Platelet-Derived Microvesicles Contribute to the Pathophysiogenesis of Human Cutaneous Leishmaniasis: A Nano-Flow Cytometric Approach in Plasma Samples from Patients before and under Antimonial Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Reagents
2.3. Blood Collection and Plasma Preparation
2.4. Nano-Flow Cytometry Analysis of Extracellular Microvesicles
2.5. Data Analysis and Statistics
3. Results
3.1. Characteristics of CL-Patient Cohorts and Healthy Individuals
3.2. Standardization of the Blood Collection Procedures for Improvement of Analysis of MVs in the Human Plasma
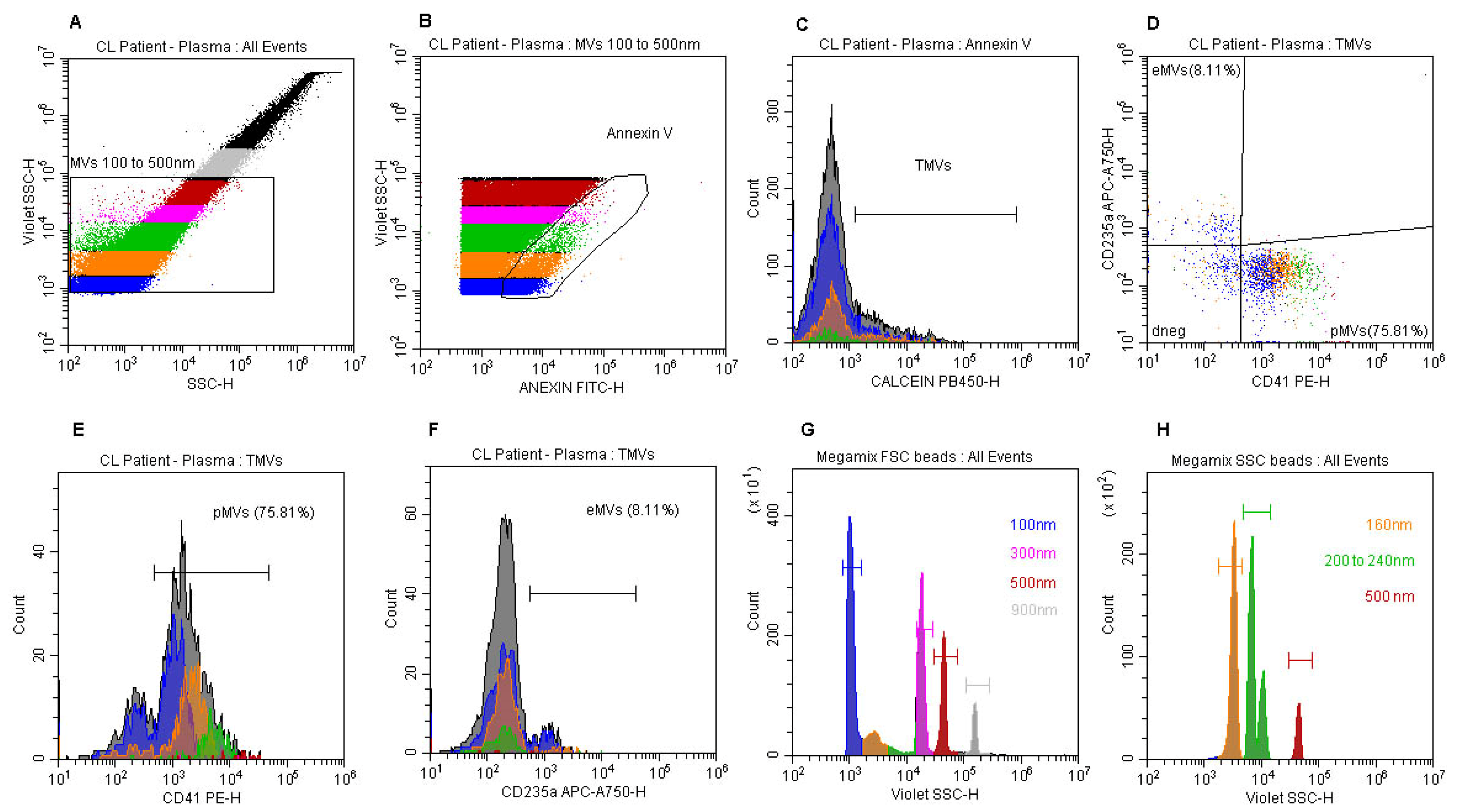
3.3. Definition of a Cytofluorimetric Gate Strategy Protocol for Quantification and Phenotyping of MVs in Platelet-Free Plasma Samples from CL Patients and Healthy Individuals
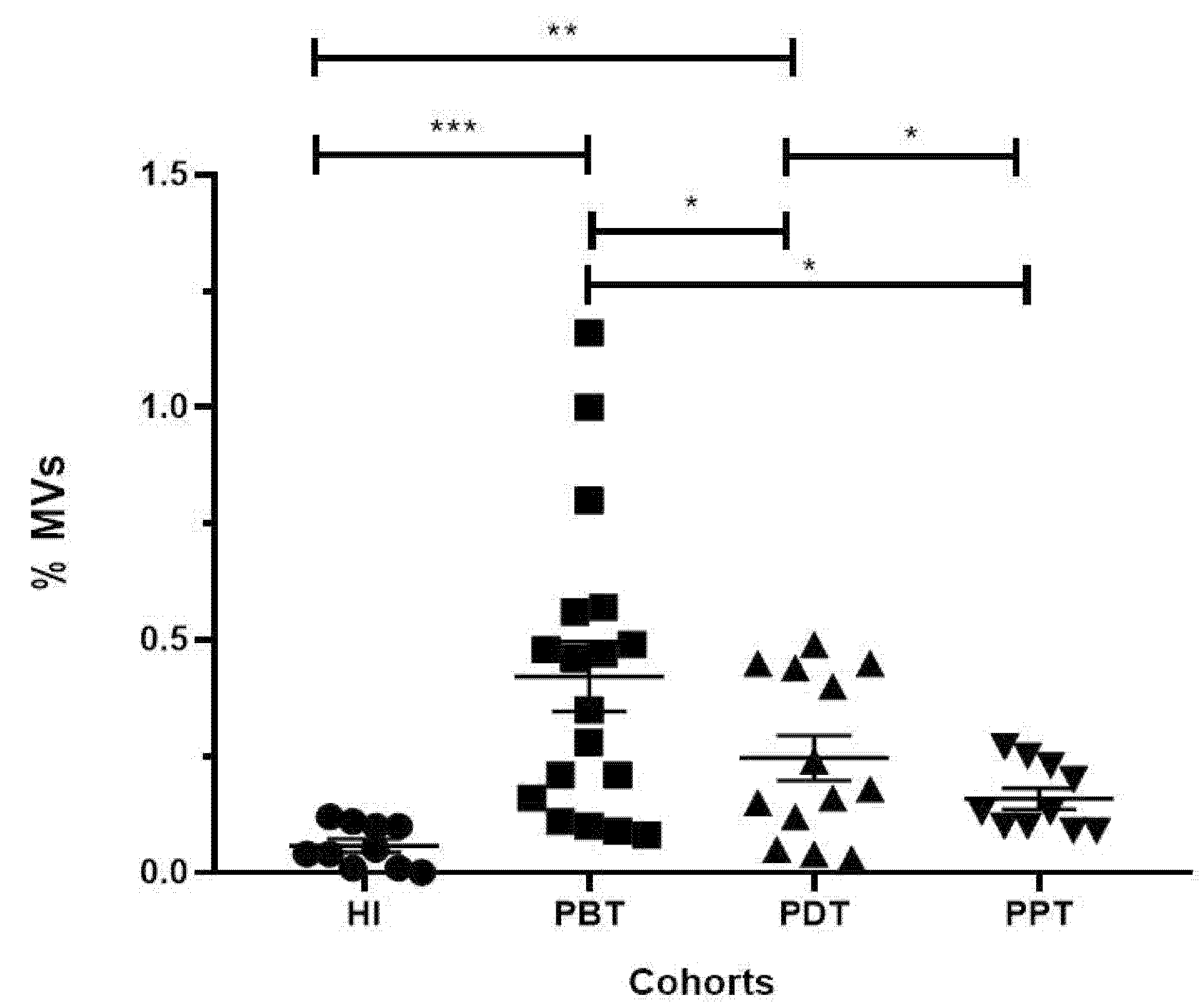
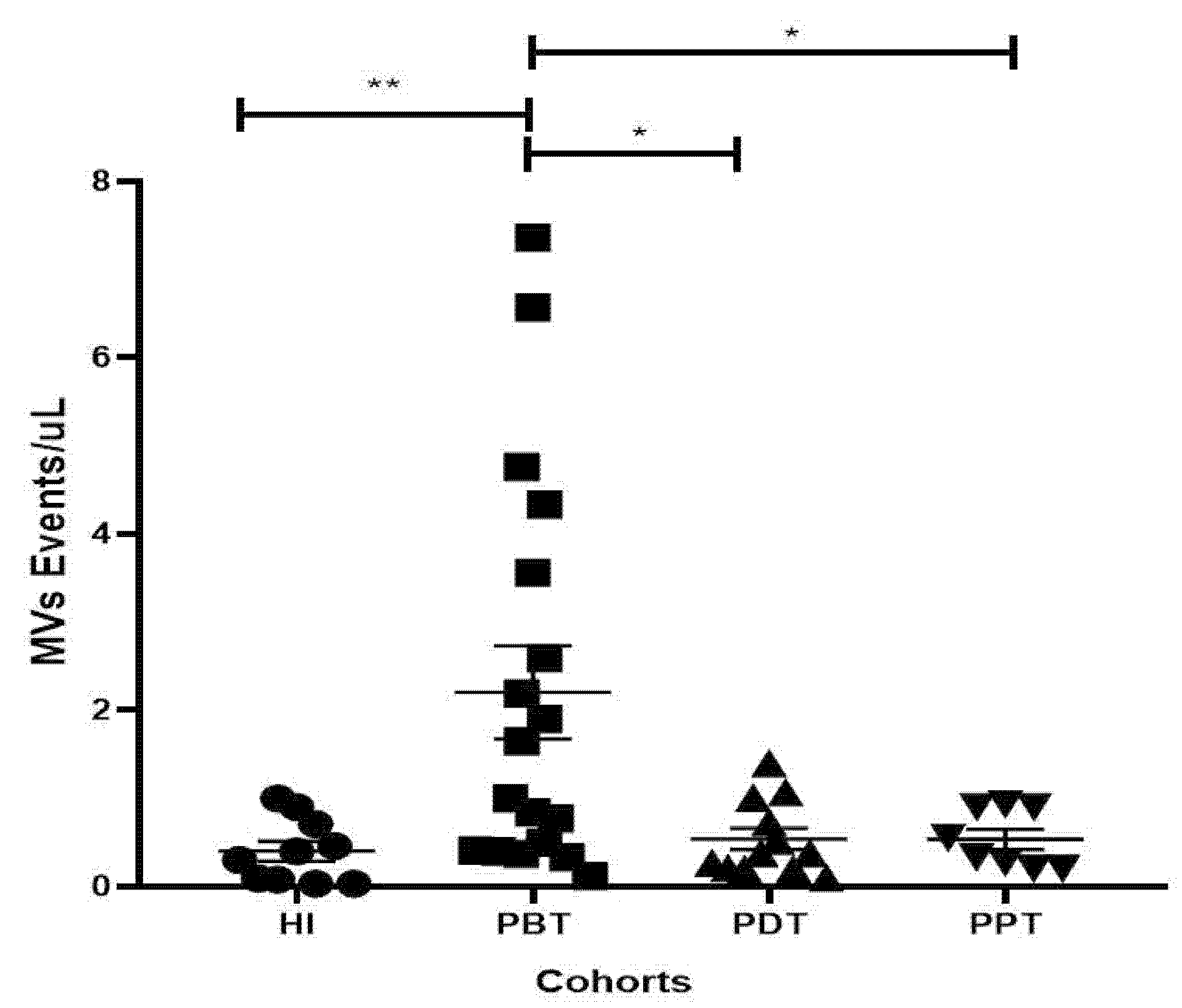
3.4. Evaluation of the Frequencies and Concentration of MVs in Plasma Samples from CL Patients and Healthy Individuals
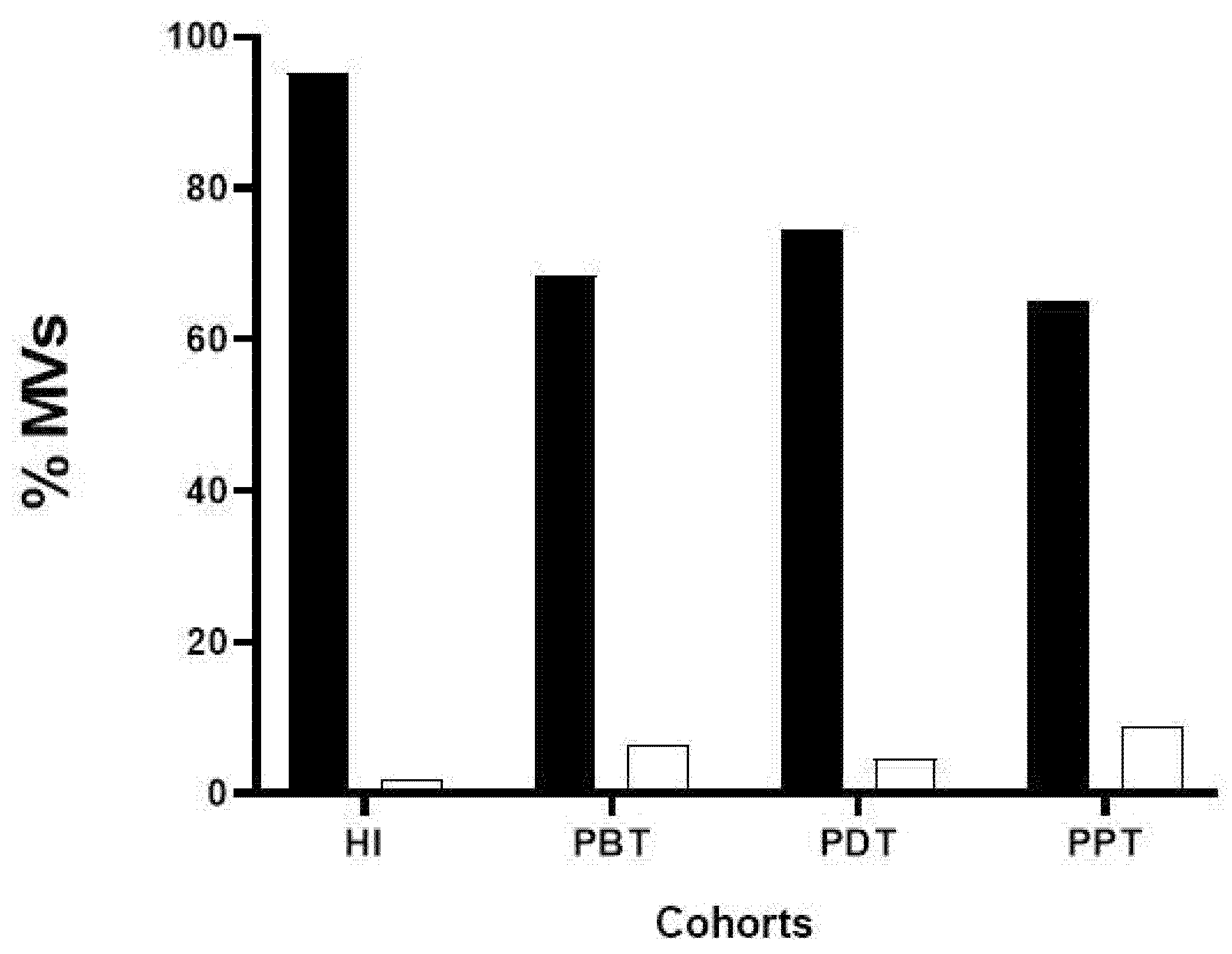
3.5. Phenotyping of MVs in Plasma Samples from CL Patients and Healthy Individuals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BRASIL, Ministério da Saúde. Manual de Vigilância da Leishmaniose Tegumentar. 2017. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar.pdf (accessed on 15 December 2023).
- OPAS, OMS Américas. Available online: https://www.paho.org/pt/topicos/leishmaniose (accessed on 23 December 2023).
- BRASIL, Ministério da Saúde. DATASUS. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/ltarj.def (accessed on 20 December 2023).
- BRASIL, Ministério da Saúde. Distribuição da Leishmaniose Tegumentar. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/l/lt/situacao-epidemiologica (accessed on 20 December 2023).
- Gabriel, A.; Valério-Bolas, A.; Palma-Marques, J.; Mourata-Gonçalves, P.; Ruas, P.; Dias-Guerreiro, T.; Santos-Gomes, G. Cutaneous Leishmaniasis: The Complexity of Host’s Effective Immune Response against a Polymorphic Parasitic Disease. J. Immunol. Res. 2019, 2019, 2603730. [Google Scholar] [CrossRef]
- Pirmez, C.; Yamamura, M.; Uyemura, K.; Paes-Oliveira, M.; Conceição-Silva, F.; Modlin, R.L. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin. Investig. 1993, 91, 1390–1395. [Google Scholar] [CrossRef]
- Santiago, M.A.; De Luca, P.M.; Bertho, Á.L.; Azeredo-Coutinho, R.B.; Coutinho, S.G. Detection of intracytoplasmic cytokines by flow cytometry. Mem. Inst. Oswaldo Cruz 2000, 95, 401–402. [Google Scholar] [CrossRef]
- Ruiz, J.H.; Becker, I. CD8 cytotoxic T cells in cutaneous leishmaniasis. Parasite Immunol. 2007, 29, 671–678. [Google Scholar] [CrossRef]
- Bertho, A.; Santiago, M.; Da-Cruz, A.; Coutinho, S. Detection of early apoptosis and cell death in T CD4+ and CD8+ cells from lesions of patients with localized cutaneous leishmaniasis. Braz. J. Med. Biol. Res. 2000, 33, 317–325. [Google Scholar] [CrossRef]
- Da-Cruz, A.M.; Oliveira-Neto, M.P.; Bertho, Á.L.; Mendes-Aguiar, C.O.; Coutinho, S.G. T Cells Specific to Leishmania and Other Nonrelated Microbial Antigens Can Migrate to Human Leishmaniasis Skin Lesions. J. Investig. Dermatol. 2010, 130, 1329–1336. [Google Scholar] [CrossRef]
- Ferraz, R.; Cunha, C.F.; Gomes-Silva, A.; O Schubach, A.; Pimentel, M.I.F.; Lyra, M.R.; Mendonça, S.C.; Valete-Rosalino, C.M.; Da-Cruz, A.M.; Bertho, Á.L. Apoptosis and frequency of total and effector CD8+ T lymphocytes from cutaneous leishmaniasis patients during antimonial therapy. BMC Infect. Dis. 2015, 15, 74–80. [Google Scholar] [CrossRef]
- Cunha, C.F.; Ferraz, R.; Pimentel, M.I.F.; Lyra, M.R.; Schubach, A.O.; Da-Cruz, A.M.; Bertho, Á.L. Cytotoxic cell involvement in human cutaneous leishmaniasis: Assessments in active disease, under therapy and after clinical cure. Parasite Immunol. 2016, 38, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.F.; Ferraz-Nogueira, R.; Costa, V.F.A.; Pimentel, M.I.F.; Chometon, T.Q.; Lyra, M.R.; Schubach, A.O.; Da-Cruz, A.M.; Bertho, A.L. Contribution of Leishmania braziliensis antigen-specific CD4+ T, CD8+ T, NK and CD3+CD56+NKT cells in the immunopathogenesis of cutaneous leishmaniasis patients: Cytotoxic, activation and exhaustion profiles. PLoS ONE 2020, 15, e0229400. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Cunha, C.F.; Pimentel, M.I.F.; Lyra, M.R.; Pereira-Da-Silva, T.; Schubach, A.O.; Da-Cruz, A.M.; Bertho, A.L. CD3+CD4negCD8neg (double negative) T lymphocytes and NKT cells as the main cytotoxic-related-CD107a+ cells in lesions of cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis. Parasites Vectors 2017, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Faria, D.R.; Souza, P.E.A.; Durães, F.V.; Carvalho, E.M.; Gollob, K.J.; Machado, P.R.; Dutra, W.O. Recruitment of CD8+ T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite Immunol. 2009, 31, 432–439. [Google Scholar] [CrossRef]
- de Oliveira, C.I.; Brodskyn, C.I. The immunobiology of Leishmania braziliensis infection. Front. Immunol. 2012, 3, 145. [Google Scholar] [CrossRef] [PubMed]
- Brahim, L.R.; Valete-Rosalino, C.M.; Antônio, L.d.F.; Pimentel, M.I.F.; Lyra, M.R.; Paes, L.E.d.C.; da Costa, A.D.; Vieira, I.F.; Dias, C.M.G.; Duque, M.C.d.O.; et al. Low dose systemic or intralesional meglumine antimoniate treatment for American tegumentary leishmaniasis results in low lethality, low incidence of relapse, and low late mucosal involvement in a referral centre in Rio de Janeiro, Brazil (2001–2013). Mem. Inst. Oswaldo Cruz 2017, 112, 838–843. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cataldo, J.I.; Conceição-Silva, F.; Antônio, L.d.F.; Schubach, A.d.O.; Marzochi, M.C.d.A.; Valete-Rosalino, C.M.; Pimentel, M.I.F.; Lyra, M.R.; Oliveira, R.d.V.C.d.; Barros, J.H.d.S.; et al. Favorable responses to treatment with 5 mg Sbv/kg/day meglumine antimoniate in patients with American tegumentary leishmaniasis acquired in different Brazilian regions. Rev. Soc. Bras. Med. Trop. 2018, 51, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Saheki, M.N.; Lyra, M.R.; Bedoya-Pacheco, S.J.; Antônio, L.d.F.; Pimentel, M.I.F.; Salgueiro, M.d.M.; Vasconcellos, É.d.C.F.e.; Passos, S.R.L.; dos Santos, G.P.L.; Ribeiro, M.N.; et al. Low versus high dose of antimony for American cutaneous leishmaniasis: A randomized controlled blind non-inferiority trial in Rio de Janeiro, Brazil. PLoS ONE 2017, 12, e0178592. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; Zhao, G.; Liu, R.; Du, Y.; Cai, Z.; Luan, J.; Shen, Y.; Chen, B. Current applications of platelet gels in wound healing—A review. Wound Repair Regen. 2021, 29, 370–379. [Google Scholar] [CrossRef]
- Shadmand, E.; Solhjoo, K.; Taghipour, A.; Tayer, A.H.; Sadeghi, F.; Meshkin, A. Healing effects of autologous platelet gel and growth factors on cutaneous leishmaniasis wounds in addition to antimony; a self-controlled clinical trial with randomized lesion assignment. BMC Res. Notes 2023, 16, 200. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, O.; Sonmez, M. Role of platelets in immune system and inflammation. Porto Biomed. J. 2017, 2, 311–314. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Heijnen, H.F.; E Schiel, A.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef]
- Puhm, F.; Boilard, E.; Machlus, K.R. Platelet Extracellular Vesicles. Arter. Thromb. Vasc. Biol. 2021, 41, 87–96. [Google Scholar] [CrossRef]
- Mabrouk, M.; Guessous, F.; Naya, A.; Merhi, Y.; Zaid, Y. The Pathophysiological Role of Platelet-Derived Extracellular Vesicles. Semin. Thromb. Hemost. 2022, 49, 279–283. [Google Scholar] [CrossRef]
- Puhm, F.; Flamand, L.; Boilard, E. Platelet extracellular vesicles in COVID-19: Potential markers and makers. J. Leukoc. Biol. 2022, 111, 63–74. [Google Scholar] [CrossRef] [PubMed]
- French, S.L.; Butov, K.R.; Allaeys, I.; Canas, J.; Morad, G.; Davenport, P.; Laroche, A.; Trubina, N.M.; Italiano, J.E., Jr.; Moses, M.A.; et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020, 4, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Lyonb, C.J.; Fletcher, J.K.; Tanga, W.; Wana, M.; Hub, T.Y. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm. Sin. B 2021, 11, 1493–1512. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Clos, J.; Horakova, E.; Wang, A.Y.; Wiesgigl, M.; Kelly, I.; Lynn, M.A.; McMaster, W.R.; Foster, L.J.; Levings, M.K.; et al. Leishmania Exosomes Modulate Innate and Adaptive Immune Responses through Effects on Monocytes and Dendritic Cells. J. Immunol. 2010, 185, 5011–5022. [Google Scholar] [CrossRef]
- Silverman, J.M.; Reiner, N.E. Leishmania Exosomes Deliver Preemptive Strikes to Create an Environment Permissive for Early Infection. Front. Cell. Infect. Microbiol. 2012, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Emerson, L.E.; Gioseffi, A.; Barker, H.; Sheppe, A.; Morrill, J.K.; Edelmann, M.J.; Kima, P.E. Leishmania infection-derived extracellular vesicles drive transcription of genes involved in M2 polarization. Front. Cell. Infect. Microbiol. 2022, 12, 934611. [Google Scholar] [CrossRef] [PubMed]
- Leite-Silva, J.; Oliveira-Ribeiro, C.; Morgado, F.N.; Pimentel, M.I.F.; Lyra, M.R.; Fagundes, A.; Miranda, L.F.C.; Valete-Rosalino, C.M.; Schubach, A.O.; Conceição-Silva, F. Is There Any Difference in the In Situ Immune Response in Active Localized Cutaneous Leishmaniasis That Respond Well or Poorly to Meglumine Antimoniate Treatment or Spontaneously Heal? Microorganisms 2023, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, C.P.B.; Moreira, O.C.; Cantanhêde, L.M.; de Farias, H.M.T.; Porrozzi, R.; Britto, C.; Boité, M.C.; Cupolillo, E. Comparison and clinical validation of qPCR assays targeting Leishmania 18S rDNA and HSP70 genes in patients with American Tegumentary Leishmaniasis. PLoS Neglected Trop. Dis. 2020, 14, e0008750. [Google Scholar] [CrossRef]
- Wisgrill, L.; Lamm, C.; Hartmann, J.; Preißing, F.; Dragosits, K.; Bee, A.; Hell, L.; Thaler, J.; Ay, C.; Pabinger, I.; et al. Peripheral blood microvesicles secretion is influenced by storage time, temperature, and anticoagulants. Cytom. Part A 2016, 89, 663–672. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Welsh, J.A.; Arkesteijn, G.J.A.; Bremer, M.; Cimorelli, M.; Dignat-George, F.; Giebel, B.; Görgens, A.; Hendrix, A.; Kuiper, M.; Lacroix, R.; et al. A compendium of single extracellular vesicle flow cytometry. J. Extracell. Vesicles 2023, 12, e12299. [Google Scholar] [CrossRef]
- Brittain, G.C.; Chen, Y.Q.; Martinez, E.; Tang, V.A.; Renner, T.M.; Langlois, M.-A.; Gulnik, S. A Novel Semiconductor-Based Flow Cytometer with Enhanced Light-Scatter Sensitivity for the Analysis of Biological Nanoparticles. Sci. Rep. 2019, 9, 16039. [Google Scholar] [CrossRef]
- Carnino, J.M.; Lee, H.; Jin, Y. Isolation and characterization of extracellular vesicles from Broncho-alveolar lavage fluid: A review and comparison of different methods. Respir. Res. 2019, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Mullier, F.; Bailly, N.; Chatelain, C.; Chatelain, B.; Dogné, J. Pre-analytical issues in the measurement of circulating microparticles: Current recommendations and pending questions. J. Thromb. Haemost. 2013, 11, 693–696. [Google Scholar] [CrossRef]
- Gray, W.D.; Mitchell, A.J.; Searles, C.D. An accurate, precise method for general labeling of extracellular vesicles. MethodsX 2015, 2, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet microvesicles in health and disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Conceição, J.; Davis, R.; Carneiro, P.P.; Giudice, A.; Muniz, A.C.; Wilson, M.E.; Carvalho, E.M.; Bacellar, O. Characterization of Neutrophil Function in Human Cutaneous Leishmaniasis Caused by Leishmania braziliensis. PLoS Neglected Trop. Dis. 2016, 10, e0004715. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.; Bezerra, C.; Medina, L.S.; Ramasawmy, R.; Scheriefer, A.; Bacellar, O.; de Carvalho, E.M. Leishmania braziliensis isolated from disseminated leishmaniasis patients downmodulate neutrophil function. Parasite Immunol. 2019, 41, e12620. [Google Scholar] [CrossRef] [PubMed]
| Clinical Status | |||
|---|---|---|---|
| Variable | PBT | PDT | PPT |
| (n) | 18 | 12 | 10 |
| Sex | |||
| Female | 7 | 5 | 5 |
| Male | 11 | 7 | 5 |
| Age | |||
| (mean ± SD) (Min–Max) | 34.06 ± 14.25 (18–60) | 42.83 ± 15.60 (20–60) | 31.60 ± 14.40 (18–57) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, V.F.d.A.; Chometon, T.Q.; de Castro, K.K.G.; Ponte, M.S.G.; Pimentel, M.I.F.; Lyra, M.R.; Bertho, A.L. Platelet-Derived Microvesicles Contribute to the Pathophysiogenesis of Human Cutaneous Leishmaniasis: A Nano-Flow Cytometric Approach in Plasma Samples from Patients before and under Antimonial Treatment. Microorganisms 2024, 12, 526. https://doi.org/10.3390/microorganisms12030526
Costa VFdA, Chometon TQ, de Castro KKG, Ponte MSG, Pimentel MIF, Lyra MR, Bertho AL. Platelet-Derived Microvesicles Contribute to the Pathophysiogenesis of Human Cutaneous Leishmaniasis: A Nano-Flow Cytometric Approach in Plasma Samples from Patients before and under Antimonial Treatment. Microorganisms. 2024; 12(3):526. https://doi.org/10.3390/microorganisms12030526
Chicago/Turabian StyleCosta, Vanessa Fernandes de Abreu, Thaize Quiroga Chometon, Katherine Kelda Gomes de Castro, Melissa Silva Gonçalves Ponte, Maria Inês Fernandes Pimentel, Marcelo Rosandiski Lyra, and Alvaro Luiz Bertho. 2024. "Platelet-Derived Microvesicles Contribute to the Pathophysiogenesis of Human Cutaneous Leishmaniasis: A Nano-Flow Cytometric Approach in Plasma Samples from Patients before and under Antimonial Treatment" Microorganisms 12, no. 3: 526. https://doi.org/10.3390/microorganisms12030526
APA StyleCosta, V. F. d. A., Chometon, T. Q., de Castro, K. K. G., Ponte, M. S. G., Pimentel, M. I. F., Lyra, M. R., & Bertho, A. L. (2024). Platelet-Derived Microvesicles Contribute to the Pathophysiogenesis of Human Cutaneous Leishmaniasis: A Nano-Flow Cytometric Approach in Plasma Samples from Patients before and under Antimonial Treatment. Microorganisms, 12(3), 526. https://doi.org/10.3390/microorganisms12030526







