A New Strain of Metarhizium robertsii Isolated from Loess Plateau and Its Virulence and Pathological Characteristics against Monochamus alternatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of the Metarhizium robertsii Strain GQH6
2.1.1. Specimen Collection and Isolation
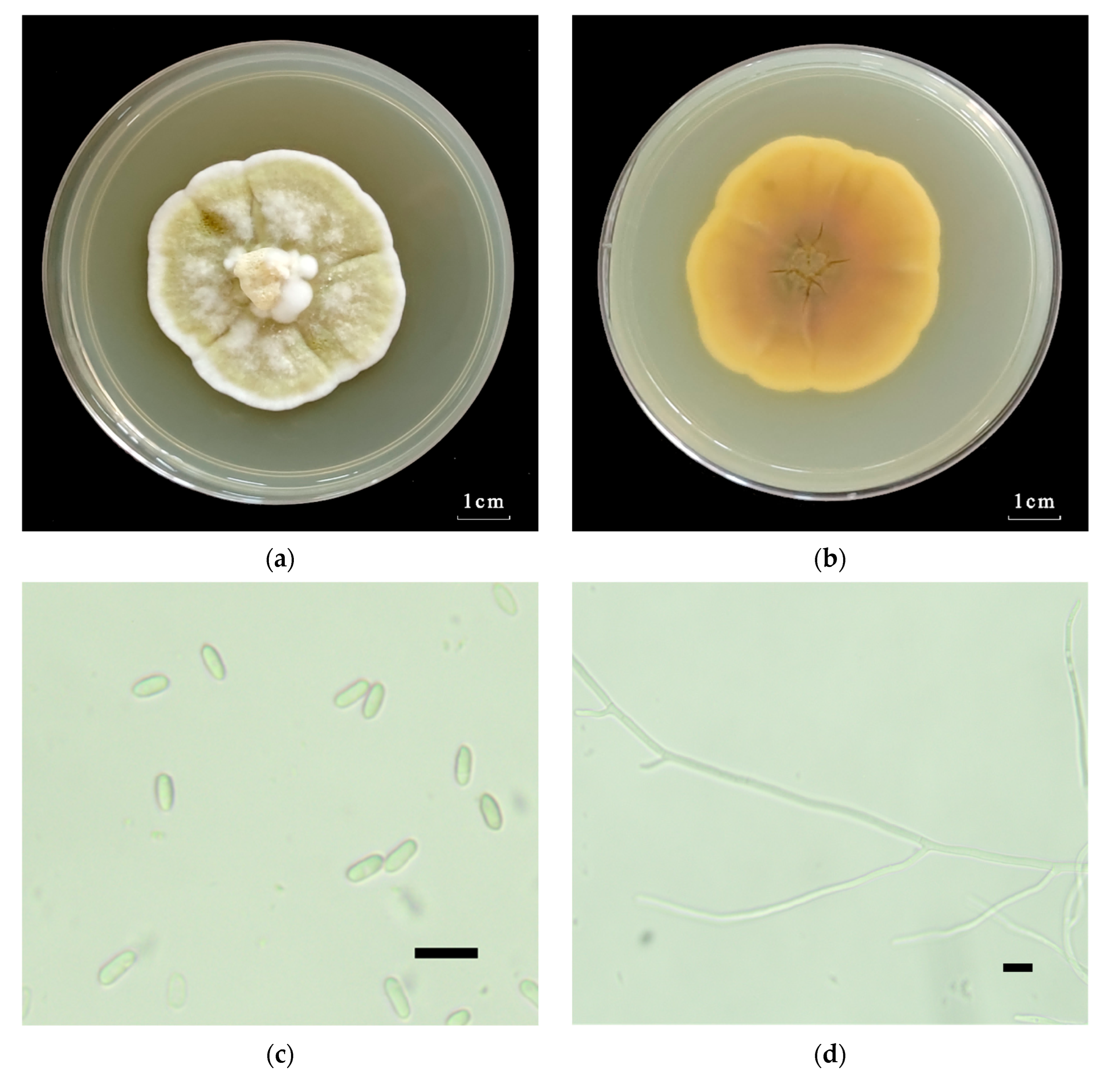
2.1.2. Morphological Identification of the Metarhizium robertsii Strain GQH6
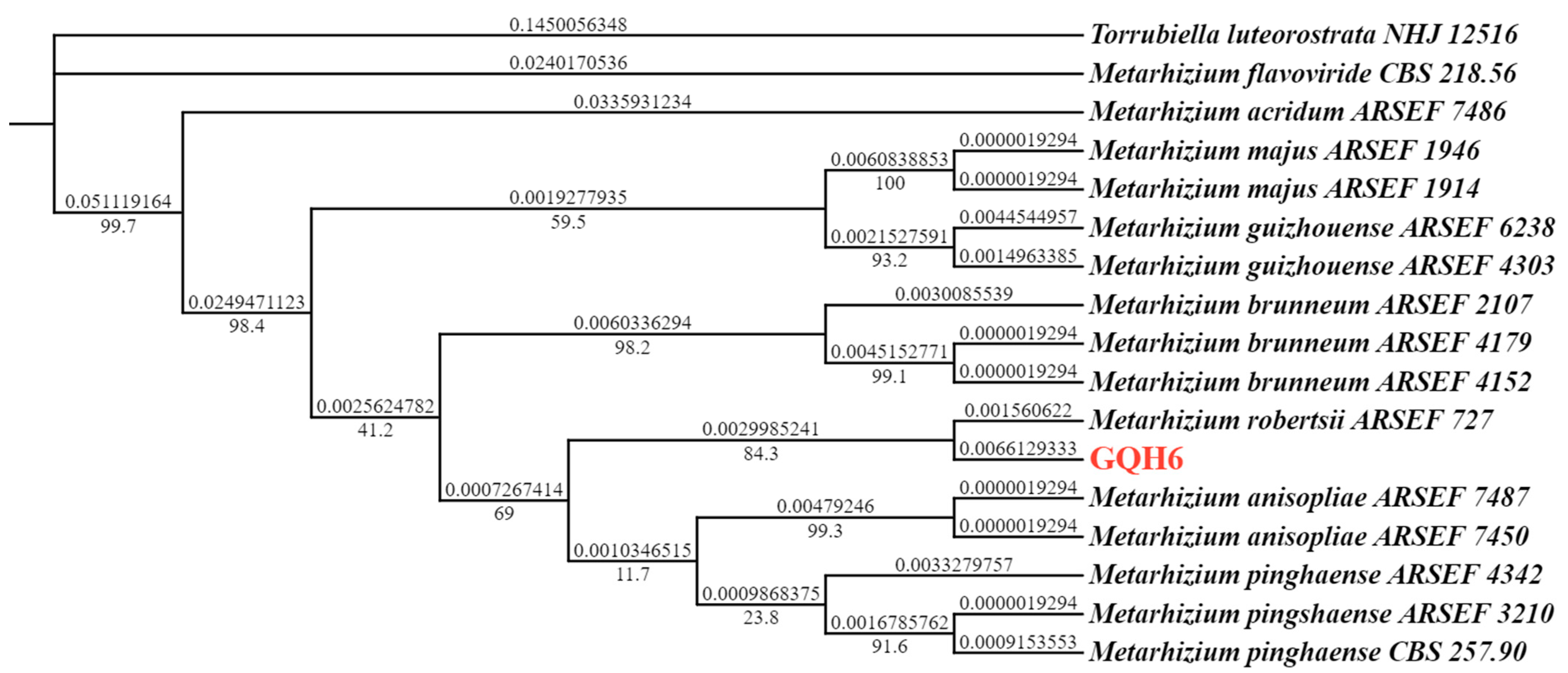
2.1.3. Molecular Identification of the Metarhizium robertsii Strain GQH6
2.2. Bioassay of the Metarhizium robertsii Strain GQH6
2.2.1. Experimental Insects
2.2.2. Preparation of Fungal Suspension
2.2.3. Bioassay
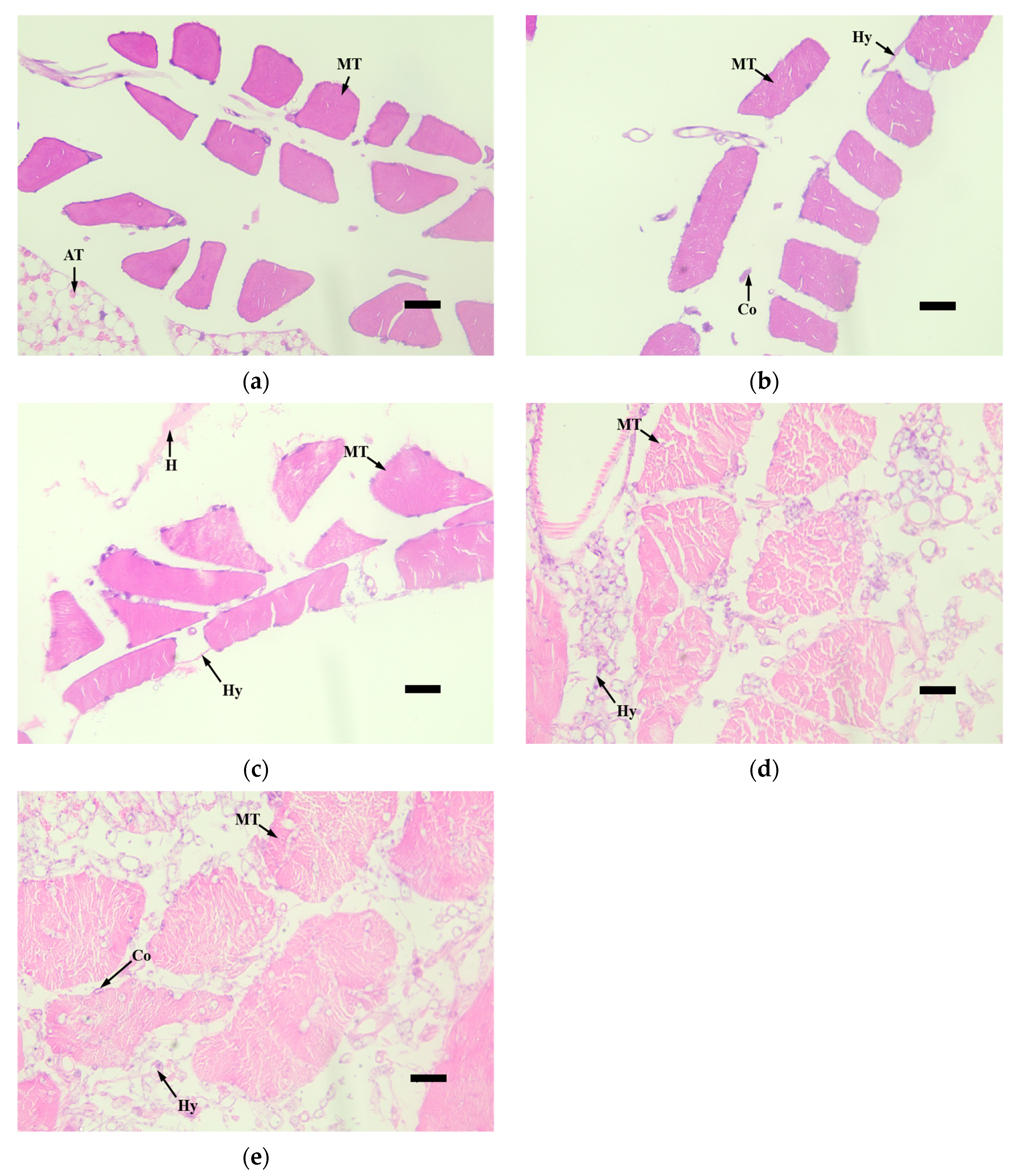
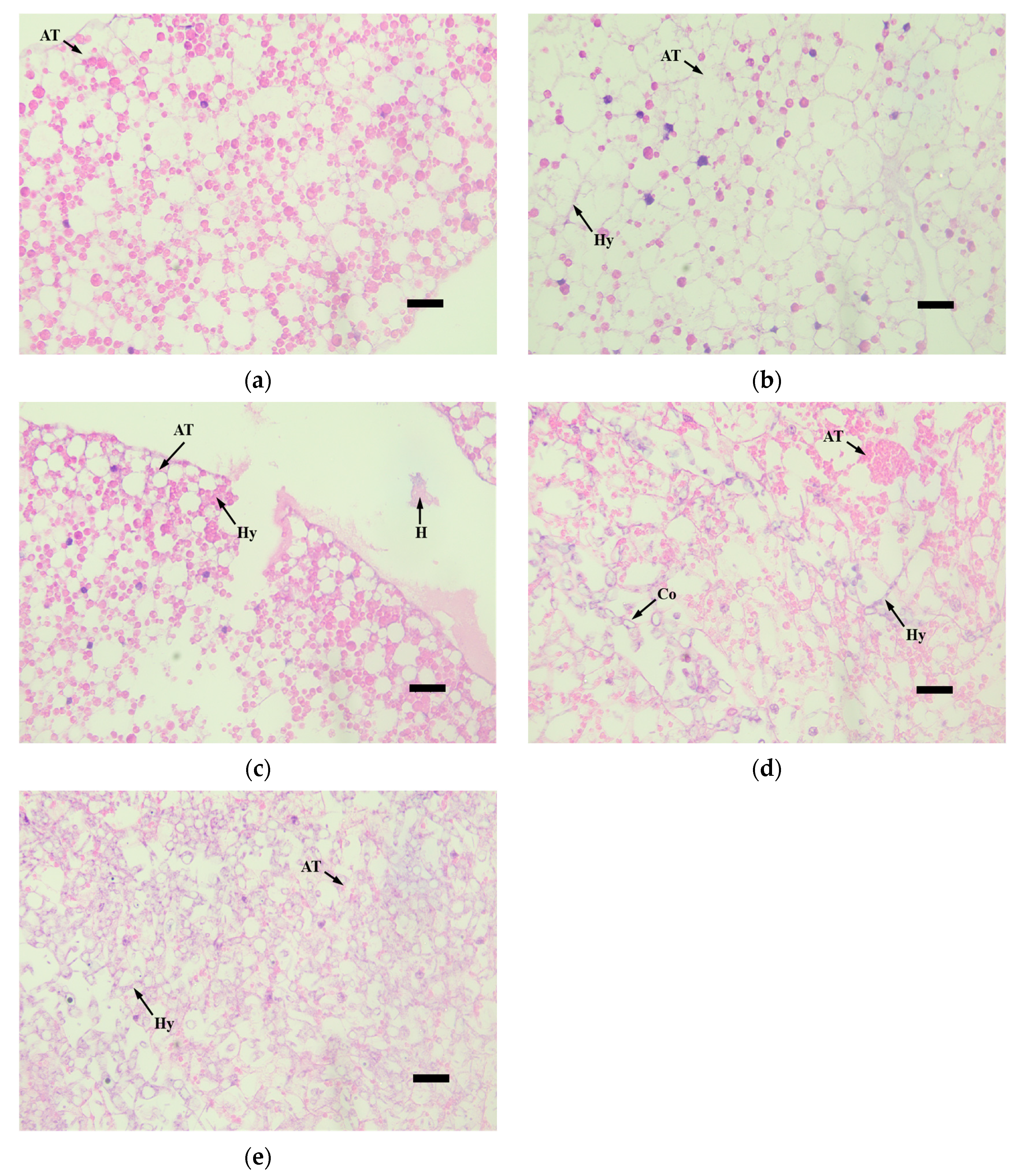
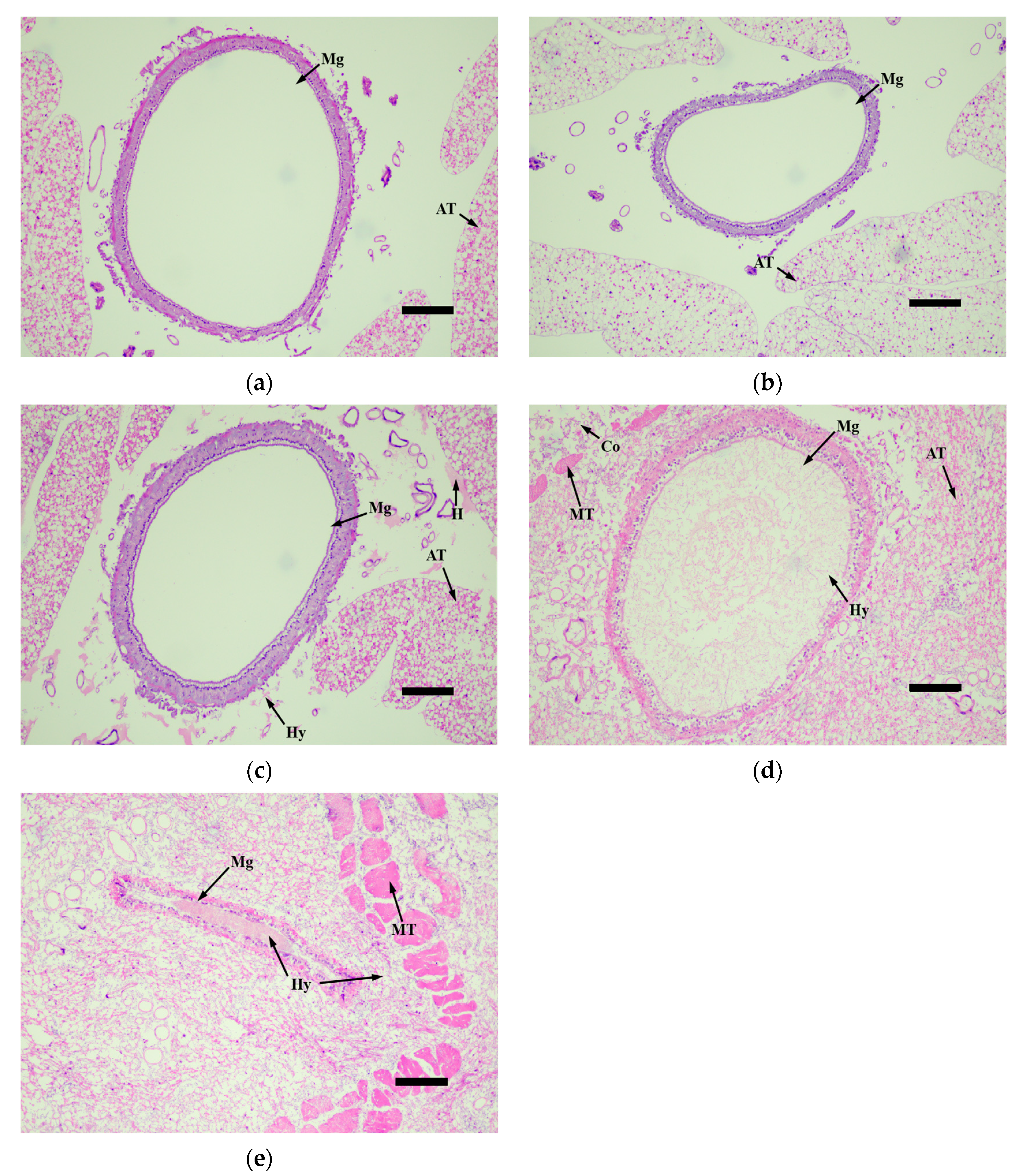
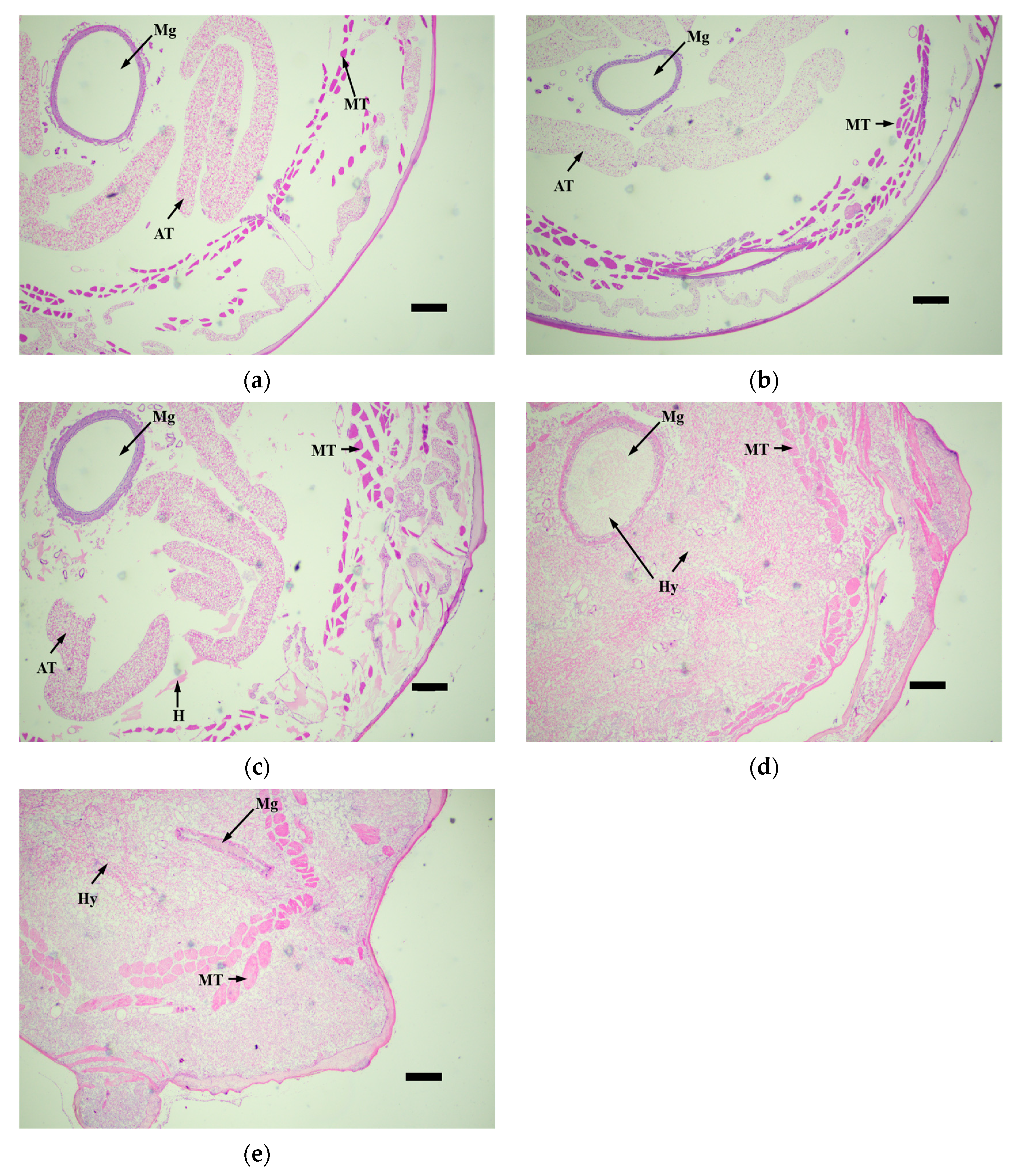
2.3. Symptom Observations and Histopathological Observations
2.4. Statistical Analysis
3. Results
3.1. Identification of the Metarhizium robertsii Strain GQH6
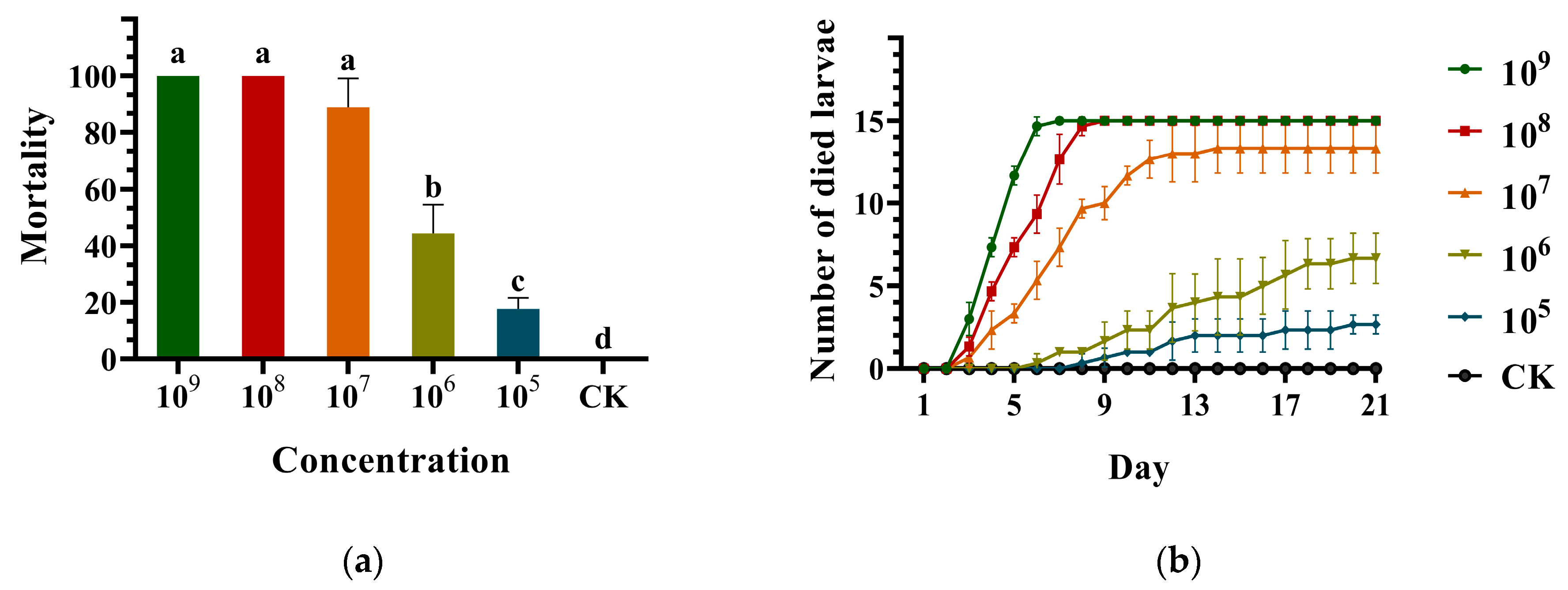
3.2. Virulence of the Metarhizium robertsii Strain GQH6 against Monochamus alternatus Larvae
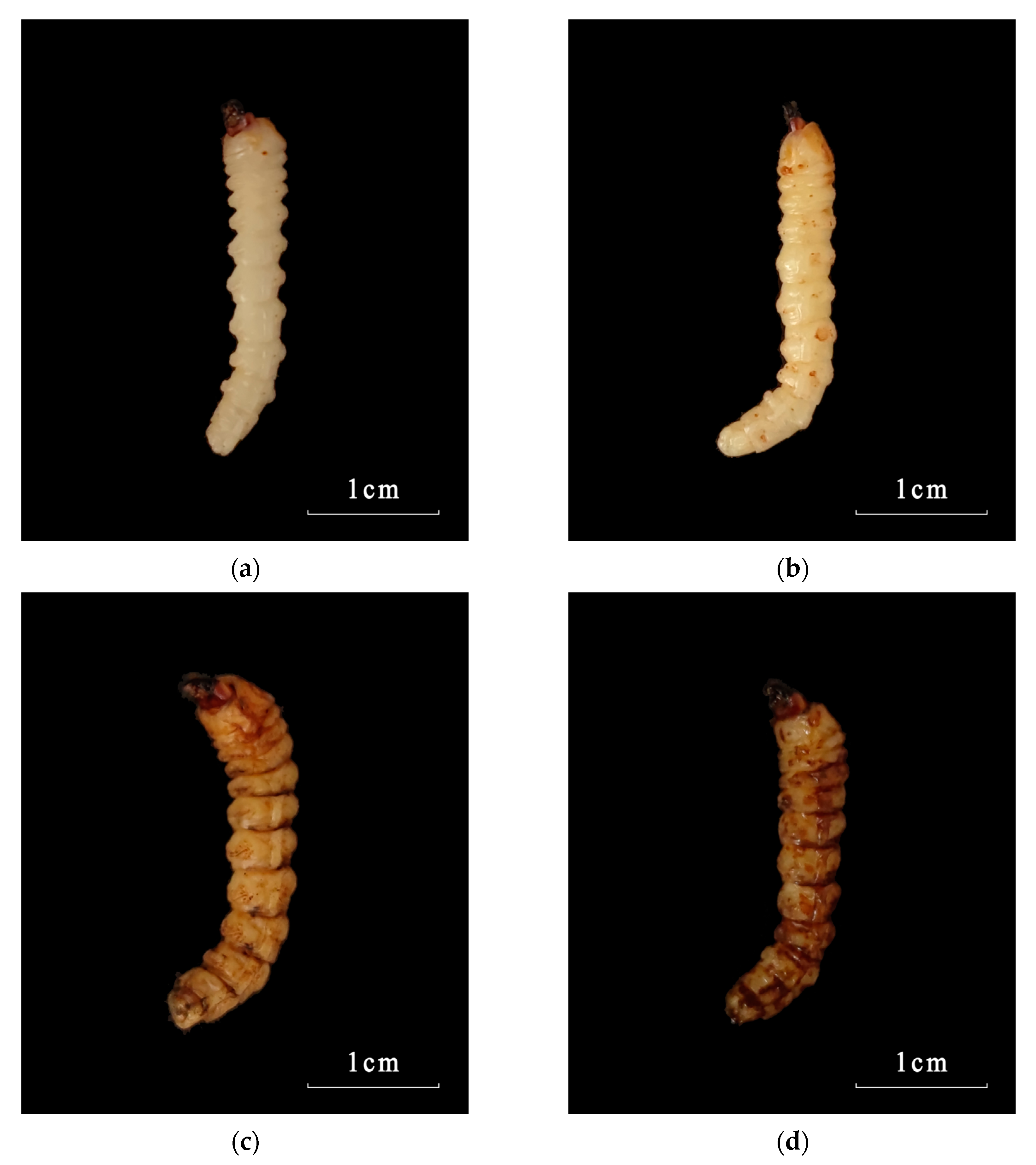
3.3. Symptom Observation of Infected Larvae
3.4. Histopathological Observation of Infected Larvae
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantzoukas, S.; Lagogiannis, I.; Mpekiri, M.; Pettas, I.; Eliopoulos, P.A. Insecticidal action of several isolates of entomopathogenic fungi against the granary weevil Sitophilus granarius. Agriculture 2019, 9, 9100222. [Google Scholar] [CrossRef]
- Gül, E.; Babarolu, N.E.; Demirci, F. Characterization and virulence of entomopathogenic fungi from sunn pests in Turkey. J. Asia-Pac. Entomol. 2021, 24, 215–223. [Google Scholar] [CrossRef]
- Gürlek, S.; Sevim, A.; Sezgin, F.M.; Sevim, E. Isolation and characterization of Beauveria and Metarhizium spp. from walnut fields and their pathogenicity against the codling moth, Cydia pomonella (NL.) (Lepidoptera: Tortricidae). Egypt. J. Biol. Pest Control. 2018, 28, 50. [Google Scholar] [CrossRef]
- Botelho, A.B.R.Z.; Alves-Pereira, A.; Prado, R.C.; Zucchi, M.I.; Delalibera, I. Metarhizium species in soil from Brazilian biomes: A study of diversity, distribution, and association with natural and agricultural environments. Fungal Ecol. 2019, 41, 289–300. [Google Scholar] [CrossRef]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future ? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C.G. Use of entomopathogenic fungi for the control of stored-product insects: Can fungi protect durable commodities? J. Pest Sci. 2017, 90, 839–854. [Google Scholar] [CrossRef]
- Fernández-Bravo, M.; Gschwend, F.; Mayerhofer, J.; Hug, A.; Widmer, F.; Enkerli, J. Land-use type drives soil population structures of the entomopathogenic fungal genus Metarhizium. Microorganisms 2021, 9, 1380. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Yadav, P.K. Entomopathogenic fungi and their relevance in sustainable agriculture: A review. Cogent Food Agric. 2023, 9, 2180857. [Google Scholar] [CrossRef]
- Fang, W.G.; Azimzadeh, P.; St Leger, R.J. Strain improvement of fungal insecticides for controlling insect pests and vector-borne diseases. Curr. Opin. Microbiol. 2012, 15, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Wang, S.B. Insect pathogenic fungi: Genomics, molecular interactions, and genetic improvements. Annu. Rev. Entomol. 2017, 62, 73–90. [Google Scholar] [CrossRef]
- Xie, X.Y.; Wang, Y.L.; Jin, S.X.; He, L.L.; Jia, Z.F.; Huang, B. MrCreC, a carbon catabolite repression gene, is required for the growth, conidiation, stress tolerance and virulence of Metarhizium robertsii. J. Invertebr. Pathol. 2023, 201, 108009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Jiang, Y.Y.; Li, Y.D.; Feng, J.Y.; Huang, B. MrArk1, an actin-regulating kinase gene, is required for endocytosis and involved in sustaining conidiation capacity and virulence in Metarhizium robertsii. Appl. Microbiol. Biotechnol. 2019, 103, 4859–4868. [Google Scholar] [CrossRef] [PubMed]
- Linit, M.J. Nematode–vector relationships in the pine wilt disease system. J. Nematol. 1988, 20, 227–235. [Google Scholar] [PubMed]
- Akbulut, S.; Stamps, W.T. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Linnakoski, R.; Forbes, K.M. Pathogens–the hidden face of forest invasions by wood-boring insect pests. Front. Plant Sci. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Lee, S.J.; Kim, S.; Lee, M.R.; Baek, S.; Park, S.E.; Kim, J.; Shin, T.Y.; Kim, J.S. Management of pine wilt disease vectoring Monochamus alternatus adults using spray and soil application of Metarhizium anisopliae JEF isolates. J. Asia-Pac. Entomol. 2020, 23, 224–233. [Google Scholar] [CrossRef]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, J.J.; Yan, J.; Fang, G.F.; Huang, J.X. Economic loss assessment of pine wilt disease in mainland China in 2017. J. Beijing For. Univ. 2020, 42, 96–106. [Google Scholar]
- Lee, S.M.; Chung, Y.J.; Moon, Y.S.; Lee, S.G.; Lee, C.K. Insecticidal activity and fumigation conditions of several insecticides against Japanese pine sawyer (Monochamus alternatus) larvae. J. Korean For. Soc. 2003, 92, 191–198. [Google Scholar]
- Jung, J.K.; Lee, U.G.; Cha, D.; Kim, D.S.; Jung, C. Can insecticide applications used to kill vector insects prevent pine wilt disease? Pest Manag. Sci. 2021, 77, 4923–4929. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef] [PubMed]
- Lira, E.C.; Bolzan, A.; Nascimento, A.R.B.; Amaral, F.S.A.; Kanno, R.H.; Kaiser, I.S.; Omoto, C. Resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to spinetoram: Inheritance and cross-resistance to spinosad. Pest Manag. Sci. 2020, 76, 2674–2680. [Google Scholar] [CrossRef]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Carlesso, D.; Smargiassi, S.; Sassoli, L.; Cappa, F.; Cervo, R.; Baracchi, D. Exposure to a biopesticide interferes with sucrose responsiveness and learning in honey bees. Sci. Rep. 2020, 10, 19929. [Google Scholar] [CrossRef] [PubMed]
- Novgorodova, T.; Vladimirova, N.; Marchenko, I.; Sadokhina, T.; Tyurin, M.; Ashmarina, L.; Bakshaev, D.; Lednev, G.; Danilov, V. The effect of bean seed treatment with entomopathogenic fungus Metarhizium robertsii on soil microarthropods (Acari, Collembola). Insects 2022, 13, 807. [Google Scholar] [CrossRef]
- Leite, M.O.G.; Alves, D.A.; Lecocq, A.; Malaquias, J.B.; Delalibera, I.; Jensen, A.B. Laboratory risk assessment of three entomopathogenic fungi used for pest control toward social bee pollinators. Microorganisms 2022, 10, 1800. [Google Scholar] [CrossRef]
- Mazra’awi, M.S.A.; Shipp, J.L.; Broadbent, A.B.; Kevan, P.G. Dissemination of Beauveria bassiana by honey bees (Hymenoptera: Apidae) for control of tarnished plant bug (Hemiptera: Miridae) on canola. Environ. Entomol. 2006, 35, 1569–1577. [Google Scholar]
- Koller, J.; Sutter, L.; Gonthier, J.; Collatz, J.; Norgrove, L. Entomopathogens and parasitoids allied in biocontrol: A systematic review. Pathogens 2023, 12, 957. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, M.R.; Kim, S.; Park, S.E.; Lee, S.J.; Shin, T.Y.; Kim, W.J.; Kim, J. Transcriptome analysis of the Japanese pine sawyer beetle, Monochamus alternatus, infected with the entomopathogenic fungus Metarhizium anisopliae JEF-197. J. Fungi 2021, 7, 373. [Google Scholar] [CrossRef]
- Shanley, R.P.; Keena, M.; Wheeler, M.M.; Leland, J.; Hajek, A.E. Evaluating the virulence and longevity of non-woven fiber bands impregnated with Metarhizium anisopliae against the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Biol. Control 2009, 50, 94–102. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I.; Ntoukas, A.; Eliopoulos, P.A.; Kouretas, D.; Karpouzas, D.G.; Poulas, K. Trapping entomopathogenic fungi from vine terroir soil samples with insect baits for controlling serious pests. Appl. Sci. 2020, 10, 3539. [Google Scholar] [CrossRef]
- Almudena, O.; Nemat, O.K. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar]
- Han, P.F.; Han, J.; Zhang, M.; Fan, J.Q.; Gong, Q.T.; Ma, E.B.; Zhang, J.Z. 20-Hydroxyecdysone enhances Immulectin-1 mediated immune response against entomogenous fungus in Locusta migratoria. Pest Manag. Sci. 2020, 76, 304–313. [Google Scholar] [CrossRef]
- Zimmermann, G. The galleria bait method for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols 1990, 18, 315–322. [Google Scholar]
- Bischoff, J.F.; Chaverri, P.; White, J.F. Clarification of the host substrate of Ascopolyporus and description of Ascopolyporus philodendrus sp. nov. Mycologia 2005, 97, 710–717. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, X.; Zhi, J.; Xie, J.; Jiang, T. Fast recognition of Lecanicillium spp. and its virulence against Frankliniella occidentalis. Front. Microbiol. 2020, 11, 561381. [Google Scholar] [CrossRef]
- Zhang, D.F.; Gao, I.; Jakovlic, H.; Zou, J.; Zhang, W.; Li, X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Apirajkamol, N.; Hogarty, T.M.; Mainali, B.; Taylor, P.W.; Walsh, T.K.; Tay, W.T. Virulence of Beauveria sp. and Metarhizium sp. fungi towards fall armyworm (Spodoptera frugiperda). Arch. Microbiol. 2023, 205, 328. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Butler, M.J.; Torabinejad, J.; Anderson, A.J.; Braga, G.U.L.; Day, A.W.; Roberts, D.W. Mutants and isolates of Metarhizium anisopliae are diverse in their relationships between conidial pigmentation and stress tolerance. J. Invertebr. Pathol. 2006, 93, 170–182. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Khonsanit, A.; Thanakitpipattana, D.; Tasanathai, K.; Noisripoom, W.; Lamlertthon, S.; Himaman, W.; Houbraken, J.; Samson, R.A.; Luangsa-ard, J. Revisiting Metarhizium and the description of new species from Thailand. Stud. Mycol. 2020, 95, 171–251. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, M.R.; Yu, J.S.; Park, S.E.; Ha, P.; Kim, J.S. Management of overwintering pine sawyer beetle, Monochamus alternatus with colonized Beauveria bassiana ERL836. PLoS ONE 2022, 17, e0274086. [Google Scholar] [CrossRef]
- Kim, H.M.; Jeong, S.G.; Choi, I.S.; Yang, J.E.; Lee, K.H.; Kim, J.; Kim, J.C.; Kim, J.S.; Park, H.W. Mechanisms of insecticidal action of Metarhizium anisopliae on adult Japanese pine sawyer beetles (Monochamus alternatus). ACS Omega 2020, 5, 25312–25318. [Google Scholar] [CrossRef]
- Kim, J.C.; Baek, S.; Park, S.E.; Kim, S.; Lee, M.R.; Jo, M.; Im, J.S.; Ha, P.; Kim, J.S.; Shin, T.Y. Colonization of Metarhizium anisopliae on the surface of pine tree logs: A promising biocontrol strategy for the Japanese pine sawyer, Monochamus alternatus. Fungal Biol. 2020, 124, 125–134. [Google Scholar] [CrossRef]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Phenotypic, molecular, and virulence characterization of entomopathogenic fungi, Beauveria bassiana (Balsam) Vuillemin, and Metarhizium anisopliae (Metschn.) Sorokin from soil samples of Ethiopia for the development of mycoinsecticide. Heliyon 2021, 7, e07091. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Bateman, R.; Charnley, A.K. Role of cuticle-degrading proteases in the virulence of Metarhizium spp. for the desert locust, Schistocerca gregaria. J. Invertebr. Pathol. 1998, 71, 128–137. [Google Scholar] [CrossRef]
- Gibson, D.M.; Donzelli, B.G.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Lu, H.L.; Sheng, H.Y.; St Leger, R.J. A Drosophila melanogaster model shows that fast growing Metarhizium species are the deadliest despite eliciting a strong immune response. Virulence 2023, 14, 2275493. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Barek, H. Critical analysis of the melanogenic pathway in insects and higher animals. Int. J. Mol. Sci. 2016, 17, E1753. [Google Scholar] [CrossRef]
- Boucias, D.G.; Zhou, Y.H.; Huang, S.S.; Keyhani, N.O. Microbiota in insect fungal pathology. Appl. Microbiol. Biotechnol. 2018, 102, 5873–5888. [Google Scholar] [CrossRef]
- Ali, S.; Huang, Z.; Ren, S.X. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biological agent against diamondback moth. J. Pest Sci. 2010, 83, 361–370. [Google Scholar] [CrossRef]
- Duan, Y.L.; Wu, H.; Ma, Z.Y.; Yang, L.; Ma, D.Y. Scanning electron microscopy and histopathological observations of Beauveria bassiana infection of Colorado potato beetle larvae. Microb. Pathog. 2017, 111, 435–439. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Saeed, S.; Wakil, W.; Nawaz, A.; Iqbal, N.; Yasin, M.; Chaurdhry, M.A.; Bashir, M.A.; Ahmed, N.; Riaz, H.; et al. Diversity and correlation of entomopathogenic and associated fungi with soil factors. J. King Saud Univ. Sci. 2021, 33, 101520. [Google Scholar] [CrossRef]
- Uzman, D.; Pliester, J.; Leyer, I.; Entling, M.H.; Reineke, A. Drivers of entomopathogenic fungi presence in organic and conventional vineyard soils. Appl. Soil Ecol. 2019, 133, 89–97. [Google Scholar] [CrossRef]
- Goble, T.A.; Dames, J.F.; Hill, M.P.; Moore, S.D. Investigation of native isolates of entomopathogenic fungi for the biological control of three citrus pests. Biocontrol Sci. Technol. 2011, 21, 1193–1211. [Google Scholar] [CrossRef]
- Tang, H.Y.; Zheng, J.Y.; Wang, D. An effective method for biological control of Anoplophora glabripennis (Coleoptera: Cerambycidae) by using a vector mite, Pyemotes moseri (Acarina: Pyemotidae), carrying insecticidal fungi. Sci. Silvae Sin. 2022, 58, 157–164. [Google Scholar]
- Sánchez-Gómez, T.; Harte, S.J.; Zamora, P.; Bareyre, M.; Díez, J.J.; Herrero, B.; Niño-Sánchez, J.; Martín-García, J. Nematicidal effect of Beauveria species and the mycotoxin beauvericin against pinewood nematode Bursaphelenchus xylophilus. Front. For. Glob. Chang. 2023, 6, 1229456. [Google Scholar] [CrossRef]

| Fungal Number | LC50 (Spore/mL) | 95% Confidence Interval | LC90 (Spore/mL) | 95% Confidence Interval |
|---|---|---|---|---|
| GQH6 | 1.93 × 106 | 1.40 × 106–2.69 × 106 | 1.35 × 107 | 3.85 × 106–4.41 × 107 |
| Fungal Number | Conidial Concentration | LT50 (Days) | 95% Confidence Interval | LT90 (Days) | 95% Confidence Interval |
|---|---|---|---|---|---|
| GQH6 | 109 | 3.96 | 3.85–4.08 | 5.45 | 4.83–6.43 |
| 108 | 4.99 | 4.79–5.18 | 7.78 | 5.66–12.99 | |
| 107 | 6.49 | 6.19–6.78 | 11.37 | 10.12–13.18 | |
| 106 | 11.83 | 10.99–12.66 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.-Y.; Shi, H.-L.; Wang, D. A New Strain of Metarhizium robertsii Isolated from Loess Plateau and Its Virulence and Pathological Characteristics against Monochamus alternatus. Microorganisms 2024, 12, 514. https://doi.org/10.3390/microorganisms12030514
Zheng J-Y, Shi H-L, Wang D. A New Strain of Metarhizium robertsii Isolated from Loess Plateau and Its Virulence and Pathological Characteristics against Monochamus alternatus. Microorganisms. 2024; 12(3):514. https://doi.org/10.3390/microorganisms12030514
Chicago/Turabian StyleZheng, Ji-Yang, He-Liang Shi, and Dun Wang. 2024. "A New Strain of Metarhizium robertsii Isolated from Loess Plateau and Its Virulence and Pathological Characteristics against Monochamus alternatus" Microorganisms 12, no. 3: 514. https://doi.org/10.3390/microorganisms12030514
APA StyleZheng, J.-Y., Shi, H.-L., & Wang, D. (2024). A New Strain of Metarhizium robertsii Isolated from Loess Plateau and Its Virulence and Pathological Characteristics against Monochamus alternatus. Microorganisms, 12(3), 514. https://doi.org/10.3390/microorganisms12030514







