. Fungi 2021, 7, 728. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Preto, M.; Grosso, C.; Vieira, M.; Delerue-Matos, C.; Vasconcelos, V.; Reis, M.; Barros, L.; Martins, R. Tracing the path between mushrooms and Alzheimer’s disease—A literature review. Molecules 2023, 28, 5614. [Google Scholar] [CrossRef] [PubMed]
- Cairney, J.W.G. Pisolithus–death of the pan-global super fungus. New Phytol. 2002, 153, 199–201. [Google Scholar] [CrossRef]
- Plett, J.M.; Miyauchi, S.; Morin, E.; Plett, K.; Wong-Bajracharya, J.; Pereira, M.F.; Kuo, A.; Henrissat, B.; Drula, E.; Wojtalewicz, D.; et al. Speciation underpinned by unexpected molecular diversity in the mycorrhizal fungal genus Pisolithus. Mol. Biol. Evol. 2023, 40, msad045. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.H. Tree host range and world distribution of the ectomycorrhizal fungus Pisolithus tinctorius. Can. J. Microbiol. 1977, 23, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Cairney, J.; Chambers, S. Interactions between Pisolithus tinctorius and its hosts: A review of current knowledge. Mycorrhiza 1997, 7, 117–131. [Google Scholar] [CrossRef]
- Ramos, M.A.; Sousa, N.R.; Franco, A.R.; Costa, V.; Oliveira, R.S.; Castro, P.M.L. Effect of diflubenzuron on the development of Pinus pinaster seedlings inoculated with the ectomycorrhizal fungus Pisolithus tinctorius. Environ. Sci. Pollut. Res. 2013, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.R.; Franco, A.R.; Ramos, M.A.; Oliveira, R.S.; Castro, P.M.L. Reforestation of burned stands: The effect of ectomycorrhizal fungi on Pinus pinaster establishment. Soil Biol. Biochem. 2011, 43, 2115–2120. [Google Scholar] [CrossRef]
- Sousa, N.R.; Franco, A.R.; Oliveira, R.S.; Castro, P.M.L. Ectomycorrhizal fungi as an alternative to the use of chemical fertilisers in nursery production of Pinus pinaster. J. Environ. Manage 2012, 95, S269–S274. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.R.; Sousa, N.R.; Ramos, M.A.; Oliveira, R.S.; Castro, P.M.L. Diversity and persistence of ectomycorrhizal fungi and their effect on nursery-inoculated Pinus pinaster in a post-fire plantation in Northern Portugal. Microb. Ecol. 2014, 68, 761–772. [Google Scholar] [CrossRef]
- Sousa, N.; Ramos, M.; Franco, A.; Oliveira, R.S.; Castro, P.M.L. Mycorrhizal symbiosis affected by different genotypes of Pinus pinaster. Plant Soil 2012, 359, 245–253. [Google Scholar] [CrossRef]
- Upadhayay, A.; Ling, J.; Pal, D.; Xie, Y.; Ping, F.-F.; Kumar, A. Resistance-proof antimicrobial drug discovery to combat global antimicrobial resistance threat. Drug Resist. Updates 2023, 66, 100890. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.K.; Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Wilson, M.; Wilson, P.J.K. Microbes and infectious diseases. In Close Encounters of the Microbial Kind; Wilson, M., Wilson, P.J.K., Eds.; Springer: Cham, Switzerland, 2021; pp. 3–48. [Google Scholar]
- Cella, E.; Giovanetti, M.; Benedetti, F.; Scarpa, F.; Johnston, C.; Borsetti, A.; Ceccarelli, G.; Azarian, T.; Zella, D.; Ciccozzi, M. Joining forces against antibiotic resistance: The one health solution. Pathogens 2023, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Mcelwain, T.F.; Thumbi, S.M. Animal pathogens and their impact on animal health, the economy, food security, food safety and public health. Rev. Sci. Tech. 2017, 36, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, R.W.; Mustafa, M.; Charles, K.; Wagara, I.W.; Kappel, N. Selected emerging and reemerging plant pathogens affecting the food basket: A threat to food security. J. Agric. Food Res. 2023, 14, 100827. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Hai, P.; Gao, Y.; Yang, L.; Chen, N.; Jia, H.; Wang, M.; Li, H.; Jiang, W.; Yang, J.; Li, R. Two new compounds from the endophytic fungi of Dryopteris crassirhizoma and their antimicrobial activities. Molecules 2023, 28, 8043. [Google Scholar] [CrossRef]
- Chakraverty, R.; Samanta, K.; Mandal, P.; Karmakar, S.; Karmakar, S. Chapter 18—Mechanisms of action of antibacterial agents (AMA). In How Synthetic Drugs Work; Kazmi, I., Karmakar, S., Shaharyar, M.A., Afzal, M., Al-Abbasi, F.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 421–429. [Google Scholar]
- Kope, H.H.; Fortin, J.A. Antifungal activity in culture filtrates of the ectomycorrhizal fungus Pisolithus tinctorius. Can. J. Bot. 1990, 68, 1254–1259. [Google Scholar] [CrossRef]
- Kope, H.H.; Tsantrizos, Y.S.; Fortin, J.A.; Ogilvie, K.K. p-Hydroxybenzoylformic acid and (R)-(-)-p-hydroxymandelic acid, two antifungal compounds isolated from the liquid culture of the ectomycorrhizal fungus Pisolithus arhizus. Can. J. Microbiol. 1991, 37, 258–264. [Google Scholar] [CrossRef]
- Tsantrizos, Y.S.; Kope, H.H.; Fortin, J.A.; Ogilvie, K.K. Antifungal antibiotics from Pisolithus tinctorius. Phytochemistry 1991, 30, 1113–1118. [Google Scholar] [CrossRef]
- Shrestha, V.G.; Shrestha, K.; Wallander, H. Antagonistic study of ectomycorrhizal fungi isolated from Baluwa forest (Central Nepal) against with pathogenic fungi and bacteria. Sci. World 2005, 3, 49–52. [Google Scholar]
- Ameri, A.; Ghadge, C.; Vaidya, J.G.; Deokule, S.S. Anti-Staphylococcus aureus activity of Pisolithus albus from Pune, India. J. Med. Plant Res. 2011, 5, 527–532. [Google Scholar]
- Mamede, A.C.P.B. Atividade Biológica do Fungo Pisolithus microcarpus. Master Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2015. [Google Scholar]
- Khadhri, A.; Aouadhi, C.; Aschi-Smiti, S. Screening of bioactive compounds of medicinal mushrooms collected on Tunisian territory. Int. J. Med. Mushrooms 2017, 19, 127–135. [Google Scholar] [CrossRef]
- Martins, T.; Machado-Carvalho, L.; Aires, A.; Saavedra, M.J.; Marques, G. Antioxidant, antimicrobial and cosmeceutical potential of wild mushroom extracts. Appl. Microbiol. 2023, 3, 562–579. [Google Scholar] [CrossRef]
- Carmo, G. Isolamento, Determinação Estrutural e Atividades Biológicas de Metabolitos dos Fungos Phellinotus piptadeniae e Pisolitus tinctorius. Ph.D. Thesis, Universidade Federal de Santa Maria, Santa Maria, Brazil, 2019. [Google Scholar]
- Ganesh, P.; Krishnamoorthy, A.S.; Sangeetha, C.; Nakkeeran, S.; Thiribhuvanamala, G.; Akshaya, S.B. Exploration of biomolecules from Pisolithus tinctorius (Pers.) against major soil-borne plant pathogens. Ann. Phytomed. 2021, 10, 311–318. [Google Scholar] [CrossRef]
- Ganeshkumar, P.; Krishnamoorthy, A.S.; Sangeetha, C.; Nakkeeran, S.; Sivakumar, U.; Thiribhuvanamala, G. Antimicrobial metabolites from ectomycorrhizal fungus, Pisolithus tinctorius (Pers.) Coker against soil borne plant pathogens. Madras Agric. J. 2021, 108, 234–241. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- WHO. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Mittra, I.; Pal, K.; Pancholi, N.; Shaikh, A.; Rane, B.; Tidke, P.; Kirolikar, S.; Khare, N.K.; Agrawal, K.; Nagare, H.; et al. Prevention of chemotherapy toxicity by agents that neutralize or degrade cell-free chromatin. Ann. Oncol. 2017, 28, 2119–2127. [Google Scholar] [CrossRef]
- Pathak, K.; Pathak, M.P.; Saikia, R.; Gogoi, U.; Sahariah, J.J.; Zothantluanga, J.H.; Samanta, A.; Das, A. Cancer chemotherapy via natural bioactive compounds. Curr. Drug Discov. Technol. 2022, 19, e310322202888. [Google Scholar] [CrossRef]
- Sengupta, M.; Guha, A.; Bhowmik, R.; Kazmi, I.; Hosawi, S.B.I.; Al-Abbasi, F.; Kaleem, M. Chapter 21—Insight into the molecular mechanism of action of anticancer drugs. In How Synthetic Drugs Work; Kazmi, I., Karmakar, S., Shaharyar, M.A., Afzal, M., Al-Abbasi, F.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 477–502. [Google Scholar]
- Gill, M.; Kiefel, M.J.; Skelton, B.W.; White, A.H. The structure and absolute stereochemistry of pisosterol, the principal triterpenoid from fruitbodies of the fungus Pisolithus tinctorius. Aust. J. Chem. 1989, 42, 995–1001. [Google Scholar] [CrossRef]
- Montenegro, R.C.; Jimenez, P.C.; Farias, R.A.F.; Andrade-Neto, M.; Bezerra, F.S.; Moraes, M.E.A.; de Moraes, M.O.; Pessoa, C.; Costa-Lotufo, L.V. Cytotoxic activity of pisosterol, a triterpene isolated from Pisolithus tinctorius (Mich.: Pers.) Coker & Couch, 1928. Z. Naturforsch. C 2004, 59, 519–522. [Google Scholar]
- Adonin, L.; Drozdov, A.; Barlev, N.A. Sea urchin as a universal model for studies of gene networks. Front. Genet. 2021, 11, 627259. [Google Scholar] [CrossRef]
- Eurtivong, C.; Semenov, V.; Semenova, M.; Konyushkin, L.; Atamanenko, O.; Reynisson, J.; Kiselyov, A. 3-Amino-thieno [2,3-b]pyridines as microtubule-destabilising agents: Molecular modelling and biological evaluation in the sea urchin embryo and human cancer cells. Bioorg. Med. Chem. 2017, 25, 658–664. [Google Scholar] [CrossRef]
- Montenegro, R.C.; Vasconcellos, M.C.; Bezerra, F.S.; Andrade-Neto, M.; Pessoa, C.; Moraes, M.O.; Costa-Lotufo, L.V. Pisosterol induces monocytic differentiation in HL-60 cells. Toxicol. In Vitro 2007, 21, 795–800. [Google Scholar] [CrossRef]
- Burbano, R.R.; Lima, P.D.L.; Bahia, M.O.; Khayat, A.S.; Silva, T.C.R.; Bezerra, F.S.; Neto, M.A.; de Moraes, M.O.; Montenegro, R.C.; Costa-Lotufo, L.V.; et al. Cell cycle arrest induced by pisosterol in HL60 cells with gene amplification. Cell Biol. Toxicol. 2009, 25, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.L.; Lima, P.D.; Khayat, A.S.; Bahia, M.O.; Bezerra, F.S.; Andrade-Neto, M.; Montenegro, R.C.; Pessoa, C.; Costa-Lotufo, L.V.; Moraes, M.O.; et al. Inhibitory effect of pisosterol on human glioblastoma cell lines with C-MYC amplification. J. Appl. Toxicol. 2011, 31, 554–560. [Google Scholar] [CrossRef]
- Ferreira, W.A.S.; Burbano, R.R.; Pessoa, C.O.; Harada, M.L.; Borges, B.N.; de Oliveira, E.H.C. Pisosterol induces G2/M cell cycle arrest and apoptosis via the ATM/ATR signaling pathway in human glioma cells. Anticancer Agents Med. Chem. 2020, 20, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, R.C.; Farias, R.A.F.; Pereira, M.R.P.; Alves, A.P.N.N.; Bezerra, F.S.; Andrade-Neto, M.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor activity of pisosterol in mice bearing with S180 tumor. Biol. Pharm. Bull. 2008, 31, 454–457. [Google Scholar] [CrossRef]
- Abreu, P.M. Produtos Naturais do Fungo Pisolithus Tinctorius. Ph.D. Thesis, Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa, Lisbon, Portugal, 1987. [Google Scholar]
- Narayanan, A.; Baskaran, S.A.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Anticarcinogenic Properties of nedium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef]
- Lappano, R.; Sebastiani, A.; Cirillo, F.; Rigiracciolo, D.C.; Galli, G.R.; Curcio, R.; Malaguarnera, R.; Belfiore, A.; Cappello, A.R.; Maggiolini, M. The lauric acid-activated signaling prompts apoptosis in cancer cells. Cell Death Discov. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Sheela, D.L.; Narayanankutty, A.; Nazeem, P.A.; Raghavamenon, A.C.; Muthangaparambil, S.R. Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor downregulation: An in silico and in vitro study. Hum. Exp. Toxicol. 2019, 38, 753–761. [Google Scholar] [CrossRef]
- Fujimoto, H.; Nakayama, M.; Nakayama, Y.; Yamazaki, M. Isolation and characterization of immunosuppressive components of three mushrooms, Pisolithus tinctorius, Microporus flabelliformis and Lenzites betulina. Chem. Pharm. Bull. 1994, 42, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Nocera, R.; Franceschelli, S.; Tedesco, C.; De Riccardis, F.; Braca, A.; De Tommasi, N.; Donadio, G. Cytotoxic triterpenoids from the ectomycorrhizal fungus Pisolithus arhizus. Phytochemistry 2023, 209, 113635. [Google Scholar] [CrossRef] [PubMed]
- Baumert, A.; Schumann, B.; Porzel, A.; Schmidt, J.; Strack, D. Triterpenoids from Pisolithus tinctorius isolates and ectomycorrhizas. Phytochemistry 1997, 45, 499–504. [Google Scholar] [CrossRef]
- Alves, R.; Preto, M.; Vasconcelos, V.; Oliveira, R.S.; Martins, R. Cytotoxicity induced by extracts of Pisolithus tinctorius spores on human cancer and normal cell lines—Evaluation of the anticancer potential. J. Toxicol. Environ. Health A 2015, 78, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Siddiqui, M.; Abdellatif, B.; Liskova, A.; Kubatka, P.; Büsselberg, D. Natural compounds in glioblastoma therapy: Preclinical insights, mechanistic pathways, and outlook. Cancers 2021, 13, 2317. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Thomas Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. [Google Scholar]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Kibel, A.; Lukinac, A.M.; Dambic, V.; Juric, I.; Selthofer-Relatic, K. Oxidative stress in ischemic heart disease. Oxid. Med. Cell. Longev. 2020, 2020, 6627144. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Wang, L.; Wang, G.; Wang, Z.; Dong, X.; Wen, B.; Zhang, Z. The pathogenesis of diabetes mellitus by oxidative stress and inflammation: Its inhibition by berberine. Front. Pharmacol. 2018, 9, 782. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Forcados, G.E.; James, D.B.; Sallau, A.B.; Muhammad, A.; Mabeta, P. Oxidative stress and carcinogenesis: Potential of phytochemicals in breast cancer therapy. Nutr. Cancer 2017, 69, 365–374. [Google Scholar] [CrossRef]
- Strzelczyk, J.; Wiczkowski, A. Oxidative damage and carcinogenesis. Contemp. Oncol. 2012, 16, 230–233. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 Indonesian indigenous herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Pringle, N.; Van de Venter, M.; Boukes, G.J.; Koekemoer, T. Therapeutic potential of selected South African macrofungi in diabetic wound healing: An in vitro evaluation. S. Afr. J. Bot. 2021, 138, 337–347. [Google Scholar] [CrossRef]
- Campi, M.; Mancuello, C.; Ferreira, F.; Maubet, Y.; Cristaldo, E.; Benítez, D. Preliminary evaluation of phenolic compounds, antioxidant activity and bioactive compounds in some species of basidiomycetes fungi from Paraguay. Steviana 2019, 11, 26–41. [Google Scholar] [CrossRef]
- Reis, F.S.; Ferreira, I.C.F.R.; Barros, L.; Martins, A. A comparative study of tocopherols composition and antioxidant properties of in vivo and in vitro ectomycorrhizal fungi. LWT-Food Sci. Technol. 2011, 44, 820–824. [Google Scholar] [CrossRef][Green Version]
- Camara, A.G.N. Pigmentos Produzidos pelo Fungo Basidiomiceto Pisolithus tinctorius: Desreplicação, Isolamento e Estudo do Potencial Biotecnológico. Master Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2023. [Google Scholar]
- Sevindik, M.; Ajaz, M.; Özdemir, B.; Akata, I.; Selamoglu, Z. Oxidant/antioxidant potentials and heavy metal levels of Pisolithus arhizus and its effects on cardiovascular diseases. Indian J. Nat. Prod. Resour. 2021, 12, 600–604. [Google Scholar]
- Onbaşlı, D.; Yuvali, G.; Aslım, B. Medicinal potential of ectomycorrhizal mushroom Pisolithus arhizus extracts from Turkey. Fresenius Environ. Bull. 2020, 29, 9455–9464. [Google Scholar]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Wimmerová, M.; Wimmer, Z. Saponins of selected triterpenoids as potential therapeutic agents: A review. Pharmaceuticals 2023, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kinghorn, A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-planktonic and anti-biofilm properties of pentacyclic triterpenes—Asiatic acid and ursolic acid as promising antibacterial future pharmaceuticals. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Darshani, P.; Sarma, S.S.; Srivastava, A.K.; Baishya, R.; Kumar, D. Anti-viral triterpenes: A review. Phytochem. Rev. 2022, 21, 1761–1842. [Google Scholar] [CrossRef]
- Hardianto, A.; Mardetia, S.S.; Destiarani, W.; Budiman, Y.P.; Kurnia, D.; Mayanti, T. Unveiling the anti-cancer potential of onoceranoid triterpenes from Lansium domesticum Corr. cv. kokosan: An in silico study against estrogen receptor alpha. Int. J. Mol. Sci. 2023, 24, 15033. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).