Abstract
The gastrointestinal tract’s microbiota plays a crucial role in human health, with dysbiosis linked to the development of diseases such as inflammatory bowel disease (IBD). Whilst the pathogenic mechanisms underlying IBD remain poorly characterised, adherent-invasive Escherichia coli (AIEC) has been implicated as a microbiological factor in disease pathogenesis. These strains show an enhanced ability to diffusely adhere to and invade intestinal epithelial cells, along with the ability to survive and replicate within macrophages. Probiotics, such as Lactobacillus strains, have been identified as potential treatment options due to their abilities to compete with pathogens for binding sites and regulate the host immune response. In this study, we used four well-characterised Lactobacillus strains and their combination to test their ability to inhibit the adhesion, invasion, and translocation of a well-characterized AIEC strain, F44A-1, in a co-culture of Caco-2 and HT29-MTX cell lines representing the gut epithelium. The results demonstrated that the pre-inoculation of the probiotic candidates 90 min prior to the introduction of the AIEC was more effective in inhibiting AIEC interaction than the co-inoculation of the strains. While the individual probiotic strains greatly reduced AIEC colonisation and invasion of the co-cultured cells, their combination was only more effective in reducing the translocation of the AIEC. These results suggest that probiotics are more effective when used prophylactically against pathogens and that the combination of strains may enhance their efficacy against AIEC translocation once used as a prophylactic measure.
1. Introduction
The mucosal layer and microbiota of the gastrointestinal (GI) tract have a substantial impact on human health. When functioning normally, the GI tract is responsible for digestion, absorption, secretion, homeostasis, and acting as a protective barrier against luminal contents and enteric pathogens [1,2]. However, any abnormalities or dysbiosis in the mucosal layer or gut microbiota may lead to the development diseases such as inflammatory bowel disease (IBD), presenting as Crohn’s disease or ulcerative colitis [3,4,5,6]. Whilst the aetiology of IBD is yet to be confirmed, studies have found associations between dysbiosis in the GI tract or a decrease in beneficial bacteria and lower diversity of the microbiota with the pathogenesis of these disorders [7,8,9].
Escherichia coli strains, although constituting less than 1% of the gut microbiota, have been found in the GI tract and faeces of patients suffering from forms of IBD, with studies suggesting it has a contributing role in the aetiology of the disease [3,10,11]. Over the past two decades, a pathotype of E. coli referred to as adherent-invasive E. coli (AIEC) has been frequently isolated from the mucosal membrane of patients with Crohn’s disease [12,13]. These strains can adhere to and invade mucosal membranes and survive and multiply within macrophages [13,14,15]. AIEC causes further dysbiosis through competitive exclusion of both commensal and beneficial bacteria, as well as expressing certain virulence genes that aid its adherence and invasion capabilities [3,8,14,16]. Once AIEC adheres to the mucosal membrane, it can trigger the release of tumour necrosis factor (TNF) and other pro-inflammatory cytokines, leading to inflammation and enhanced epithelial permeability, allowing for further colonisation by the pathogen [7,8,10].
Currently, there are no curative treatments for these disorders, only management options that have small reductive impacts on patients’ symptoms [17,18,19]. The significance of the lack of treatment options, combined with problems arising with increased antibiotic-resistant bacteria has led to research into the use of beneficial bacteria [17,20,21,22]. Probiotics have been described by the World Health Organisation (WHO) as “live organisms that when administered in adequate amounts, can confer health benefits onto the host” [23]. Probiotic strains belonging to Lactobacillus, Bifidobacterium, and Propionibacterium have been found in the GI tract, fermented foods, and dairy products, and over the years have been commercialised to be consumed via tablets or capsules [21,24,25,26]. Studies into both approaches uncovering health benefits include alleviation of constipation and diarrhoea symptoms, improving lactose absorption and antibiotic efficacy, as well as enhancing immune responses and reducing bacterial infections [27,28,29,30].
Probiotics have also been shown to compete with pathogenic bacteria for binding sites and nutrients, leading to adherence to the epithelial or mucosal layers [31,32,33]. Competitive exclusion and adhesion are two of the most effective methods used to block pathogen colonisation, with an added benefit of enhancement of the epithelial barrier [34,35,36,37]. Secretion of antimicrobial substances is another major mechanism, with bacterial fermentation leading to the production of antimicrobial substances. Organic acids, hydrogen peroxide, diacetyl, reuterin, and bacteriocins are some of the substances that aid in inhibition and production of an antagonistic environment against pathogens [20,38,39,40]. Although probiotic strains have a variety of mechanisms to inhibit colonisation of pathogens, these abilities are strain-specific.
There is a plethora of studies that have already characterised the efficacy of single and mixtures of probiotic strains and the benefits they confer on human health, and their use as preventative or therapeutic options [41,42,43]. However, due to the heterogeneity of probiotics in these studies, there is now a move towards combinations of probiotic strains to determine if they are of a greater advantage [27,44,45]. Early research has shown that when in combination, it is possible that a higher efficacy of pathogen inhibition can occur [26,38,46], and that their greater activity is due to a synergistic effect created by the combination of strains [27,44,45,47,48]. Clinically, a higher inhibition of pathogens creates a wider range of health benefits, with some studies already showing greater probiotic adhesion rates, and is linked to alleviation of gastrointestinal disorder symptoms and increased intestinal regularity [45,49,50], although these results may be strains specific. In this study we aimed to test the efficacy of four previously characterised Lactobacillus strains with probiotic activities [51] and their combination to inhibit interaction of an E. coli strain F44A-1 with all AIEC characteristics [52], using a co-culture of Caco-2 and HT29-MTX cell lines that closely resemble the GI mucosal membrane.
2. Materials and Methods
2.1. Probiotic Strains
The four probiotic strains selected for this study were Lactobacillus brevis M4-205, L. reuteri M4-100, L. rhamnosus M4-195, and L. plantarum M4-165. The isolates were collected from faecal samples of healthy individuals aged between 2 and 40 years old [51] and identified to the species with 16S rRNA as described before [53].
Stock cultures were maintained at −80 °C in de Man, Rogosa, and Sharpe (MRS) broth (Oxoid, Thebarton, Australia) with 20% glycerol and the working cultures were cultured anaerobically in MRS broth or MRS agar plates for 48 h at 37 °C. To ensure strains were pure before each assay, a single colony was selected and inoculated into fresh MRS broth and grown anaerobically. The strain suspensions were centrifuged at 3500 rpm for 5 min and resuspended in phosphate-buffered saline (PBS; pH = 7.00). The concentration of each probiotic strain was adjusted to an optical density of 1.0 at OD600nm (approx. 109 CFU/mL) and diluted before the inoculation of wells to 107 CFU/mL. For the combination, equal concentration of each Lactobacillus strains (107 CFU/mL) was added to a tube, thoroughly mixed, and inoculated into the wells in each assay.
2.2. E. coli Strains
A wild type E. coli strain (F44A-1) initially isolated from a patient with colorectal cancer was used as a challenging strain. This strain was characterised for the presence of all virulence genes involved in the pathogenesis of AIEC strains, specifically their diffuse adhesion pattern to Caco-2 cells, as well as their intramacrophage survival and replication [52,54]. As a negative control for adhesion and invasion assays, we used the K12 E. coli strain 46-4 [55], and for translocation experiments, we used a highly translocating E. coli strain HMLN-1, as a positive control and a non-translocating E. coli strain JM109 [55,56,57]. Stock cultures of the strains were maintained in tryptone soy broth (TSB, Oxoid) with 20% glycerol at −80 °C, before being grown in Luria-Bertoni (LB) broth (Millipore, Burlington, MA, USA) in a reciprocal shaker (138 strokes/min−1) at 37 °C for 24 h before each assay. The bacterial suspensions were centrifuged at 3500 rpm for 5 min and resuspended in PBS (pH = 7.00) and the cultures adjusted to an optical density of 1.0 at OD600nm (approx. 109 CFU/mL) and diluted before the inoculation of corresponding wells to 107 CFU/mL. The multiplicity of infection (MOI) was calculated at 100 CFU/cell, and this was achieved by dividing the number of bacteria inoculated in each well per number of cells. This MOI was used in all infection experiments described hereafter.
2.3. Co-Culture of Cells
A co-culture of two human colon adenocarcinoma cells, Caco-2 (ATCC = HTB-37) and HT29-MTX cells. The HT29-MTX cell line is a mucus producing cell lone which was differentiated from HT-29 cells (ATCC = HTB38) into mature goblet cells using methotrexate (MTX) that produce mucin (HT29-MTX). These cell lines express multiple structural and physiological features of the GI tract epithelial layer, including dense tight junctions (TJ), microvilli, and enzymatic activity. However, Caco-2 cells do not produce mucus, an essential feature of the epithelial and mucosal layers of the GI tract. In contrast, HT29-MTX cells can differentiate into mature goblet cells following methotrexate (MTX) treatment [58,59]. Initially, each cell line was grown individually in Eagles Minimum Essential Media (EMEM, Gibco, Thermo Fisher Scientific, Waltham, MA, USA), with Caco-2 cell media supplemented with 20% foetal bovine serum (FBS, Gibco) and HT29-MTX cell media with 15% FBS for growth, and 1% penicillin-streptomycin for both. The cells were incubated at 37 °C in a 5% CO2 environment, with the medium replaced every 48 h. Once confluent, each cell line was dissociated from the culture flask and their concentration adjusted to the 9:1 ratio (Caco-2:HT29-MTX) for inoculation into wells for each assay.
2.4. Adhesion Assay
The adhesion of AIEC in the presence and absence of four probiotics and their combination was tested in 8-well chamber slides (Nunc Lab-Tek II, Thermo Fisher Scientific, Waltham, MA, USA) using the method adapted from Smit et al. [54]. Co-culture cells were grown to >70% confluence, washed three times with sterile PBS and the media replaced with 200 μL of antibiotic-free EMEM. In the co-inoculation assay, 100 μL of the AIEC strain and each of the probiotic strains alone and in combination were inoculated into the wells (competitive adhesion assay) at a final concentration of 107 CFU/mL. Chamber slides were then incubated for 90 min at 37°C in a 5% CO2 environment. In the pre-inoculation assay, all probiotic strains alone and in combination were initially inoculated into wells and incubated for 90 min at 37°C in a 5% CO2 environment. Following initial probiotic incubation, 100 μL aliquots of AIEC were added to each well of the chamber slides containing probiotic strains and incubated for a further 90 min. All wells were washed three times with PBS to remove non-adhering bacteria and cells were fixed with 95% ethanol for 5 min, and Gram-stained to differentiate between Lactobacillus (Gram-positive) and AIEC (Gram-negative) strains. Slides were dried and observed under a light microscope. The ability of the AIEC and the probiotic strains to colonise the co-culture was measured by counting the number of cells showing adhesion among 100 randomly selected cells, whilst the number of adhering bacteria per cell was determined by counting the number of bacteria attached to 25 randomly selected cells showing adhesion. All tests were completed in triplicate, and all results are expressed as mean ± SEM.
2.5. Invasion Assay
Invasion of the AIEC alone and in the presence of four probiotics and their combination was tested as described previously [60]. The co-cultured cells are grown in a flat-bottom 96-well plate to confluence over 4–5 days. Before inoculating the bacterial strains, the media was replaced with antibiotic-free media and the wells were inoculated either with AIEC alone, or AIEC with probiotic strains. Co-inoculation and pre-inoculation protocols were the same as described for the adhesion assays. Following incubation, the medium was removed, and cells washed three times with PBS and wells inoculated with 200 µL of EMEM containing gentamycin (150 μg/mL) and the plate was incubated for a further 60 min to kill any extracellular bacteria. The medium was then removed, and cells lysed with 0.1% Triton-X-100 for 15 min to release invading bacteria. The lysed suspension was then serially diluted and plated on MacConkey agar No. 3 plates (Oxoid) and incubated overnight and the number of colonies counted. All tests were done in triplicate and the results recorded as mean ± SEM of invading bacteria (CFU) per well.
2.6. Translocation Assay
Translocation of the AIEC alone and in the presence of four probiotics and their combination were tested as described previously by Smit et al. [54] with some modifications. The co-culture of Caco-2:HT29-MTX cells was grown on cell inserts (pore size = 8 µm) (Millipore) placed into 12 well plates and incubated under 5% CO2 at 37 °C. The co-culture was grown until a fully confluent monolayer was achieved. The cell layer integrity was determined by monitoring the trans-epithelial electrical resistance (TEER) value (Ω) until it began to plateau, between 14–21 days of growth. Cell-inserts and wells are washed three times with PBS and 200 μL of antibiotic-free EMEM was added in the inserts and 600 μL in the outer wells. Like the adhesion and invasion assays, co-inoculation and pre-inoculation testing took take place, with 100 μL of the probiotic candidates added to the inserts 90 min prior to adding 100 μL of AIEC in pre-inoculation, and simultaneously in co-inoculation experiments. After 30 min of incubation, 100 µL of medium in the outer well was serially diluted and inoculated overnight at 37 °C onto MacConkey agar No. 3 plates. At the same time 100 μL of antibiotic-free EMEM was added to the outer well to replace the sampled medium and the wells were incubated again for a further 90 min for a total of 120 min of incubation after which the number of translocated E. coli was counted in 100 μL of the medium from the outer well. All tests were done in triplicate, with the plates to be incubated for 24 h and counted for CFU. Results were expressed as mean ± SEM.
2.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism statistical software (Version 9.0.0). Two-way ANOVA analysis was followed by Tukey’s multiple comparisons testing to determine differences in the number of adhering, invading, and translocating bacteria amongst all treatment groups and controls. Relationships between adhesion, invasion, and translocation was evaluated using Pearson’s correlation coefficient. Differences are considered statistically significant if p < 0.05.
3. Results
In general, both AIEC and probiotic strains individually colonized the co-culture at a similar level with some variations among the probiotic strains that ranged from 50% to 70%. In the presence of probiotics, there was a significant reduction in the percentage of cells colonized when the wells were pre-inoculated with probiotics. For the co-inoculation studies, strain M4-205 and the combination of the four strains had the greater effect; however, the most effective strain was M4-195 when pre-inoculated, resulting in a reduction in AIEC of 14% (Figure 1a). This made M4-195 the most successful candidate overall in reducing AIEC colonisation in both co- and pre-inoculation assays (Table 1). The number of AIEC adhering to individual co-culture cells was also reduced in the presence of all probiotics, in both pre- and co-inoculation assays. Pre-inoculation of the probiotic strains resulted in a significantly greater reduction in the number of AIEC adhering individual co-culture cells compared to co-inoculation of the same strain (Figure 1b). In the co-inoculation studies, the combination of strains was able to competitively exclude the AIEC better than the individual strains, with a 34% reduction (Table 1). In all, the highest inhibition of the AIEC was seen with strains M4-100, M4-205 and M4-195 in pre-inoculation experiments (Figure 1b).
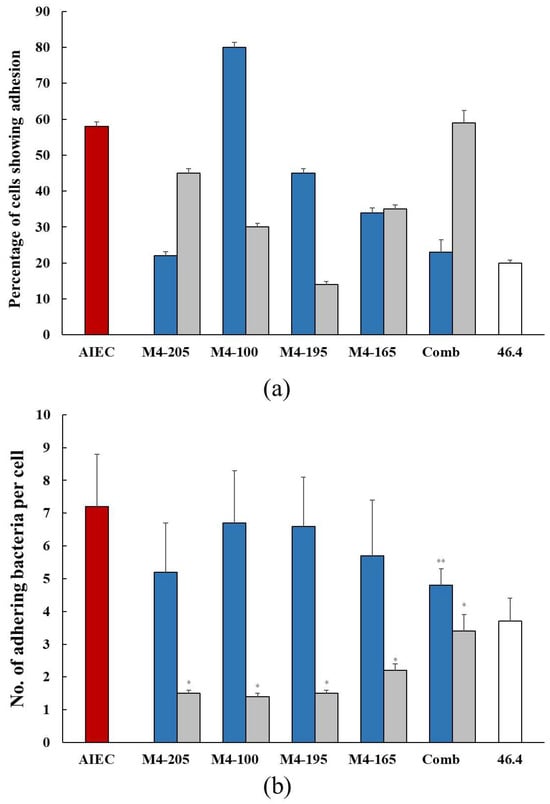
Figure 1.
(a) The percentage (mean ± SEM) colonization of the co-culture of Caco-2:HT29-MTX cells by the AIEC alone  and in the presence of the Lactobacillus strains when co-inoculated
and in the presence of the Lactobacillus strains when co-inoculated  and pre-inoculated
and pre-inoculated  . (b) The number (mean ± SEM) of adhering AIEC alone
. (b) The number (mean ± SEM) of adhering AIEC alone  and in the presence of the Lactobacillus strains when co-inoculated
and in the presence of the Lactobacillus strains when co-inoculated  and pre-inoculated
and pre-inoculated  . E. coli strain 46.4 used as negative control
. E. coli strain 46.4 used as negative control  . * p < 0.0001 co-inoculation versus pre-inoculation ** p < 0.0001 combination versus individual strain.
. * p < 0.0001 co-inoculation versus pre-inoculation ** p < 0.0001 combination versus individual strain.
 and in the presence of the Lactobacillus strains when co-inoculated
and in the presence of the Lactobacillus strains when co-inoculated  and pre-inoculated
and pre-inoculated  . (b) The number (mean ± SEM) of adhering AIEC alone
. (b) The number (mean ± SEM) of adhering AIEC alone  and in the presence of the Lactobacillus strains when co-inoculated
and in the presence of the Lactobacillus strains when co-inoculated  and pre-inoculated
and pre-inoculated  . E. coli strain 46.4 used as negative control
. E. coli strain 46.4 used as negative control  . * p < 0.0001 co-inoculation versus pre-inoculation ** p < 0.0001 combination versus individual strain.
. * p < 0.0001 co-inoculation versus pre-inoculation ** p < 0.0001 combination versus individual strain.

Table 1.
Percentage of reduction in colonising AIEC bacteria, and adhesion and invasion of AIEC strain, in the presence of individual probiotic strains and their combination.
The addition of probiotic strains greatly reduced the invasion of the AIEC strain. Reduction of invading bacteria (per well) varied little between the probiotics, with all inhibiting AIEC invasion in both co- and pre-inoculation studies (Figure 2). Pre-inoculation of the candidates resulted in greater inhibition of AIEC invasion, and overall, strain M4-205 had the best combined efficacy overall, followed by the combination of strains (Table 1).
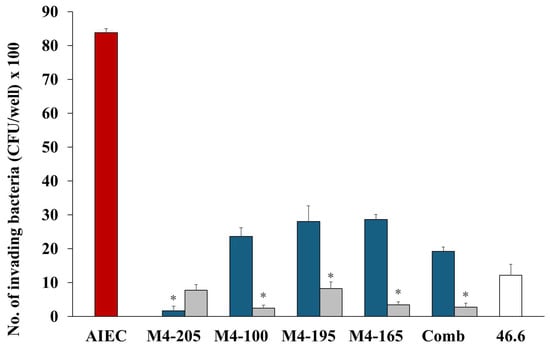
Figure 2.
The number (mean ± SEM) of invading AIEC (CFU) alone  and in the presence of Lactobacillus strains when co-inoculated
and in the presence of Lactobacillus strains when co-inoculated  and pre-inoculated
and pre-inoculated  . E. coli strain 46.4 used as negative control
. E. coli strain 46.4 used as negative control  . * p < 0.0001 co-inoculation versus pre-inoculation.
. * p < 0.0001 co-inoculation versus pre-inoculation.
 and in the presence of Lactobacillus strains when co-inoculated
and in the presence of Lactobacillus strains when co-inoculated  and pre-inoculated
and pre-inoculated  . E. coli strain 46.4 used as negative control
. E. coli strain 46.4 used as negative control  . * p < 0.0001 co-inoculation versus pre-inoculation.
. * p < 0.0001 co-inoculation versus pre-inoculation.
The translocation of AIEC strains over 120 min was similar to that of the positive control, HMLN-1 strain, which has been shown in our previous studies to be an efficient translocating strain [57]. In all instances, the presence of probiotics reduced the translocation of the AIEC strain; there were no significant differences between the probiotics or their interaction times (Figure 3). Co-inoculation of the AIEC and the candidates showed that Lactobacillus strain M4-205 was the most successful in its inhibition of the AIEC, with nearly complete inhibition of AIEC translocation after 30 min and an 88% reduction after 120 min (see Figure 3a,b). However, all candidates demonstrated significant efficacies against the pathogen, including the combination of strains. Pre-inoculation testing was the more successful method, with consistent reductions in AIEC translocation occurring, and the combination demonstrating the highest inhibitions, apart from Lactobacillus strain M4-100, with a 95% reduction after 120 min interaction with the AIEC (see Figure 3c,d).
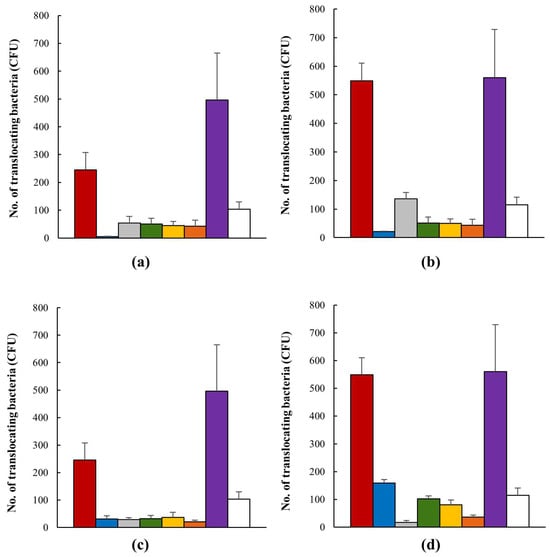
Figure 3.
The number (mean ± SEM) of translocating AIEC (CFU) alone  , when in the presence of Lactobacillus strains M4-205
, when in the presence of Lactobacillus strains M4-205  , M4-100
, M4-100  , M4-195
, M4-195  , M4-165
, M4-165  , and their combination
, and their combination  . E. coli strain HMLN1 was used a positive control
. E. coli strain HMLN1 was used a positive control  and E. coli strain JM109 as a negative control
and E. coli strain JM109 as a negative control  . (a) Co-inoculation of AIEC and probiotic candidates after 30 min interaction; (b) co-inoculation of AIEC and probiotic candidates after 120 min interaction; (c) pre-inoculation of AIEC and probiotic candidates after 30 min interaction; (d) pre-inoculation of AIEC and probiotic candidates after 120 min interaction.
. (a) Co-inoculation of AIEC and probiotic candidates after 30 min interaction; (b) co-inoculation of AIEC and probiotic candidates after 120 min interaction; (c) pre-inoculation of AIEC and probiotic candidates after 30 min interaction; (d) pre-inoculation of AIEC and probiotic candidates after 120 min interaction.
 , when in the presence of Lactobacillus strains M4-205
, when in the presence of Lactobacillus strains M4-205  , M4-100
, M4-100  , M4-195
, M4-195  , M4-165
, M4-165  , and their combination
, and their combination  . E. coli strain HMLN1 was used a positive control
. E. coli strain HMLN1 was used a positive control  and E. coli strain JM109 as a negative control
and E. coli strain JM109 as a negative control  . (a) Co-inoculation of AIEC and probiotic candidates after 30 min interaction; (b) co-inoculation of AIEC and probiotic candidates after 120 min interaction; (c) pre-inoculation of AIEC and probiotic candidates after 30 min interaction; (d) pre-inoculation of AIEC and probiotic candidates after 120 min interaction.
. (a) Co-inoculation of AIEC and probiotic candidates after 30 min interaction; (b) co-inoculation of AIEC and probiotic candidates after 120 min interaction; (c) pre-inoculation of AIEC and probiotic candidates after 30 min interaction; (d) pre-inoculation of AIEC and probiotic candidates after 120 min interaction.
4. Discussion
It is generally accepted that probiotics are beneficial bacteria, competing with pathogens for binding sites and resources, essentially inhibiting them from colonising the gut epithelium [17,20]. Previous studies have shown probiotic strains as both a valuable prevention strategy and a therapeutic treatment option, reducing bacterial interaction with the gut epithelium [54,61,62]. Whilst many studies use a single cell line such as Caco-2 or HT-29 to assess the efficacy of probiotic strains in vitro, the lack of mucin production in these cell lines renders them suboptimal as a representation of the cell model of the GI tract when measuring bacterial colonisation [63]. In this study, we used a co-culture of the Caco-2 cells and HT29-MTX cells, as this cell model is a closer representation of the gut epithelium. This allowed for a much better environment for evaluating probiotic abilities, particularly for determining how well they can competitively bind to receptor sites and exclude pathogenic bacteria from colonisation in the presence of mucin [20,64]. The four Lactobacillus strains used in this study have previously been shown to meet all selection criteria for potential probiotics in previous studies and are highly adherent to this cell model [51,65]. The AIEC strain F44A-1 used in this study has been shown to possess all virulence characteristics associated with AIEC, including survival and replication within macrophages [52]. Here, we demonstrated that this strain is highly adherent and invasive; and was able to translocate across the co-culture cell lines, making it an effective representative of AIEC pathotype.
In this study, the probiotic strains were able to colonise the co-culture of cells at a similar level to the AIEC strain, although the level of adhesion varied amongst the Lactobacillus strains. Individually, the probiotic strains were able to reduce the AIEC adherence to the co-culture cells, demonstrating their competitive capabilities. However, combining the strains did not significantly improve the efficacy of the probiotics. L. rhamnoses M4-195 and L. brevis M4-205 were the most efficient at reducing AIEC colonisation and AIEC adherence per cell, respectively. This is consistent with findings from a systematic review which found that single strains were, in general, as effective as multi-strain combinations [43]. Furthermore, there appears to be no relationships between the probiotic strains’ ability to colonise the cells nor their adhering numbers per cell with their inhibitory effects on the pathogen. These findings suggest that the competitiveness of probiotic strains against the pathogen may not be entirely dependent on direct competition with binding sites, with some factors such as their secretion of antimicrobial substances or their ability to regulate the immune response [40,53,66,67] being involved in the efficacy of individual strain. Van den Abbeele et al. have previously shown that over a 24 h period Lactobacillus strains could not inhibit an AIEC strain’s adhesion to mucus but did affect the growth and survival of the pathogen [68]. This suggests that the reduction in adhesion observed in this study and others [69,70] occurs at the epithelial layer, not in the mucus compartment.
The results of the invasion assay demonstrated that despite the highly invasive capability of the AIEC strain, all the probiotics strains were able to significantly reduce the invasion of the AIEC strain with L. brevis M4-205 being the most successful strain followed by the combination of strains. This links well with the findings from the adhesion assays where the L. brevis M4-205 was the most successful inhibitor of AIEC adhesion. This strain has also been shown to be very effective in inhibiting adhesion of other enteric pathogens to cell lines representing the intestinal epithelium [53]. However, we have previously shown that there was no correlation between the F44A-1 AIEC adhesion and invasion of the co-culture cell model, and that this also did not correlate with the adhesion capabilities of live biotherapeutics products tested in that study [54]. For translocation assays we used E. coli strain HMLN-1, a highly efficient translocating strain as shown before [56,57,71], as a positive control. The L. brevis M4-205 was again the most effective strain in reducing translocation of AIEC F44A-1 strain, but only in co-inoculation experiments as opposed to pre-inoculation experiments. Despite this, all probiotics inhibited or dramatically reduced AIEC translocation. Translocation across intestinal epithelial cells is a key component of AIEC pathogenesis [72,73], so we propose that when investigating the inhibitory effects of probiotics their effect on translocation of pathogens as well as their adhesion and invasion should also be examined.
Nonetheless, the use of co-inoculation and pre-inoculation assays allowed us to assess the ability of probiotics, alone or in combination, for their competitive and prophylactic abilities against the interaction of the AIEC strain with the gut epithelium. Indeed, pre-inoculation of the co-culture with the probiotic strains generally reduced AIEC interaction with the co-culture more than when the AIEC and probiotic strains were co-inoculated. The higher levels of reduction obtained during pre-inoculation assays indicate that probiotics could be better used as a prophylactic measure, suggestive of them having a greater efficacy to interact with the gut epithelium and bind to mucosal layer prior to invasion of pathogens. The effect of pre-inoculation having a greater inhibitory effect compared to co-inoculation has also been reported in other strains [74] and may suggest that probiotic metabolites have or the early interaction with the co-culture cells have a role in the inhibitory action.
The probiotic strains used in this study had previously demonstrated their heterogeneity and varying efficacy to inhibit pathogens, with each strain expressing differing characteristics [53,75,76,77]. These studies also suggest the use of a combination of strains to fill in the gaps for a more widespread efficacy against pathogenic bacteria [51,65]. Other studies also suggest that combinations of probiotic strains, can have a greater colonisation ability and higher levels of inhibition of pathogens compared to using the same strains individually [35,50,78,79]. In vivo studies have also found that combinations of probiotic strains can alleviate physical symptoms, such as diarrhoea in patients suffering from GI disorders [44]. However, these studies either used single cell lines to represent the gut epithelium when assessing the efficacy of their strains or used laboratory animals to mimic to assess the therapeutic abilities of their probiotic strains [80]. The co-culture cell lines, i.e., Caco-2:HT29-MTX that we used in this study has been postulated to reflect the cellular components of the gut environment more effectively than the monoculture [81], such as Caco-2 cells or HT-29 cells that are void of mucin. This improved in vitro model for testing interaction of pathogens with the gut epithelium provided a robust approach to investigate the impact of probiotics against pathogens such as AIEC F44A-1 as also shown in other studies [54].
Using this co-culture of cell lines, we evaluated the ability of four known probiotic strains alone and in combination to work synergistically to compete with invading pathogens for colonisation of the gut epithelium. However, our results suggest that the combination of the strains, although had a better efficacy during the translocation assay, they did not significantly increase the efficacy of the individual probiotic strains to inhibit or reduce interaction of the AIEC strain with the gut epithelium. These data suggest that the combination of probiotics may work differently in different cell lines and/or against different pathogens. We also postulate the differences in our results with those reported for combination of probiotic might also be due to the differences in the ability of probiotics in combination to compete for receptor sites or in production of antimicrobial substances. From a clinical point of view the use of these strains alone or in combination can alleviate the sign and symptoms of the disease in patients with ulcerative colitis. Although at this stage there are no direct clinical applications from this study, further strain characterisation and in vivo experiments are required to assess the clinical application of these probiotics. In this study we combined all four probiotic strains, which may have resulted in probiotic-to-probiotic competition and thereby not resulted in an increased inhibitory action against AIEC. A future direction for this project is to genetically characterise the probiotic strain and formulate combinations based on the knowledge of the presence of different characteristics (e.g., bacteriocins), etc. Despite this, in our study, we found a greater reduction in adhesion, invasion, and translocation of AIEC when the mixture of probiotics was pre-inoculated. These data were consistent in our previous pre-inoculation studies [54,82], suggesting that probiotics as an individual or combined can be more effective when used as a prophylactic measure. Further strain characterisation is needed to determine a proper combination of strains, as different combinations of strains can produce different results [26,83].
5. Conclusions
In conclusion, we demonstrated that the four probiotic strains effectively reduced the interaction of the AIEC strain in a co-culture of Caco-2:HT29-MTX cells, with this effect more pronounced when the strains were pre-inoculated onto the cells. This suggests that probiotic strains are more effective when they are used prophylactically. This study also found that, there was no significant difference in the reduction in pathogen interaction when the combination of the strains was used, apart from during translocation. Different levels of inhibition were observed amongst the probiotic strains, with strain L. brevis M4-205 showing the greatest reductions against the AIEC strain in both the co- and pre-inoculation studies. Further characterisation of these strains can help towards formulating a more powerful combination of the strains to be used as a treatment and prophylactics option.
Author Contributions
Conceptualization, M.K., E.H. and A.K.; methodology, formal analysis and investigation, G.B., B.A. and B.S.; data curation, M.K. and G.B.; writing—original draft preparation, G.B.; writing—review and editing, G.B., B.A., B.S., E.H., A.K. and M.K.; visualization, G.B.; supervision, E.H., A.K. and M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Training Program (RTP) scholarship provided to Georgia Bradford by the University of the Sunshine Coast and the Australian Commonwealth Government.
Data Availability Statement
Data available upon request.
Acknowledgments
We thank the University of the Sunshine Coast and the School of Science, Technology and Engineering for their support during the completion of this study.
Conflicts of Interest
Author Anna Kuballa was employed by the company Servatus Biopharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Guzman, J.R.; Conlin, V.S.; Jobin, C. Diet, microbiome, and the intestinal epithelium: An essential triumvirate? BioMed Res. Int. 2013, 2013, 425146. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Chervy, M.; Barnich, N.; Denizot, J. Adherent-invasive E. coli: Update on the lifestyle of a troublemaker in Crohn’s disease. Int. J. Mol. Sci. 2020, 21, 3734. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Gen. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Darfeuillemichaud, A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1720–1728.e3. [Google Scholar] [CrossRef] [PubMed]
- Palmela, C.; Chevarin, C.; Xu, Z.; Torres, J.; Sevrin, G.; Hirten, R.; Barnich, N.; Ng, S.C.; Colombel, J.F. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 2018, 67, 574–587. [Google Scholar] [CrossRef]
- Conte, M.P.; Longhi, C.; Marazzato, M.; Conte, A.L.; Aleandri, M.; Lepanto, M.S.; Zagaglia, C.; Nicoletti, M.; Aloi, M.; Totino, V.; et al. Adherent-invasive Escherichia coli (AIEC) in pediatric Crohn’s disease patients: Phenotypic and genetic pathogenic features. BMC Res. Notes 2014, 7, 748. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, Y.; Ma, Y.; Gong, W.; Zhu, G. The role of major virulence factors of AIEC involved in inflammatory bowl disease—A mini-review. Appl. Microbiol. Biotechnol. 2017, 101, 7781–7787. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiocchi, C. Etiopathogenesis of inflammatory bowel diseases. World J. Gastroenterol. 2006, 12, 4807–4812. [Google Scholar] [CrossRef] [PubMed]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.L.; Barnich, N.; Bringer, M.A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Aldeguer, X.; Lopez-Siles, M.; González-Huix, F.; López-Oliu, C.; Dahbi, G.; Bianco, J.E.; Blanco, J.; Garcia-Gil, L.J.; Darfeuille-Michaud, A. Molecular diversity of Escherichia coli in the human gut: New ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Sharma, V.; Elmén, L.; Peterson, S.N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 2015, 179, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Hertzberger, R.Y.; Knaus, U.G. Hydrogen peroxide production by lactobacilli promotes epithelial restitution during colitis. Redox Biol. 2018, 16, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.L.; Farias, A.Q.; Rezaie, A. Gastrointestinal motility and absorptive disorders in patients with inflammatory bowel diseases: Prevalence, diagnosis and treatment. World J. Gastroenterol. 2019, 25, 4414–4426. [Google Scholar] [CrossRef]
- McLean, L.P.; Cross, R.K. Integrin antagonists as potential therapeutic options for the treatment of Crohns disease. Expert Opin. Investig. Drugs 2016, 25, 263–273. [Google Scholar] [CrossRef]
- Varankovich, N.V.; Nickerson, M.T.; Korber, D.R. Probiotic-based strategies for therapeutic and prophylactic use against multiple gastrointestinal diseases. Front. Microbiol. 2015, 6, 685. [Google Scholar] [CrossRef]
- Nami, Y.; Haghshenas, B.; Abdullah, N.; Barzegari, A.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Probiotics or antibiotics: Future challenges in medicine. J. Med. Microbiol. 2015, 64, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-term antibiotic treatment has differing long- term impacts on the human throat and gut microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef] [PubMed]
- Hotel, A. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- McFarland, L.V. From Yaks to Yogurt: The History, Development, and Current Use of Probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Adriana, N.; Ilona, M.; Katarzyna, Ś.; Zdzisława, L.; Elżbieta, K. Adherence of probiotic bacteria to human colon epithelial cells and inhibitory effect against enteric pathogens—In vitro study. Int. J. Dairy. Technol. 2016, 69, 532–539. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. In vitro analysis of probiotic strain combinations to inhibit pathogen adhesion to human intestinal mucus. Food Res. Int. 2007, 40, 629–636. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Development of new probiotics by strain combinations: Is it possible to improve the adhesion to intestinal mucus? J. Dairy. Sci. 2007, 90, 2710–2716. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: Probiotics and their potential as antimicrobials. Expert. Rev. Anti-Infect. Ther. 2019, 17, 635–645. [Google Scholar] [CrossRef]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Muñoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013, 109 (Suppl. S2), S35–S50. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Laksemi, D.A.A.S.; Ketut Tunas, I.; Diarthini, N.L.P.E. A brief review of probiotic: Action mechanism, benefits, and clinical application on human health. J. Glob. Pharma Technol. 2018, 10, 19–29. [Google Scholar]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Tuomola, E.; Crittenden, R.; Playne, M.; Isolauri, E.; Salminen, S. Quality assurance criteria for probiotic bacteria. Am. J. Clin. Nutr. 2001, 73 (Suppl. S2), 393S–398S. [Google Scholar] [CrossRef] [PubMed]
- Piatek, J.; Gibas-Dorna, M.; Olejnik, A.; Krauss, H.; Wierzbicki, K.; Zukiewicz-Sobczak, W.; Głowacki, M. The viability and intestinal epithelial cell adhesion of probiotic strain combination—In vitro study. Ann. Agric. Environ. Med. 2012, 19, 99–102. [Google Scholar]
- Shewale, R.N.; Sawale, P.D.; Khedkar, C.D.; Singh, A. Selection criteria for probiotics: A review. Int. J. Probiotics Prebiotics 2014, 9, 17–22. [Google Scholar]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett. Appl. Microbiol. 2007, 45, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Nybom, S.; Meriluoto, J.; Collado, M.C.; Vesterlund, S.; El-Nezami, H. Interaction of probiotics and pathogens-benefits to human health? Curr. Opin. Biotechnol. 2010, 21, 157–167. [Google Scholar] [CrossRef]
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.V.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef]
- Roy, D. Probiotics. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 649–661. [Google Scholar]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Arena, M.P.; Capozzi, V.; Russo, P.; Drider, D.; Spano, G.; Fiocco, D. Immunobiosis and probiosis: Antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl. Microbiol. Biotechnol. 2018, 102, 9949–9958. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Efficacy of Single-Strain Probiotics Versus Multi-Strain Mixtures: Systematic Review of Strain and Disease Specificity. Dig. Dis. Sci. 2021, 66, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Kajander, K.; Korpela, R. Clinical studies on alleviating the symptoms of irritable bowel syndrome with a probiotic combination. Asia Pac. J. Clin. Nutr. 2006, 15, 576–580. [Google Scholar] [PubMed]
- Wang, Y.; Xie, Q.; Zhang, Y.; Ma, W.; Ning, K.; Xiang, J.Y.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020, 104, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; De Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef]
- Soni, R.; Jain, N.K.; Shah, V.; Soni, J.; Suthar, D.; Gohel, P. Development of probiotic yogurt: Effect of strain combination on nutritional, rheological, organoleptic and probiotic properties. J. Food Sci. Technol. 2020, 57, 2038–2050. [Google Scholar] [CrossRef]
- Chapman, C.M.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Verdenelli, M.C.; Silvi, S.; Cecchini, C.; Orpianesi, C.; Cresci, A. Influence of a combination of two potential probiotic strains, Lactobacillus rhamnosus IMC 501® and Lactobacillus paracasei IMC 502® on bowel habits of healthy adults. Lett. Appl. Microbiol. 2011, 52, 596–602. [Google Scholar] [CrossRef]
- Collado, M.C.; Grześkowiak, Ł.; Salminen, S. Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr. Microbiol. 2007, 55, 260–265. [Google Scholar] [CrossRef]
- Rohani, M.; Noohi, N.; Talebi, M.; Katouli, M.; Pourshafie, M.R. Highly heterogeneous probiotic Lactobacillus species in healthy iranians with low functional activities. PLoS ONE 2015, 10, e0144467. [Google Scholar] [CrossRef]
- Astley, D.J.; Masters, N.; Kuballa, A.; Katouli, M. Commonality of adherent-invasive Escherichia coli isolated from patients with extraintestinal infections, healthy individuals and the environment. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Bibalan, M.H.; Eshaghi, M.; Rohani, M.; Esghaei, M.; Darban-Sarokhalil, D.; Pourshafie, M.R.; Talebi, M. Isolates of Lactobacillus plantarum and L. Reuteri display greater antiproliferative and antipathogenic activity than other Lactobacillus isolates. J. Med. Microbiol. 2017, 66, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Smit, B.; Chinaka, C.C.; Scott, A.A.; Gaiduschek, K.; Hatje, E.; Kuballa, A.; Coulson, S.; Finlayson, W.; Katouli, M. Efficacy of Selected Live Biotherapeutic Candidates to Inhibit the Interaction of an Adhesive-Invasive Escherichia coli Strain with Caco-2, HT29-MTX Cells and Their Co-Culture. Biomedicines 2022, 10, 2245. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, N.L.; Katouli, M.; Polkinghorne, A. Genomic comparison of translocating and non-translocating Escherichia coli. PLoS ONE 2015, 10, e0137131. [Google Scholar] [CrossRef] [PubMed]
- Nettelbladt, C.G.; Katouli, M.; Bark, T.; Svenberg, T.; Möllby, R.; Ljungqvist, O. Evidence of bacterial translocation in fatal hemorrhagic pancreatitis. J. Trauma. 2000, 48, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Ramos, N.L.; Lamprokostopoulou, A.; Chapman, T.A.; Chin, J.C.; Römling, U.; Brauner, A.; Katouli, M. Virulence characteristics of translocating Escherichia coli and the interleukin-8 response to infection. Microb. Pathog. 2011, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mahler, G.J.; Shuler, M.L.; Glahn, R.P. Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J. Nutr. Biochem. 2009, 20, 494–502. [Google Scholar] [CrossRef]
- Wan, L.Y.M.; Allen, K.J.; Turner, P.C.; El-nezami, H. Modulation of mucin mRNA (MUC5AC AND MUC5B) expression and protein production and secretion in Caco-2/HT29-MTX Co-cultures following exposure to individual and combined Fusarium mycotoxins. Toxicol. Sci. 2014, 139, 83–98. [Google Scholar] [CrossRef]
- Duncan, M.J.; Li, G.; Shin, J.S.; Carson, J.L.; Abraham, S.N. Bacterial Penetration of Bladder Epithelium through Lipid Rafts. J. Biol. Chem. 2004, 279, 18944–18951. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Noshad, M.; Falah, F. Inhibition of Escherichia coli adhesion to human intestinal Caco-2 cells by probiotic candidate Lactobacillus plantarum strain L15. Microb. Pathog. 2019, 136, 103677. [Google Scholar] [CrossRef]
- Gagnon, M.; Zihler Berner, A.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J. Microbiol. Methods 2013, 94, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, H.U.; Olesen, S.V.; Prehn, K.; Lahtinen, S.J.; Brix, S.; Abou Hachem, M.; Svensson, B. Mucin- and carbohydrate-stimulated adhesion and subproteome changes of the probiotic bacterium Lactobacillus acidophilus NCFM. J. Proteom. 2017, 163, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Morrin, S.T.; Hickey, R.M. New insights on the colonization of the human gut by health-promoting bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Reale, O.; Huguet, A.; Fessard, V. Co-culture model of Caco-2/HT29-MTX cells: A promising tool for investigation of phycotoxins toxicity on the intestinal barrier. Chemosphere 2020, 273, 128497. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Caetano M Antunes, L.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.; Bankole, O.; Olaitan, O. Production and characterization of antimicrobial agents by Lactic Acid Bacteria Isolated from Fermented Foods. Internet J. Microbiol. 2008, 4, 1–7. [Google Scholar]
- Van den Abbeele, P.; Marzorati, M.; Derde, M.; De Weirdt, R.; Joan, V.; Possemiers, S.; Van de Wiele, T. Arabinoxylans, inulin and Lactobacillus reuteri 1063 repress the adherent-invasive Escherichia coli from mucus in a mucosa-comprising gut model. NPJ Biofilms Microbiomes 2016, 2, 16016. [Google Scholar] [CrossRef]
- Bernet, M.F.; Brassart, D.; Neeser, J.R.; Servin, A.L. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 1994, 35, 483–489. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Prete, R.; Battista, N.; Corsetti, A. Adhesion Properties of Food-Associated Lactobacillus plantarum Strains on Human Intestinal Epithelial Cells and Modulation of IL-8 Release. Front. Microbiol. 2018, 9, 2392. [Google Scholar] [CrossRef]
- Katouli, M.; Ramos, N.L.; Nettelbladt, C.G.; Ljungdahl, M.; Robinson, W.; Ison, H.M.; Brauner, A.; Möllby, R. Host species-specific translocation of Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1095–1103. [Google Scholar] [CrossRef]
- Wine, E.; Ossa, J.C.; Gray-Owen, S.D.; Sherman, P.M. Adherent-invasive Escherichia coli target the epithelial barrier. Gut Microbes 2010, 1, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Massier, S.; Darfeuille-Michaud, A.; Billard, E.; Barnich, N. Understanding Host-Adherent-Invasive Escherichia coli Interaction in Crohn’s Disease: Opening Up New Therapeutic Strategies. BioMed Res. Int. 2014, 2014, 567929. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, I.; Leplingard, A.; Darfeuille-Michaud, A. Lactobacillus casei DN-114 001 Inhibits the Ability of Adherent-Invasive Escherichia coli Isolated from Crohn’s Disease Patients To Adhere to and To Invade Intestinal Epithelial Cells. Appl. Environ. Microbiol. 2005, 71, 2880–2887. [Google Scholar] [CrossRef]
- Ghanavati, R.; Mohammadi, F.; Javadi, A.; Shahosseini, Z.; Talebi, M.; Rohani, M. The effects of a mixture of Lactobacillus species on colorectal tumor cells activity through modulation of Hes1 pathway. PharmaNutrition 2020, 13, 100207. [Google Scholar] [CrossRef]
- Ghanavati, R.; Akbari, A.; Mohammadi, F.; Asadollahi, P.; Javadi, A.; Talebi, M.; Rohani, M. Lactobacillus species inhibitory effect on colorectal cancer progression through modulating the Wnt/β-catenin signaling pathway. Mol. Cell Biochem. 2020, 470, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammad, S.; Sepehr, A.; Miri, S.T.; Najafi, S.; Pourshafie, M.R.; Rohani, M. Anti-inflammatory and immunomodulatory effects of Lactobacillus spp. as a preservative and therapeutic agent for IBD control. Immun. Inflamm. Dis. 2022, 10, e635. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Šulc, D.; Steyer, A. Study of the In Vitro Antagonistic Activity of Various Single-Strain and Multi-Strain Probiotics against Escherichia coli. Int. J. Environ. Res. Public Health 2018, 15, 1539. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; van Hemert, S.; Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 12. [Google Scholar] [CrossRef]
- Asadollahi, P.; Ghanavati, R.; Rohani, M.; Razavi, S.; Esghaei, M.; Talebi, M. Anti-cancer effects of Bifidobacterium species in colon cancer cells and a mouse model of carcinogenesis. PLoS ONE 2020, 15, e0232930. [Google Scholar]
- Béduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef]
- Smit, B.; Kuballa, A.; Coulson, S.; Katouli, M. Interaction of Candida albicans with human gut epithelium in the presence of Live Biotherapeutic Products (LBPs). Microbiol. Aust. 2021, 42, 120–124. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Niers, L.E.M.; Ridwan, B.U.; Koning, C.J.M.; Mulder, L.; Akkermans, L.M.A.; Rombouts, F.M.; Rijkers, G.T. Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients. Clin. Nutr. 2007, 26, 450–459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).