In Vitro Cultivation for Glugea plecoglossi (Microsporidia) Isolated from Ayu (Plecoglossus altivelis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Maintenance
2.2. G. plecoglossi Spores Purification
2.3. Culture of Primary MO/MΦ, EPC Cells and RAW264.7 Cells
2.4. In Vitro Infection of Primary Cultures and Cell Lines Using G. plecoglossi Spores
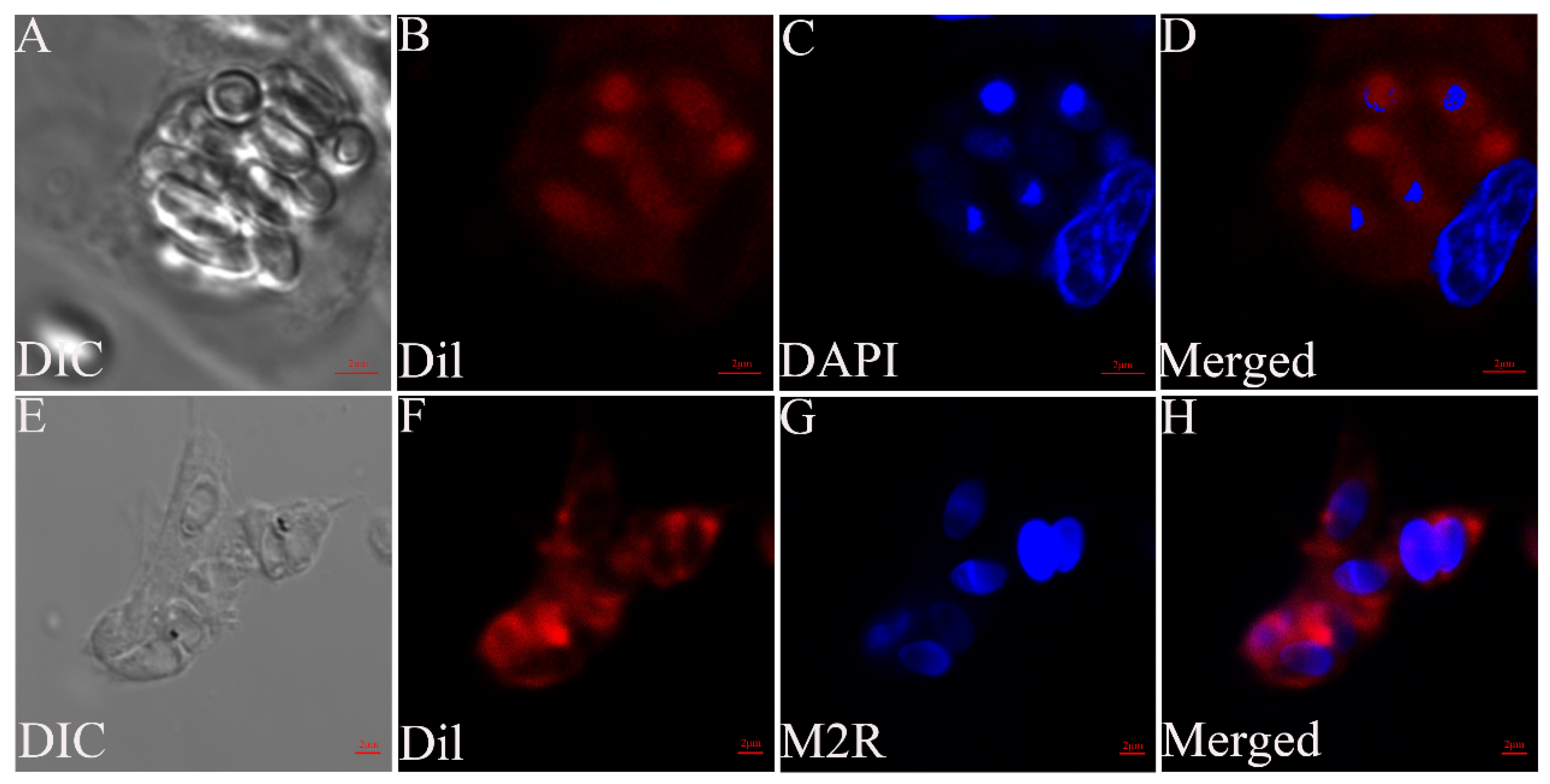
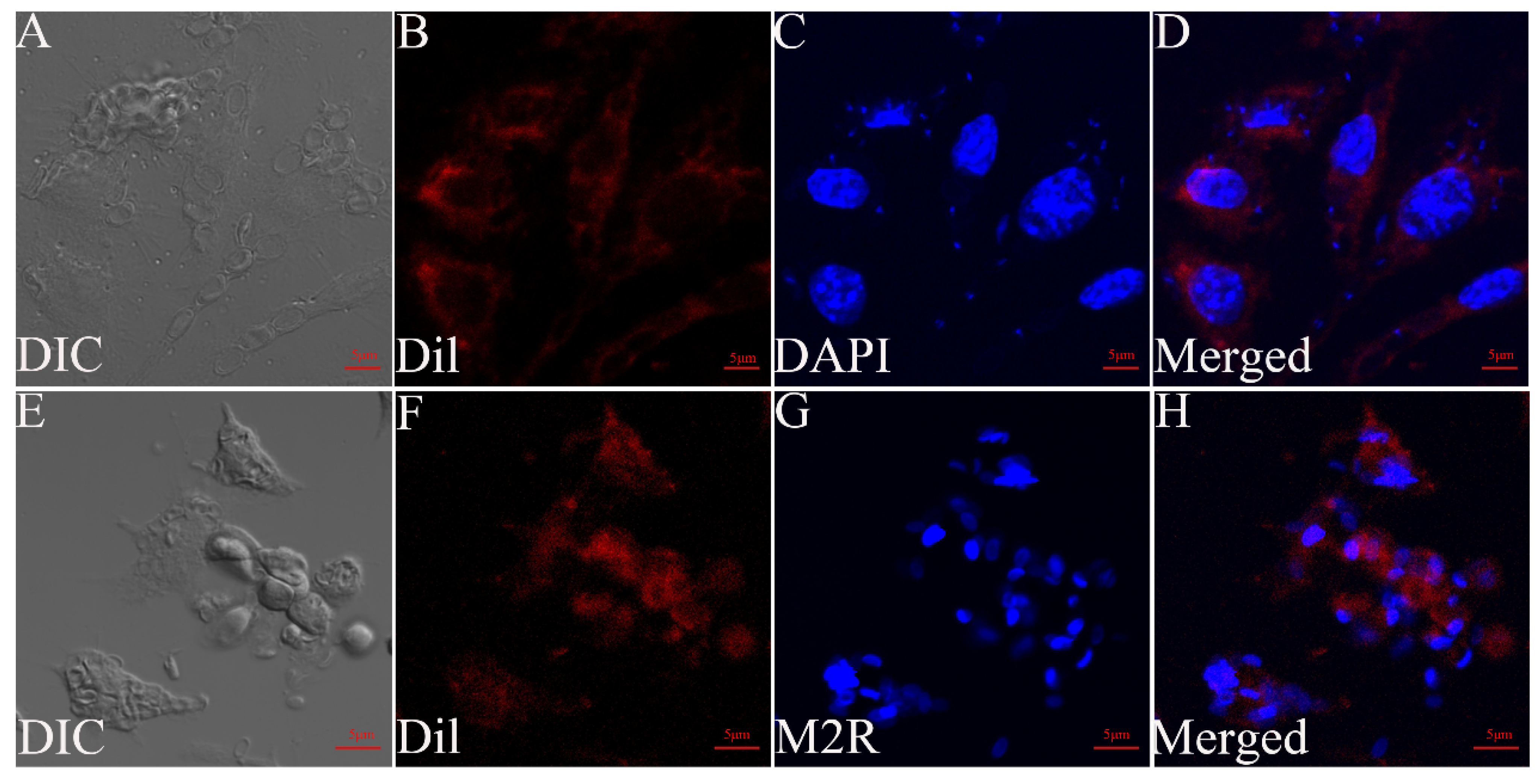
2.5. Cell Staining
2.6. Observation of G. plecoglossi Spores in Primary Cell and Cell Lines Using Transmission Electron Microscopy (TEM)
2.7. Cytokines Detection Using Real-Time Fluorescent Quantitative PCR
2.8. Re-Infection of Both Cell Lines and In Vivo Infection of Ayu Using EPC-Cultured G. plecoglossi Spores
3. Results
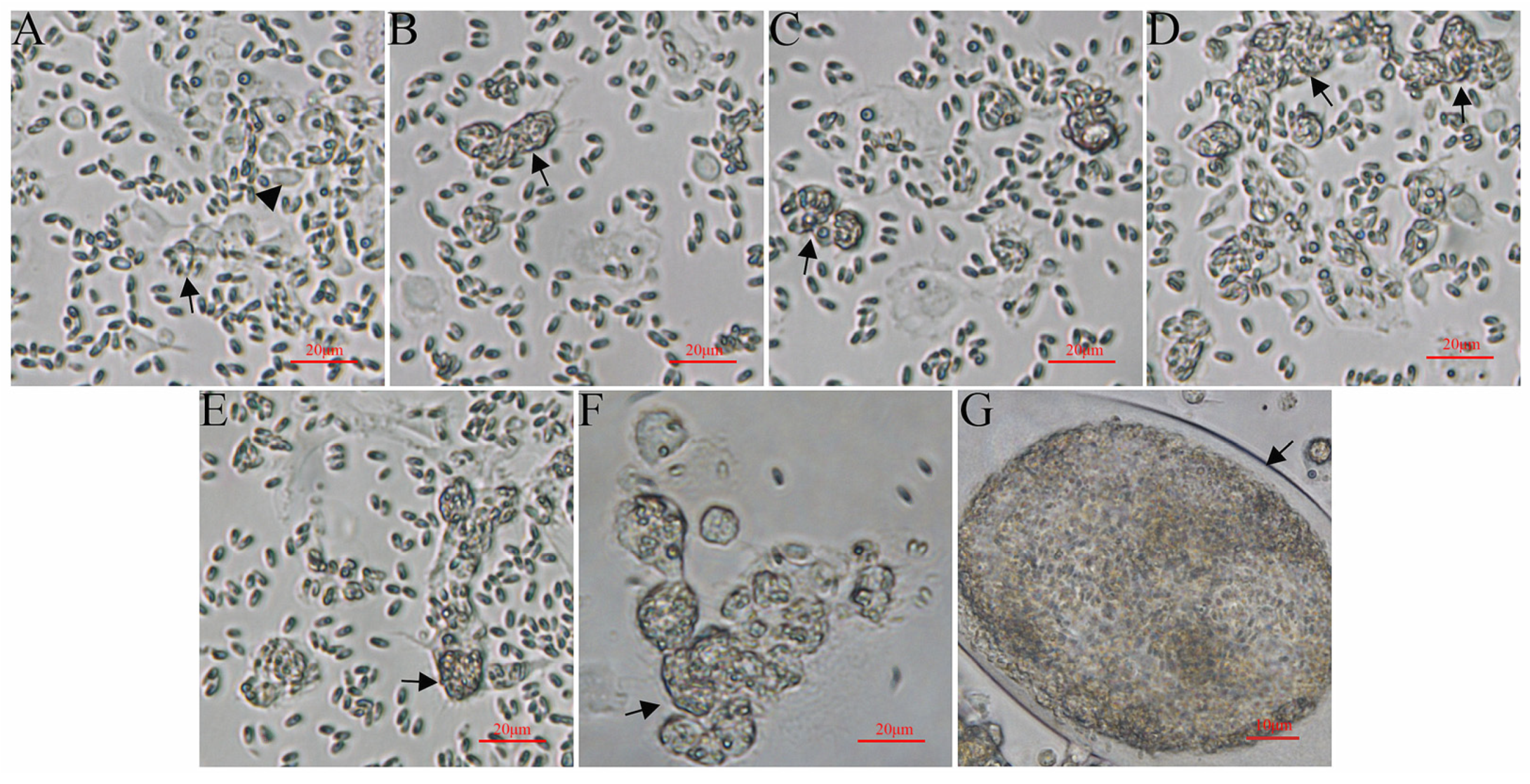
3.1. Light Microscopy Observation of Ayu MO/MΦ Infected with G. plecoglossi
3.2. Light Microscopy Observation of RAW264.7 Cells Infected with G. plecoglossi
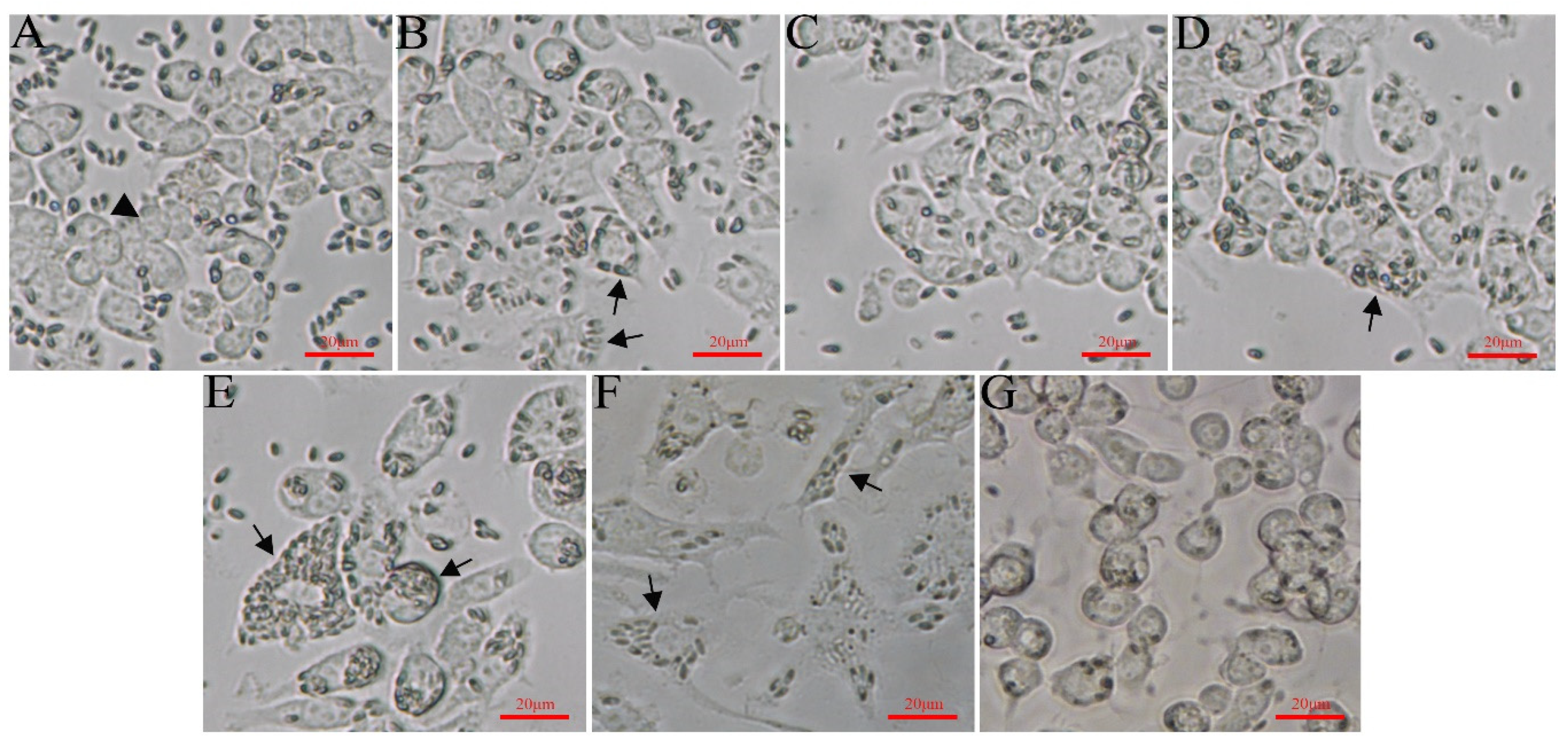
3.3. Light Microscopy Observation of EPC Cells Infected with G. plecoglossi
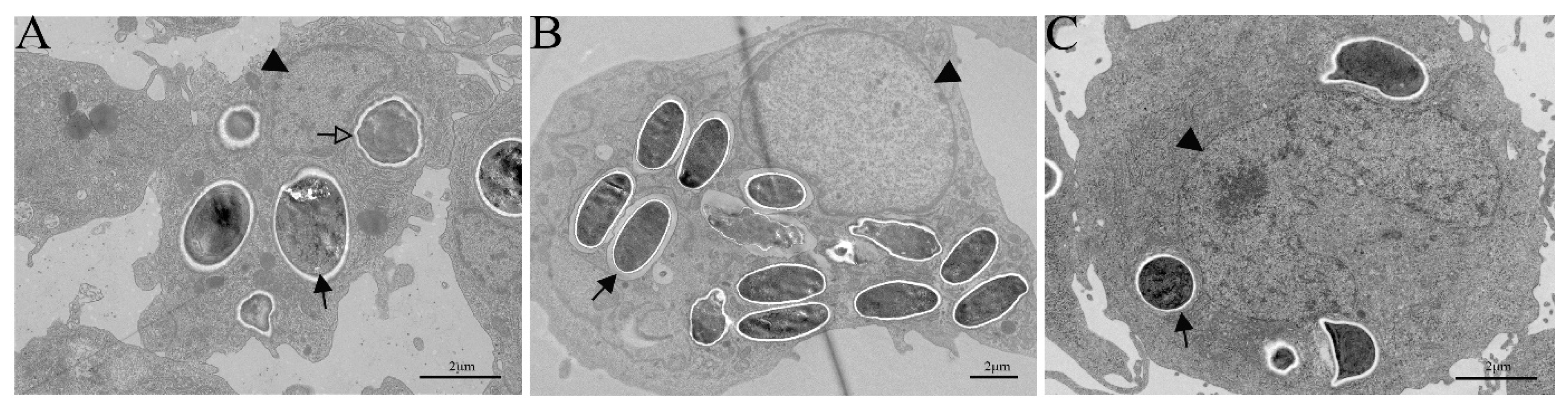
3.4. TEM Observation of G. plecoglossi Spores in Primary Cell and Cell Lines
3.5. The Cytokines Expression in MO/MΦ, RAW264.7, and EPC Cells upon G. plecoglossi Infection
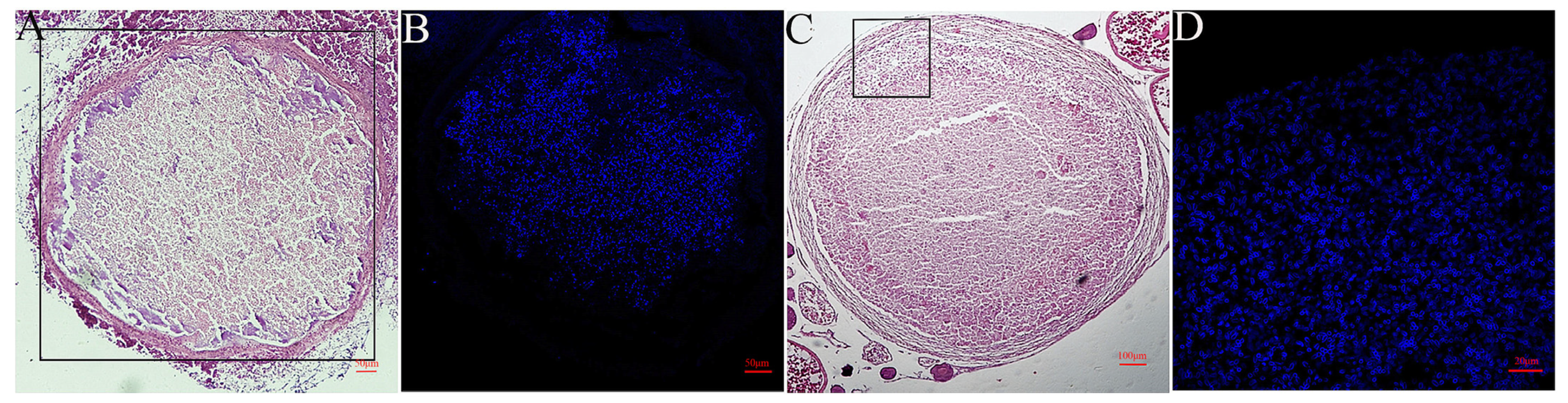
3.6. The Infectivity of Spores Cultured in EPC on Cell Lines and Ayu
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Texier, C.; Vidau, C.; Viguès, B.; El Alaoui, H.; Delbac, F. Microsporidia: A model for minimal parasite-host interactions. Curr. Opin. Microbiol. 2010, 13, 443–449. [Google Scholar] [CrossRef]
- Tamim, E.J.H.; Reinke, A.W. The ins and outs of host-microsporidia interactions during invasion, proliferation and exit. Cell. Microbiol. 2020, 22, e13247. [Google Scholar]
- Rodriguez-Tovar, L.E.; Speare, D.J.; Markham, R.J. Fish microsporidia: Immune response, immunomodulation and vaccination. Fish. Shellfish. Immunol. 2011, 30, 999–1006. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Feist, S.W.; Stone, D.M.; Bateman, K.S.; Dunn, A.M. Microsporidia: Diverse, dynamic, and emergent pathogens in aquatic systems. Trends Parasitol. 2013, 29, 567–578. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Marcet-Houben, M.; Gabaldón, T. Phylogenomics supports microsporidia as the earliest diverging clade of sequenced fungi. BMC Biol. 2012, 10, 47. [Google Scholar] [CrossRef]
- Lom, J.; Nilsen, F. Fish microsporidia: Fine structural diversity and phylogeny. Int. J. Parasitol. 2003, 33, 107–127. [Google Scholar] [CrossRef]
- Weng, M.Q.; Zhang, X.T.; Xin, Z.Z.; Xue, S.J.; Zhang, Q.Q.; Li, A.H.; Zhang, J.Y. Intraspecific genetic diversity of the fish-infecting microsporidian parasite Pseudokabatana alburnus (Microsporidia). Front. Microbiol. 2023, 14, 1129136. [Google Scholar] [CrossRef]
- Liu, X.H.; Wen, M.Q.; Zhao, Y.L.; Li, A.H.; Zhang, J.Y. Morphological and molecular characterization of a new freshwater microsporidium, Jirovecia sinensis sp. n. (Microsporidia) infecting the coelomocytes of Branchiura sowerbyi (Oligochaeta: Naididae) in China. J. Invertebr. Pathol. 2020, 173, 107368. [Google Scholar] [CrossRef]
- Couch, C.E.; Kent, M.L.; Weiss, L.M.; Takvorian, P.M.; Nervino, S.; Cummins, L.; Sanders, J.L. Enterocytozoon schreckii n. sp. Infects the Enterocytes of Adult Chinook Salmon (Oncorhynchus tshawytscha) and May Be a Sentinel of Immunosenescence. mSphere 2022, 7, e0090821. [Google Scholar] [CrossRef]
- Liu, X.H.; Stentiford, G.D.; Ren, S.S.; Yuan, X.P.; Song, R.; Yu, J.B.; Li, D.L.; Xiang, J.G.; Zhang, J.Y. Naidispora caidianensis n. gen. n. sp. infecting coelomocytes of oligochaete Branchiura sowerbyi (Oligochaeta: Naididae) in China. J. Invertebr. Pathol. 2022, 191, 107768. [Google Scholar] [CrossRef]
- Xu, L.W.; Liu, X.H.; Zhang, J.Y.; Liu, G.F.; Feng, J. Outbreak of enteric microsporidiosis of hatchery-bred juvenile groupers, Epinephelus spp., associated with a new intranuclear microporidian in China. J. Fish. Dis. 2017, 40, 183–189. [Google Scholar] [CrossRef]
- Palenzuela, O.; Redondo, M.J.; Cali, A.; Takvorian, P.M.; Alonso-Naveiro, M.; Alvarez-Pellitero, P.; Sitjà-Bobadilla, A. A new intranuclear microsporidium, Enterospora nucleophila n. sp., causing an emaciative syndrome in a piscine host (Sparus aurata), prompts the redescription of the family Enterocytozoonidae. Int. J. Parasitol. 2014, 44, 189–203. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Chai, F.C.; Chen, J. First record of Glugea plecoglossi (Takahashi & Egusa, 1977), a microsporidian parasite of ayu (Plecoglossus altivelis altivelis Temminck & Schlegel, 1846) in Mainland China. J. Fish. Dis. 2018, 41, 165–169. [Google Scholar]
- Kim, J.H.; Ogawa, K.; Wakabayashi, H. Lectin-reactive components of the microsporidian Glugea plecoglossi and their relation to spore phagocytosis by head kidney macrophages of ayu Plecoglossus altivelis. Dis. Aquat. Organ. 1999, 39, 59–63. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shiina, N.; Shinozaki, F.; Yokoyama, H.; Kominami, J.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Sugahara, K.; Kamiya, H.; Matsubara, H.; et al. Isolation and characterization of L-rhamnose-binding lectin, which binds to microsporidian Glugea plecoglossi, from ayu (Plecoglossus altivelis) eggs. Dev. Comp. Immunol. 2008, 32, 487–499. [Google Scholar] [CrossRef]
- Kim, J.H.; Ogawa, K.; Wakabayashi, H. Respiratory burst assay of head kidney macrophages of ayu, Plecoglossus altivelis, stimulated with Glugea plecoglossi (Protozoa: Microspora) spores. J. Parasitol. 1998, 84, 552–556. [Google Scholar] [CrossRef]
- Lores, B.; Rosales, M.J.; Mascaró, C.; Osuna, A. In vitro culture of Glugea sp. Vet. Parasitol. 2003, 112, 185–196. [Google Scholar] [CrossRef]
- Monaghan, S.R.; Kent, M.L.; Watral, V.G.; Kaufman, R.J.; Lee, L.E.; Bols, N.C. Animal cell cultures in microsporidial research: Their general roles and their specific use for fish microsporidia. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 135–147. [Google Scholar] [CrossRef]
- McConnachie, S.H.; Sheppard, J.; Wright, G.M.; Speare, D.J. Development of the microsporidian parasite, Loma salmonae, in a rainbow trout gill epithelial cell line (RTG-1): Evidence of xenoma development in vitro. Parasitology 2015, 142, 326–331. [Google Scholar] [CrossRef]
- MacLeod, M.J.; Vo, N.T.K.; Mikhaeil, M.S.; Monaghan, S.R.; Alexander, J.A.N.; Saran, M.K.; Lee, L.E.J. Development of a continuous cell line from larval Atlantic cod (Gadus morhua) and its use in the study of the microsporidian, Loma morhua. J. Fish. Dis. 2018, 41, 1359–1372. [Google Scholar] [CrossRef]
- Monaghan, S.R.; Rumney, R.L.; Vo, N.T.; Bols, N.C.; Lee, L.E. In Vitro growth of microsporidia Anncaliia algerae in cell lines from warm water fish. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 104–113. [Google Scholar] [CrossRef]
- Kumar, G.; Saleh, M.; Abdel-Baki, A.A.; Al-Quraishy, S.; El-Matbouli, M. In Vitro cultivation model for Heterosporis saurida (Microsporidia) isolated from lizardfish, Saurida undosquamis (Richardson). J. Fish. Dis. 2014, 37, 443–449. [Google Scholar] [CrossRef]
- Kang, J.W.; Jin, J.L.; Duan, L.J.; Zhou, Y.; Miao, L.; Zhou, Q.J.; Chen, J. A loop-mediated isothermal amplification technique combined with a lateral flow dipstick for the detection of Glugea plecoglossi. Oceanol. Limnol. Sin. 2020, 51, 1501–1512. [Google Scholar]
- Lee, S.J.; Yokoyama, H.; Ogawa, K. Modes of transmission of Glugea plecoglossi (Microspora) via the skin and digestive tract in an experimental infection model using rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish. Dis. 2004, 27, 435–444. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Q.J.; Qiao, Y.; Chen, J.; Li, M.Y. The host defense peptide β-defensin confers protection against Vibrio anguillarum in ayu, Plecoglossus altivelis. Dev. Comp. Immunol. 2020, 103, 103511. [Google Scholar] [CrossRef]
- Wu, X.Y.; Xiong, J.B.; Fei, C.J.; Dai, T.; Zhu, T.F.; Zhao, Z.Y.; Pan, J.; Nie, L.; Chen, J. Prior exposure to ciprofloxacin disrupts intestinal homeostasis and predisposes ayu (Plecoglossus altivelis) to subsequent Pseudomonas plecoglossicida-induced infection. Zool. Res. 2022, 43, 648–665. [Google Scholar]
- Huang, C.; Liu, X.J.; Zhou, Q.; Xie, J.; Ma, T.T.; Meng, X.M.; Li, J. MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264.7 macrophages. Int. Immunopharmacol. 2016, 32, 46–54. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.Y.; Wu, Z.Q.; Yao, L.J.; Wu, Y.H.; Huang, L.; Liu, K.; Zhou, X.; Gou, D.M. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef]
- Odaka, C.; Mizuochi, T.; Yang, J.X.; Ding, A.H. Murine macrophages produce secretory leukocyte protease inhibitor during clearance of apoptotic cells: Implications for resolution of the inflammatory response. J. Immunol. 2003, 171, 1507–1514. [Google Scholar] [CrossRef]
- Ma, F.; Liu, X.G.; Li, D.; Wang, P.; Li, N.; Lu, L.W.; Cao, X.T. MicroRNA-466l upregulates IL-10 expression in TLR-triggered macrophages by antagonizing RNA-binding protein tristetraprolin-mediated IL-10 mRNA degradation. J. Immunol. 2010, 184, 6053–6059. [Google Scholar] [CrossRef]
- Liu, Y.W.; Tseng, H.P.; Chen, L.C.; Chen, B.K.; Chang, W.C. Functional cooperation of simian virus 40 promoter factor 1 and CCAAT/enhancer-binding protein beta and delta in lipopolysaccharide-induced gene activation of IL-10 in mouse macrophages. J. Immunol. 2003, 171, 821–828. [Google Scholar] [CrossRef]
- Holopainen, R.; Tapiovaara, H.; Honkanen, J. Expression analysis of immune response genes in fish epithelial cells following ranavirus infection. Fish. Shellfish. Immunol. 2012, 32, 1095–1105. [Google Scholar] [CrossRef]
- Lu, J.F.; Luo, S.; Jin, T.C.; Wang, L.C.; Yang, G.J.; Lu, X.J.; Cheng, J. Betaine protects ayu (Plecoglossus altivelis) against Vibrio anguillarum infection in salinity by regulating the immunomodulatory activity of monocytes/macrophages. Aquaculture 2021, 536, 736482. [Google Scholar] [CrossRef]
- Lallo, M.A.; Vidoto Da Costa, L.F.; Alvares-Saraiva, A.M.; Rocha, P.R.; Spadacci-Morena, D.D.; Konno, F.T.; Suffredini, I.B. Culture and propagation of microsporidia of veterinary interest. J. Vet. Med. Sci. 2016, 78, 171–176. [Google Scholar] [CrossRef]
- Willis, A.R.; Reinke, A.W. Factors That Determine Microsporidia Infection and Host Specificity. Exp. Suppl. 2022, 114, 91–114. [Google Scholar]
- Molestina, R.; Weiss, L.M.; Becnel, J.J. Culture and Propagation of Microsporidia. In Microsporidia: Pathogens of Opportunity; Wiley: Hoboken, NJ, USA, 2014; pp. 457–467. [Google Scholar]
- Lom, J.; Dyková, I. Microsporidian xenomas in fish seen in wider perspective. Folia Parasitol. 2005, 52, 69–81. [Google Scholar] [CrossRef]
- Leiro, J.; Iglesias, R.; Paramá, A.; Sanmartín, M.L.; Ubeira, F.M. Effect of Tetramicra brevifilum (Microspora) infection on respiratory-burst responses of turbot (Scophthalmus maximus L.) phagocytes. Fish. Shellfish. Immunol. 2001, 11, 639–652. [Google Scholar] [CrossRef]
- Picard-Sánchez, A.; Piazzon, M.C.; Ahmed, N.H.; Del Pozo, R.; Sitjà-Bobadilla, A.; Palenzuela, O. Enterospora nucleophila (Microsporidia) in Gilthead Sea Bream (Sparus aurata): Pathological Effects and Cellular Immune Response in Natural Infections. Vet. Pathol. 2020, 57, 565–576. [Google Scholar] [CrossRef]
- Didier, E.S.; Weiss, L.M. Microsporidiosis: Current status. Curr. Opin. Infect. Dis. 2006, 19, 485–492. [Google Scholar] [CrossRef]
- Khan, I.A.; Schwartzman, J.D.; Kasper, L.H.; Moretto, M. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J. Immunol. 1999, 162, 6086–6091. [Google Scholar] [CrossRef]
- Schottelius, J.; Kuhn, E.M.; Enriquez, R. Microsporidia and Candida spores: Their discrimination by Calcofluor, trichrome-blue and methylene-blue combination staining. Trop. Med. Int. Health 2000, 5, 453–458. [Google Scholar]
- Green, L.C.; LeBlanc, P.J.; Didier, E.S. Discrimination between viable and dead Encephalitozoon cuniculi (Microsporidian) spores by dual staining with sytox green and calcofluor white M2R. J. Clin. Microbiol. 2000, 38, 3811–3814. [Google Scholar] [CrossRef]
- Chen, J.; Guo, W.; Dang, X.Q.; Huang, Y.K.; Liu, F.Y.; Meng, X.Z.; An, Y.Y.; Long, M.X.; Bao, J.L.; Zhou, Z.Y.; et al. Easy labeling of proliferative phase and sporogonic phase of microsporidia Nosema bombycis in host cells. PLoS ONE 2017, 12, e0179618. [Google Scholar] [CrossRef]
- Thepmanee, O.; Munkongwongsiri, N.; Prachumwat, A.; Saksmerprome, V.; Jitrakorn, S.; Sritunyalucksana, K.; Vanichviriyakit, R.; Chanarat, S.; Jaroenlak, P.; Itsathitphaisarn, O. Molecular and cellular characterization of four putative nucleotide transporters from the shrimp microsporidian Enterocytozoon hepatopenaei (EHP). Sci. Rep. 2023, 13, 20008. [Google Scholar] [CrossRef]
- Zhao, R.H.; Gao, W.; Qiu, L.; Chen, X.; Dong, X.; Li, C.; Huang, J. A staining method for detection of Enterocytozoon hepatopenaei (EHP) spores with calcofluor white. J. Invertebr. Pathol. 2020, 172, 107347. [Google Scholar] [CrossRef]



| Gene | GenBank No. | Primer | Nucleotide Sequence (5′→3′) | Resource |
|---|---|---|---|---|
| PaTNF-α | JP740414 | PaTNF-αF | ACATGGGAGCTGTGTTCCTC | [25] |
| PaTNF-αR | ACATGGGAGCTGTGTTCCTC | |||
| PaIL-1β | HF543937 | PaIL-1βF | TACCGGTTGGTACATCAGCA | |
| PaIL-1βR | TGACGGTAAAGTTGGTGCAA | |||
| PaTGF-β | JP742920 | PaTGF-βF | CTGGAATGCCGAGAACAAAT | |
| PaTGF-βR | CTGGAATGCCGAGAACAAAT | |||
| PaIL-10 | JP758157 | PaIL-10F | TGCTGGTGGTGCTGTTTATGTGT | |
| PaIL-10R | AAGGAGCAGCAGCGGTCAGAA | |||
| Pa18S rRNA | FN646593 | Pa18SF | GAATGTCTGCCCTATCAACT | |
| Pa18SR | GATGTGGTAGCCGTTTCT | |||
| RAWTNF-α | NM013693 | RAWTNF-αF | CCTCACACTCAGATCATCTTCTC | [27] |
| RAWTNF-αR | AGATCCATGCCGTTGGCCAG | |||
| RAWIL-1β | NM008361 | RAWIL-1βF | CTTTGAAGAAGAGCCCATCC | [28] |
| RAWIL-1βR | TTTGTCGTTGCTTGGTTCTC | |||
| RAWTGF-β | NM011577 | RAWTGF-βF | CAAGGAGACGGAATACAGGG | [29] |
| RAWTGF-βR | CGCACACAGCAGTTCTTCTC | |||
| RAWIL-10 | NM010548 | RAWIL-10F | GCTCTTACTGACTGGCATGAG | [30] |
| RAWIL-10R | CGCAGCTCTAGGAGCATGTG | |||
| RAWβ-actin | NM007393 | RAWβ-actinF | CCTAAGGCCAACCGTGAAAAG | [31] |
| RAWβ-actinR | TCTTCATGGTGCTAGGAGCCA | |||
| EPCTNF-α | JN412133 | EPCTNF-αF | CAAGCAATTGGCGAGTGTGT | [32] |
| EPCTNF-αR | CAGTTCCACTTTCCTGATTACTCTGA | |||
| EPCIL-1β | AJ245635 | EPCIL-1βF | AGACCAATCTCTACCTCGCTTGTAC | |
| EPCIL-1βR | TTAATGGTGTTTAATGTTTCACTGATCTC | |||
| EPCTGF-β | JN412135 | EPCTGF-βF | TGTATAACAGCACTGTCGAGCTAAGC | |
| EPCTGF-βR | TCCCTTCTCATTAGGATCTTCTACATC | |||
| EPCIL-10 | JN412134 | EPCIL-10F | GATGTCACGTCATGGACGAGAT | |
| EPCIL-10R | GGACTGGAAGTGGTTCTTCTGTACA | |||
| EPC40S | JN412136 | EPC40SF | TTTTGAGAAGAGGCATAAGAACATGT | |
| EPC40SR | GTAACGATGTCACCAACAGTCACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Zhang, Z.; Zhou, Q.; Song, M.; Yang, G.; Kang, J.; Xu, Z.; Chen, F.; Chen, J. In Vitro Cultivation for Glugea plecoglossi (Microsporidia) Isolated from Ayu (Plecoglossus altivelis). Microorganisms 2024, 12, 522. https://doi.org/10.3390/microorganisms12030522
Xu G, Zhang Z, Zhou Q, Song M, Yang G, Kang J, Xu Z, Chen F, Chen J. In Vitro Cultivation for Glugea plecoglossi (Microsporidia) Isolated from Ayu (Plecoglossus altivelis). Microorganisms. 2024; 12(3):522. https://doi.org/10.3390/microorganisms12030522
Chicago/Turabian StyleXu, Guizong, Zengyi Zhang, Qianjin Zhou, Mingyan Song, Guanjun Yang, Jinwei Kang, Zhongjie Xu, Fangjie Chen, and Jiong Chen. 2024. "In Vitro Cultivation for Glugea plecoglossi (Microsporidia) Isolated from Ayu (Plecoglossus altivelis)" Microorganisms 12, no. 3: 522. https://doi.org/10.3390/microorganisms12030522
APA StyleXu, G., Zhang, Z., Zhou, Q., Song, M., Yang, G., Kang, J., Xu, Z., Chen, F., & Chen, J. (2024). In Vitro Cultivation for Glugea plecoglossi (Microsporidia) Isolated from Ayu (Plecoglossus altivelis). Microorganisms, 12(3), 522. https://doi.org/10.3390/microorganisms12030522








