Selected Australian Terminalia Species Extracts Inhibit β-Lactam Drug-Resistant Bacteria Growth and Potentiate the Activity of Conventional Antibiotics: Bioactivities and Phytochemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Collection and Extraction
2.3. Antibacterial Studies
2.3.1. Bacterial Strains Screened
2.3.2. Growth of Bacterial Cultures
2.3.3. Disc Diffusion Assay and Liquid Microdilution Assay
2.4. Examination of Combinational Effects and Identifying Optimal Ratios
2.5. Non-Targeted LC-MS Conditions for Quantitative Analysis
2.6. Toxicity Studies
3. Results
3.1. Antimicrobial Susceptibility Studies
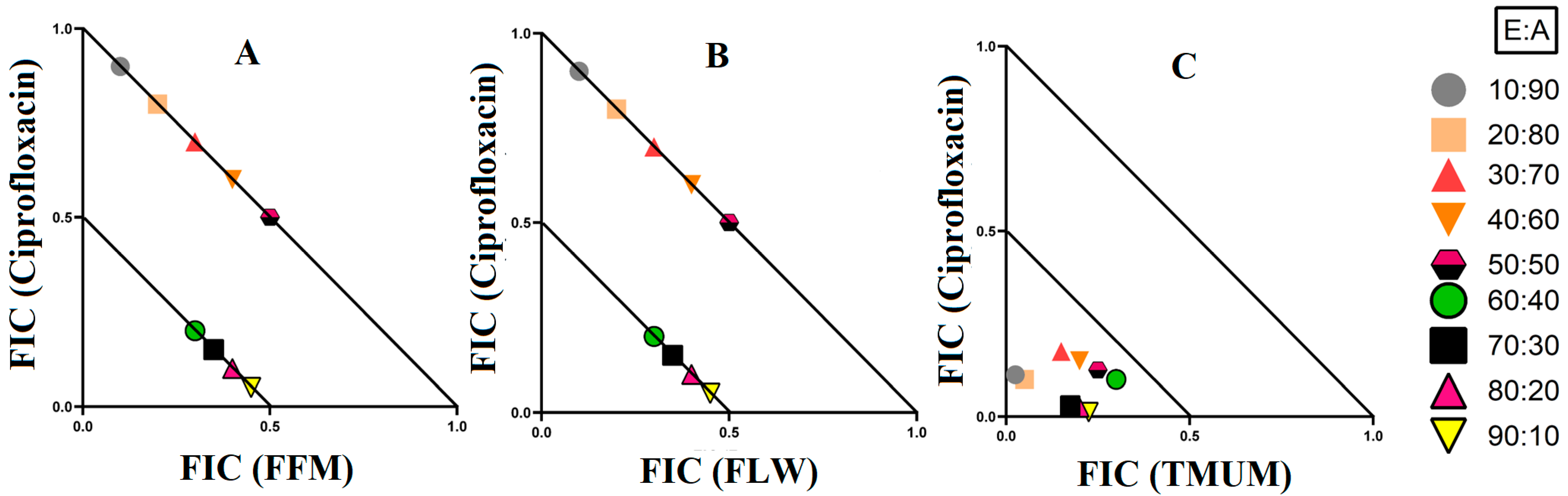
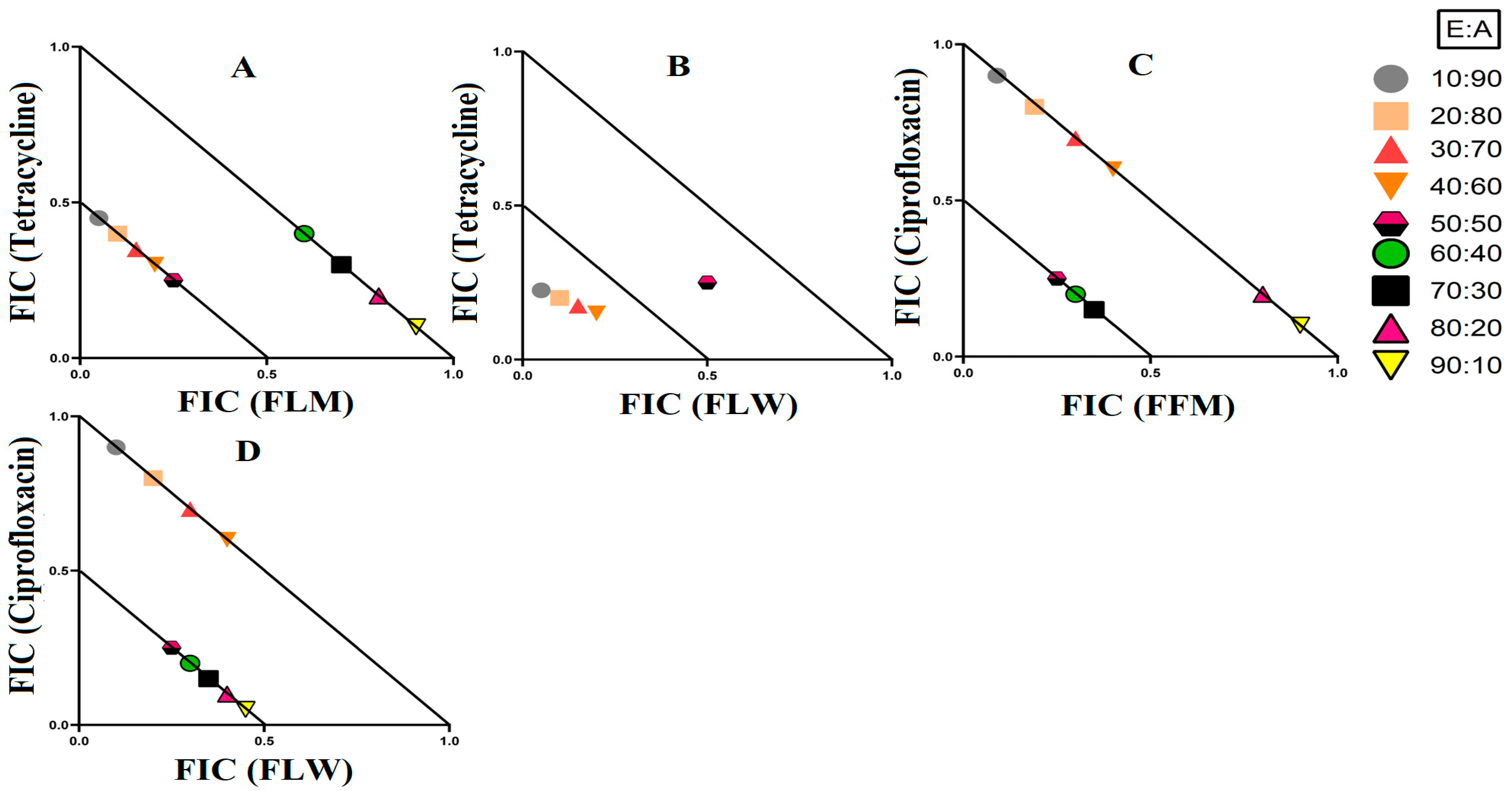
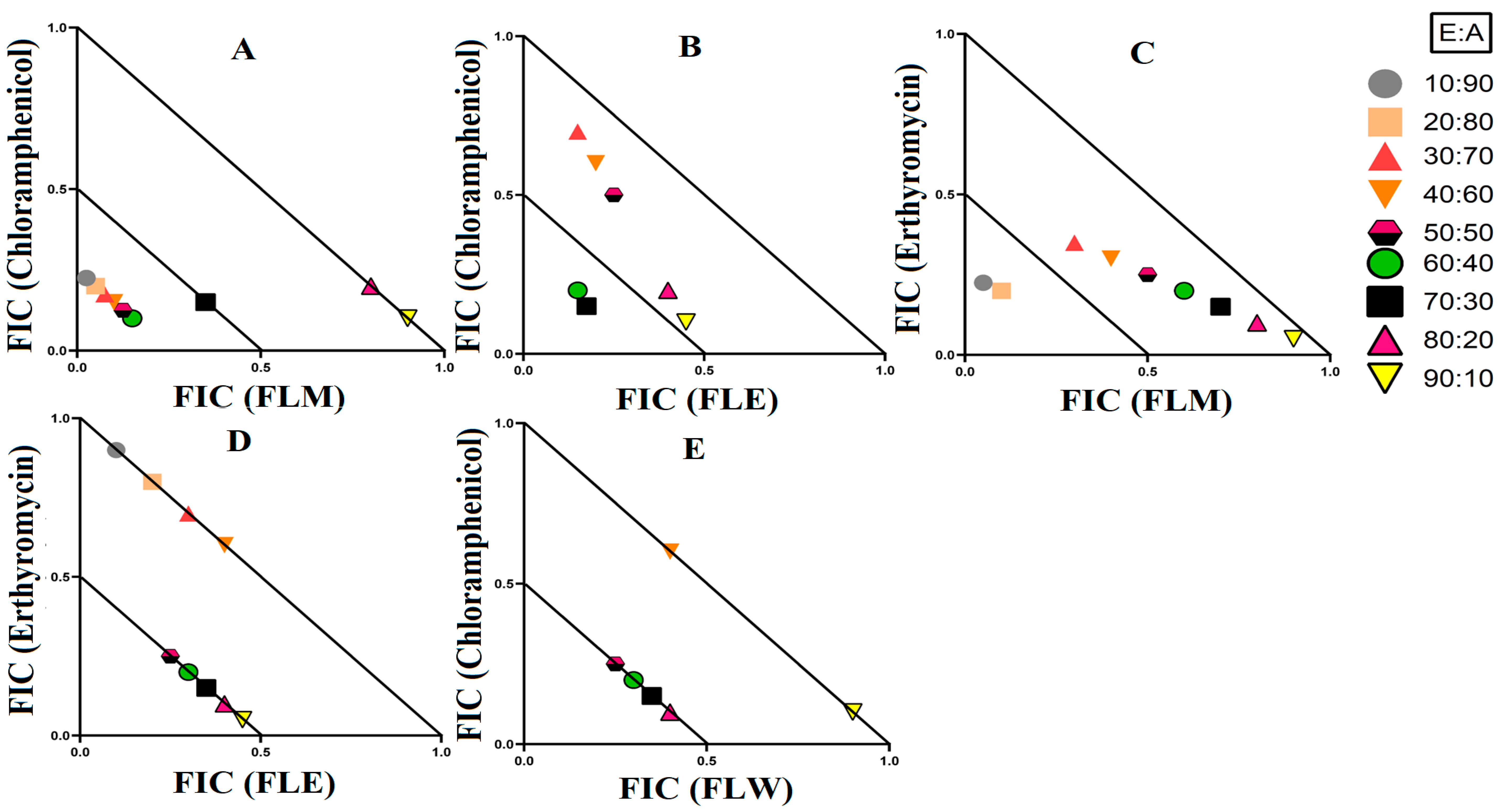
3.2. Fractional Inhibitory Concentration
3.3. Synergistic Interaction of Extract Antibiotic at Different Ratios
3.3.1. Extract and Antibiotic Synergistic Interactions against E. coli
3.3.2. Extract and Antibiotic Synergistic Interactions against S. aureus and MRSA
3.3.3. Extract and Antibiotic Interactions against K. pneumoniae and ESBL K. pneumoniae
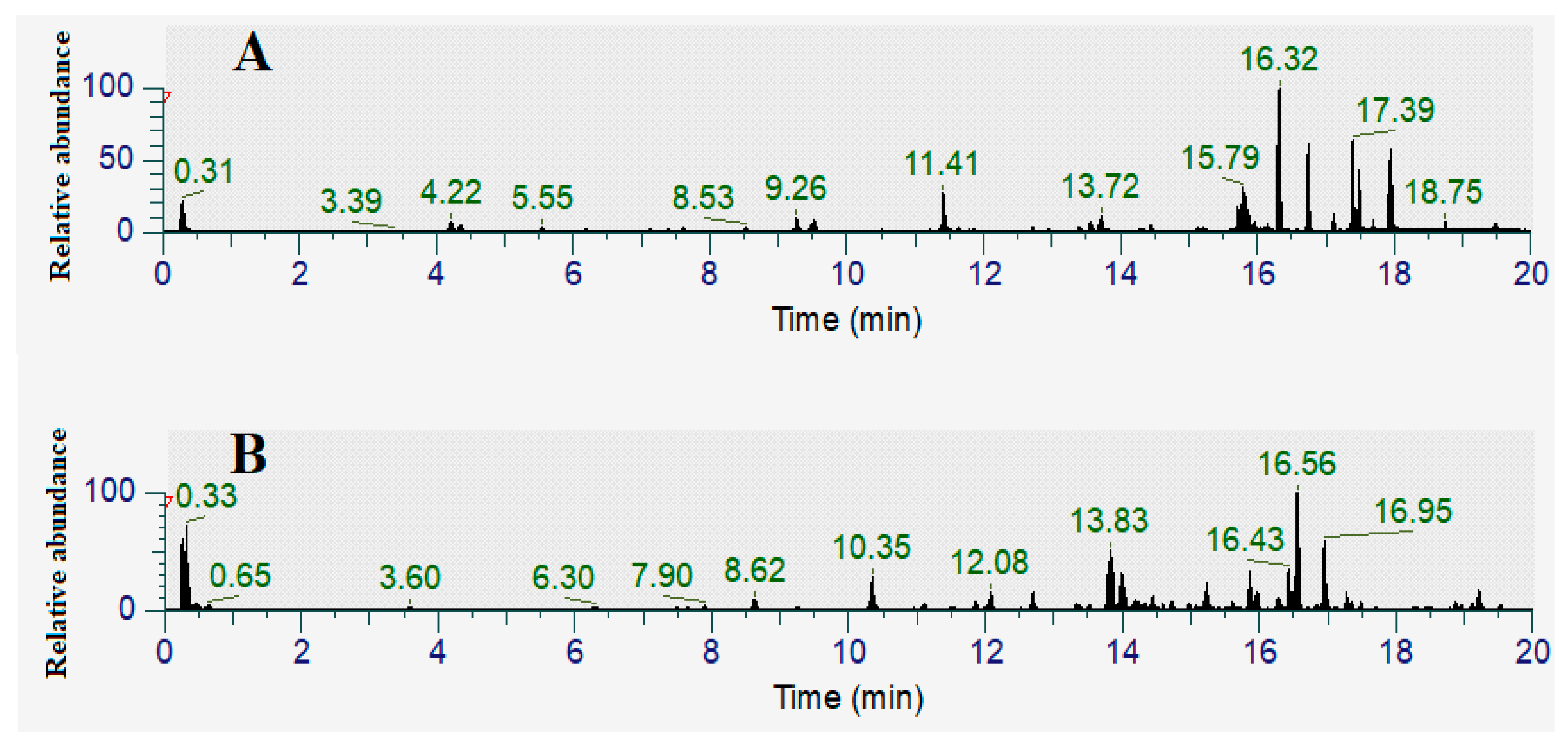
3.4. Identification of Compounds in the FLM and FLW Extracts
3.5. Quantification of Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Brown, D. Antibiotic resistance breakers: Can repurposed drugs fill the antibiotic discovery void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef]
- Rana, R.; Sharma, R.; Kumar, A. Repurposing of existing statin drugs for treatment of microbial infections: How much promising? Infect. Disord.-Drug Targets 2019, 19, 224–237. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, B.S.F.; Khameneh, B.; Ostad, M.R.Z.; Hosseinzadeh, H. In vitro evaluation of antibacterial activity of verbascoside, lemon verbena extract and caffeine in combination with gentamicin against drug-resistant Staphylococcus aureus and Escherichia coli clinical isolates. Avicenna J. Phytomed. 2018, 8, 246. [Google Scholar]
- McGaw, L.; Rabe, T.; Sparg, S.; Jäger, A.; Eloff, J.; Van Staden, J. An investigation on the biological activity of Combretum species. J. Ethnopharmacol. 2001, 75, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.; Katerere, D.; McGaw, L. The biological activity and chemistry of the southern African Combretaceae. J. Ethnopharmacol. 2008, 119, 686–699. [Google Scholar] [CrossRef]
- Garcez, F.R.; Garcez, W.S.; Miguel, D.L.; Serea, A.A.; Prado, F.C. Chemical constituents from Terminalia glabrescens. J. Braz. Chem. Soc. 2003, 14, 461–465. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Woods, B.E. A Study of the Intra-Specific Variations and Commercial Potential of Terminalia ferdinandiana (Exell) (the Kakadu Plum). Master’s Thesis, Charles Darwin University, Casuarina, Australia, 1995. [Google Scholar]
- Cheesman, M.J.; White, A.; Matthews, B.; Cock, I.E. Terminalia ferdinandiana fruit and leaf extracts inhibit methicillin-resistant Staphylococcus aureus growth. Planta Med. 2019, 85, 1253–1262. [Google Scholar] [CrossRef]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Terminalia petiolaris A. Cunn ex Benth. extracts have antibacterial activity and potentiate conventional antibiotics against β-lactam-drug-resistant bacteria. Antibiotics 2023, 12, 1643. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Eloff, J. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies—Methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Anam, K.; Suganda, A.; Sukandar, E.; Kardono, L. Antibacterial agents of Terminalia muelleri Benth. leaves. Res. J. Med. Plant 2010, 4, 197–205. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57. [Google Scholar] [PubMed]
- Fahmy, N.; Al-Sayed, E.; Singab, A. Genus Terminalia: A phytochemical and biological review. Med. Aromat. Plants 2015, 4, 1–22. [Google Scholar]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Otsuka, N.; Liu, M.-H.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Anti-methicillin resistant Staphylococcus aureus (MRSA) compounds isolated from Laurus nobilis. Biol. Pharm. Bull. 2008, 31, 1794–1797. [Google Scholar] [CrossRef]
- Šmejkal, K.; Chudík, S.; Kloucek, P.; Marek, R.; Cvacka, J.; Urbanová, M.; Julínek, O.; Kokoška, L.; Šlapetová, T.; Holubová, P. Antibacterial C-geranylflavonoids from Paulownia tomentosa fruits. J. Nat. Prod. 2008, 71, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure–activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Thakur, J.; Prakash, O.; Khan, F.; Saikia, D.; Gupta, M.M. Screening of flavonoids for antitubercular activity and their structure–activity relationships. Med. Chem. Res. 2013, 22, 2706–2716. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Hancock, V.; Dahl, M.; Vejborg, R.M.; Klemm, P. Dietary plant components ellagic acid and tannic acid inhibit Escherichia coli biofilm formation. J. Med. Microbiol. 2010, 59, 496–498. [Google Scholar] [CrossRef]
- Tinh, T.H.; Nuidate, T.; Vuddhakul, V.; Rodkhum, C. Antibacterial activity of pyrogallol, a polyphenol compound against Vibrio parahaemolyticus isolated from the central region of Thailand. Procedia Chem. 2016, 18, 162–168. [Google Scholar] [CrossRef]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforschung C 2006, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
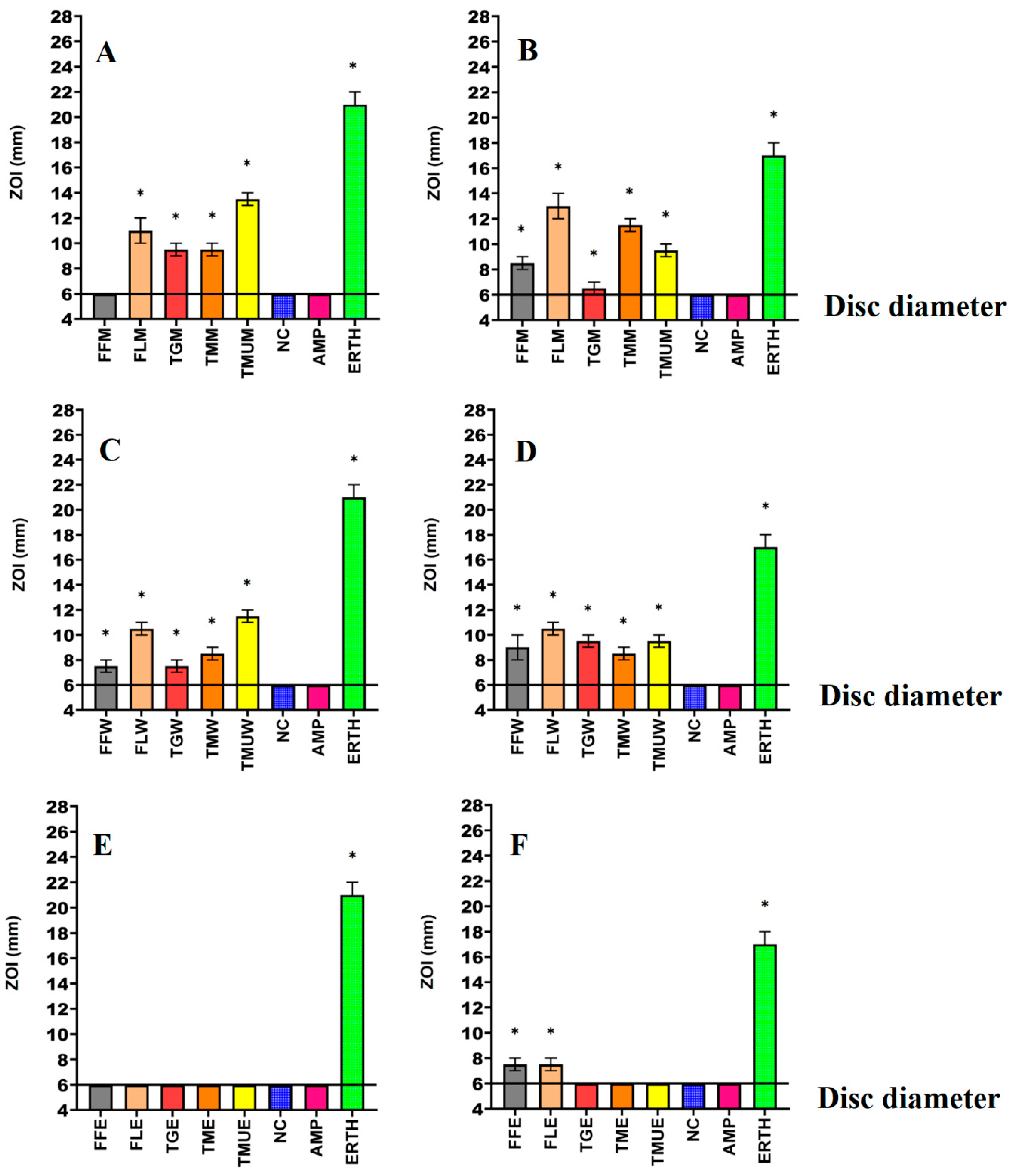
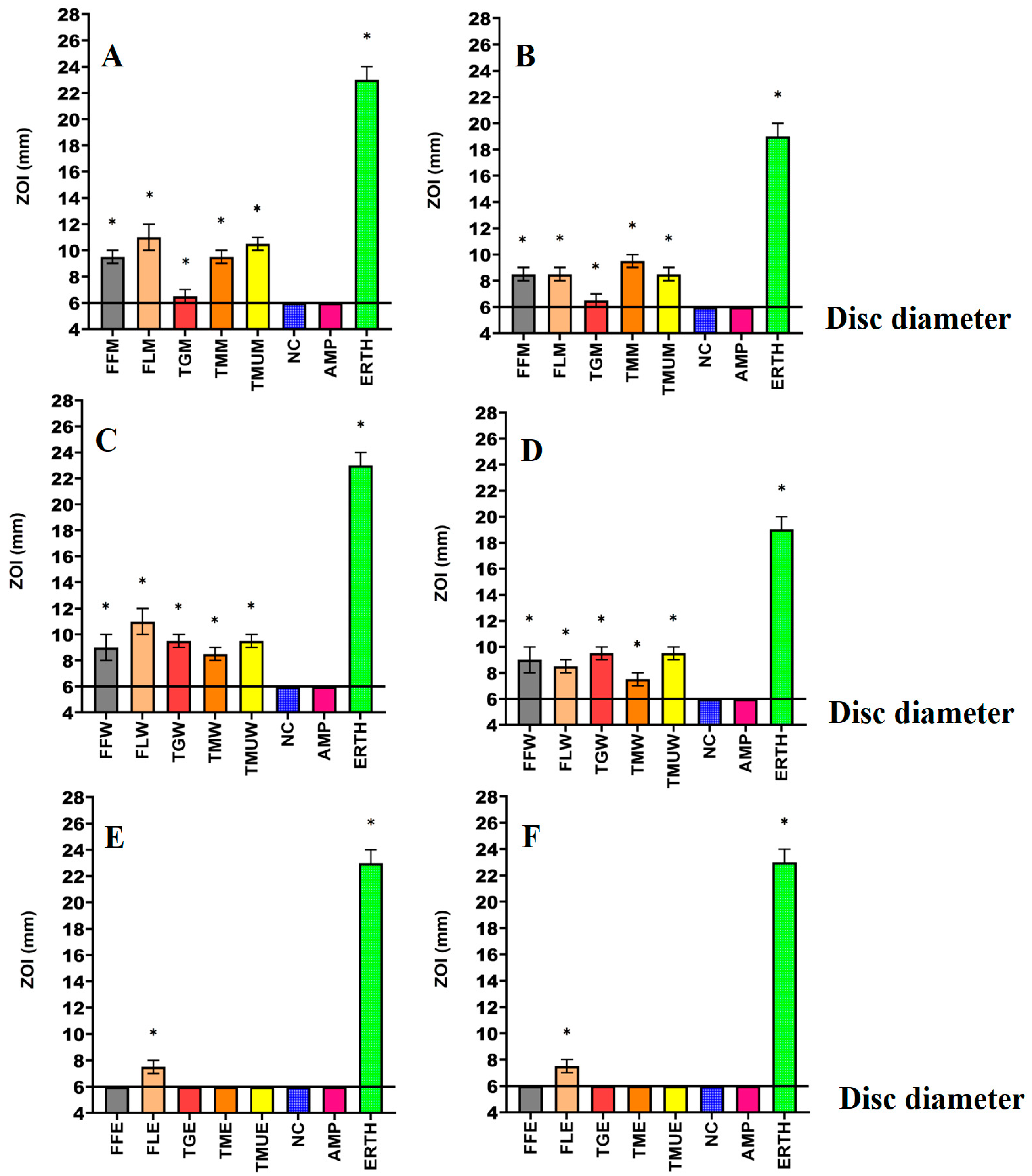
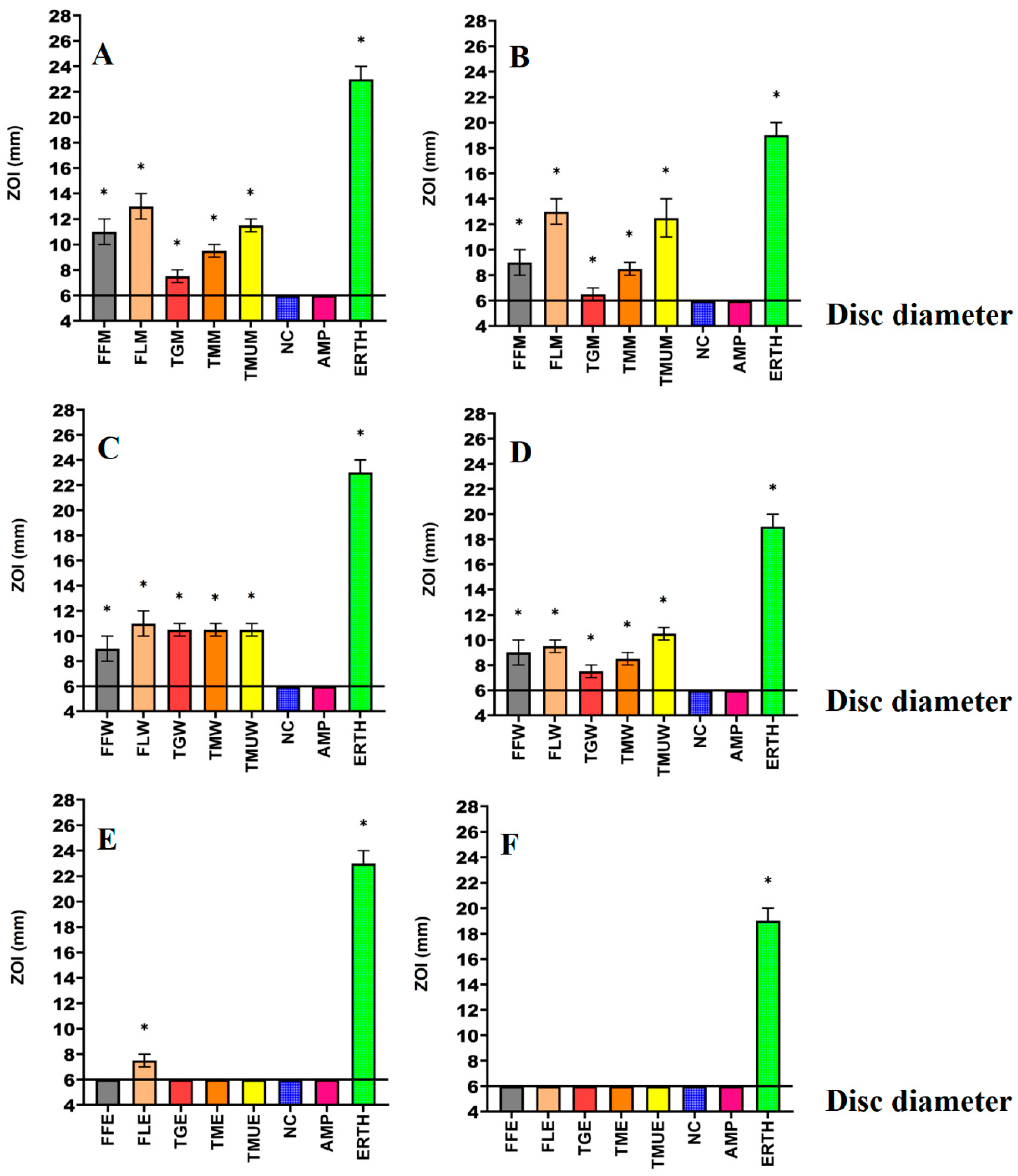

| Species | Common Name | Plant Part | Origin | Supplier | Voucher Number |
|---|---|---|---|---|---|
| Terminalia ferdinandiana Exell. | Kakadu plum, gubinge, billygoat plum | Fruit | Kakadu Nation Park, Northern Territory, Australia (collected under licence) | David Boehme, Northern Territory Wild Harvest | GU_NT_TFF21 |
| Leaves | GU_NT_TFL21 | ||||
| Terminalia gradndflora (Benth.) Kuntze | Yalu, plumwood, nutwood | Leaves | James Cook University, Cairns campus, Australia | Dr. Phurpa Wangchuk, James Cook University, Australia | GU_NQ_TGrL21 |
| Terminalia microcarpa Decne. | Damson plum, sovereign wood | Leaves | Kimberley Wild Gubinge, Western Australia | Jacinta Monck, Kimberley, Australia | GU_WA_TmicL21 |
| Terminalia muelleri Benth. | Australian almond | Leaves | James Cook University, Cairns campus, Australia | Dr. Phurpa Wangchuk, James Cook University, Australia | GU_NQ_TMueL21 |
| Extract and Antibiotic | MIC (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| E. coli | ESBL E. coli | S. aureus | MRSA | K. pneumoniae | ESBL K. pneumoniae | Yield (mg/mL) | |
| FFM | 1344 | 1344 | - | 1344 | - | - | 21.5 |
| FLM | 498 | 996 | 996 | 996 | 996 | 996 | 31.9 |
| TGM | 2550 | 2550 | - | 2550 | 2550 | 2550 | 10.2 |
| TMM | 1300 | 2600 | 1300 | 2600 | 2600 | 2600 | 20.8 |
| TMUM | 3400 | 3400 | 1700 | 6800 | 3400 | 3400 | 27.2 |
| FFW | 606 | 1212 | 606 | 1212 | 1212 | 1212 | 38.8 |
| FLW | 663 | 331 | 165 | 663 | 331 | 331 | 10.6 |
| TGW | 1600 | 1600 | 3200 | 1600 | 3200 | 3200 | 12.8 |
| TMW | 2063 | 4125 | 2063 | 2063 | 2063 | 2063 | 16.5 |
| TMUM | 2750 | 5500 | 1375 | 2750 | 2750 | 2750 | 22 |
| FFE | - | 1225 | - | - | - | - | 4.9 |
| FLE | - | 37.5 | - | - | 75 | 75 | 0.3 |
| TGE | - | - | - | - | - | - | 7 |
| TME | - | - | - | - | - | - | 6.6 |
| TMUE | - | - | - | - | - | - | 3.6 |
| Tetracycline | - | - | 1.25 | - | - | - | |
| Chloramphenicol | - | - | 0.31 | - | 1.25 | 1.25 | |
| Ciprofloxacin | 2.5 | - | 0.62 | 2.5 | 2.5 | 1.25 | |
| Gentamicin | 0.039 | 0.039 | 0.03 | 0.03 | 0.03 | 0.03 | |
| Erythromycin | - | - | 1.25 | - | 2.5 | - | |
| Negative control | - | - | - | - | - | - | |
| Bacteria | Extract | Tetracycline | Chloramphenicol | Ciprofloxacin | Gentamicin | Erythromycin |
|---|---|---|---|---|---|---|
| E. coli | FFM | - | - | 0.50 | 1.31 | - |
| FLM | - | - | 1.25 | 0.66 | - | |
| TGM | - | - | 1 | 1 | - | |
| TMM | - | - | 0.63 | 2.13 | - | |
| TMUM | - | - | 0.18 | 1.1 | - | |
| FFW | - | - | 1.25 | 1.06 | - | |
| FLW | - | - | 0.50 | 1.01 | - | |
| TGW | - | - | 1.50 | 2.1 | - | |
| TMW | - | - | 1 | 2.1 | - | |
| TMUW | - | - | 0.65 | 1.03 | - | |
| FFE | - | - | - | - | - | |
| FLE | - | - | 0.75 | 0.66 | - | |
| TGE | - | - | - | - | - | |
| TME | - | - | - | - | - | |
| TMUE | - | - | - | - | - | |
| ESBL E. coli | FFM | - | - | - | 7.65 | - |
| FLM | - | - | - | 5.33 | - | |
| TGM | - | - | - | 1.1 | - | |
| TMM | - | - | - | 2.66 | - | |
| TMUM | - | - | - | 2.66 | - | |
| FFW | - | - | - | 1.33 | - | |
| FLW | - | - | - | 0.66 | - | |
| TGW | - | - | - | 2.66 | - | |
| TMW | - | - | - | 2.63 | - | |
| TMUW | - | - | - | 2.63 | - | |
| FFE | - | - | - | 0.65 | - | |
| FLE | - | - | - | 2.66 | - | |
| TGE | - | - | - | - | - | |
| TME | - | - | - | - | - | |
| TMUE | - | - | - | - | - | |
| S. aureus | FFM | - | - | - | - | - |
| FLM | 0.50 | 1.25 | 1.5 | 1.33 | 1 | |
| TGM | - | - | - | - | - | |
| TMM | 0.75 | 3 | 2 | 22 | 1.5 | |
| TMUM | 0.75 | 3 | 0.75 | 12 | 1.5 | |
| FFW | 0.75 | 1.50 | 4 | 0.68 | 1.5 | |
| FLW | 0.50 | 1.5 | 4 | 0.68 | 1.5 | |
| TGW | 0.75 | - | 1.25 | 5.26 | 1.5 | |
| TMW | 1 | 5 | 1.5 | 42.6 | 2 | |
| TMUW | 0.75 | 3 | 2 | 22 | 1.5 | |
| FFE | - | - | - | - | - | |
| FLE | - | - | - | - | - | |
| TGE | - | - | - | - | - | |
| TME | - | - | - | - | - | |
| TMUE | - | - | - | - | - | |
| MRSA | FFM | - | - | 0.50 | 1.01 | - |
| FLM | - | - | 0.75 | 2.66 | - | |
| TGM | - | - | 1 | 21.08 | - | |
| TMM | - | - | 0.65 | 10.66 | - | |
| TMUM | - | - | 0.65 | 10.53 | - | |
| FFW | - | - | 0.75 | 2.66 | - | |
| FLW | - | - | 0.50 | 2.63 | - | |
| TGW | - | - | 1.5 | 42.66 | - | |
| TMW | - | - | 0.75 | 21.33 | - | |
| TMUW | - | - | 1 | 10.66 | - | |
| FFE | - | - | - | 5.26 | - | |
| FLE | - | - | - | 1.31 | - | |
| TGE | - | - | - | - | - | |
| TME | - | - | - | - | - | |
| TMUE | - | - | - | - | - | |
| K. pneumoniae | FFM | - | - | - | - | - |
| FLM | - | 0.25 | 0.75 | 2.66 | 0.18 | |
| TGM | - | 1.5 | 1.5 | 21.1 | 1 | |
| TMM | - | 1 | 1.25 | 21.3 | 0.75 | |
| TMUM | - | 0.75 | 2 | 21.3 | 0.60 | |
| FFW | - | 1 | 0.75 | 0.66 | 0.75 | |
| FLW | - | 1 | 0.75 | 1.03 | 1.50 | |
| TGW | - | 1.50 | 1 | 42.2 | 1 | |
| TMW | - | 2 | 0.75 | 21.3 | 0.75 | |
| TMUW | - | 2 | 2.5 | 43 | 1 | |
| FFE | - | - | - | - | - | |
| FLE | - | 0.37 | 1 | 0.65 | 0.25 | |
| TGE | - | - | - | - | - | |
| TME | - | - | - | - | - | |
| TMUE | - | - | - | - | - | |
| ESBL K. pneumoniae | FFM | - | - | - | - | - |
| FLM | - | 1 | 1 | 2.66 | - | |
| TGM | - | 1.50 | - | 21 | 1 | |
| TMM | - | 1 | - | 10.6 | - | |
| TMUM | - | 1 | - | 10.6 | - | |
| FFW | - | 2 | 1 | 0.66 | - | |
| FLW | - | 0.50 | 1 | 1.03 | 1 | |
| TGW | - | 1.50 | - | 42.16 | - | |
| TMW | - | 2 | - | 42.66 | - | |
| TMUW | - | 2 | - | 10.54 | - | |
| FFE | - | - | - | - | - | |
| FLE | - | 1 | 0.75 | 0.65 | - | |
| TGE | - | - | - | - | - | |
| TME | - | - | - | - | - | |
| TMUE | - | - | - | - | - |
| Retention Time (Min) | Empirical Formula | Molecular Mass | Putative Identification | Relative Abundance (% Total Area) | ||
|---|---|---|---|---|---|---|
| FLM | FLW | |||||
| Flavonoids | 5.84 | C21H20O10 | 432 | Vitexin | 2.20 | |
| 6.324 | C15H10O7 | 302 | Robinetin | 0.06 | ||
| 6.322 | C21H20O12 | 464 | Quercitin-3β-D-glucoside | 0.14 | ||
| 6.37 | C27 H30 O16 | 610 | Quercitin 3-O-rhamnoside-7-O-glucoside | 0.23 | ||
| 5.40 | C21 H20 O11 | 448 | Orientin | 5.42 | ||
| 8.53 | C22H20O12 | 476 | Hispidulin 7-glucuronide | 6.28 | ||
| 3.59 | C21H20O14 | 496 | Hibiscetin 3-glucoside | 0.06 | ||
| 5.96 | C21H18O13 | 478 | 6-Hydroxyluteolin 6-glucuronide | 0.06 | ||
| 7.07 | C21H20O11 | 448 | 4-(3,4-Dihydroxyphenyl)-7-hydroxy-5-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2H-chromen-2-one | 0.24 | ||
| 7.46 | C28H36O11 | 548 | 3,7-Dihydroxy-4,5-dimethoxy-8-prenylflavan 7-O-beta-D-glucopyranoside | 0.70 | ||
| 0.32 | C20H18O13 | 466 | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-8-{[(2R,3R,4S,5S,6R)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one | 1.47 | ||
| 6.19 | C21H20O10 | 432 | 1,5-Anhydro-1-[5,7-dihydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl]hexitol | 3.05 | ||
| 5.55 | C28H24O11 | 448 | (1ξ)-1,5-Anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-D-galactitol | 4.74 | ||
| 8.77 | C28H24O12 | 552 | Vitexin 2″-p-hydroxybenzoate | 0.02 | ||
| 6.30 | C21H20O11 | 448 | Orientin | 1.65 | ||
| 4.06 | C16H14O7 | 318 | 5,7,2′,5′-Tetrahydroxy-6-methoxyflavanone | 0.01 | ||
| 7.38 | C21H20O10 | 432 | 1,5-Anhydro-1-[5,7-dihydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl]hexitol | 0.80 | ||
| 6.97 | C28H24O15 | 600 | (2S,3R,4R,5S,6S)-2-{[2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl]oxy}-3,5-dihydroxy-6-methyloxan-4-yl 3,4,5-trihydroxybenzoate | 0.40 | ||
| Tannins | 6.09 | C14H6O8 | 303 | Ellagic acid (Isomer 1) | 3.17 | |
| 7.47 | C14H6O8 | 303 | Ellagic acid (Isomer 2) | 3.37 | 2.12 | |
| 1.64 | C6H6O3 | 126 | Pyrogallol (Isomer 1) | 0.47 | ||
| 0.64 | C6H6O3 | 126 | Pyrogallol (Isomer 2) | 0.92 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zai, M.J.; Cheesman, M.J.; Cock, I.E. Selected Australian Terminalia Species Extracts Inhibit β-Lactam Drug-Resistant Bacteria Growth and Potentiate the Activity of Conventional Antibiotics: Bioactivities and Phytochemistry. Microorganisms 2024, 12, 498. https://doi.org/10.3390/microorganisms12030498
Zai MJ, Cheesman MJ, Cock IE. Selected Australian Terminalia Species Extracts Inhibit β-Lactam Drug-Resistant Bacteria Growth and Potentiate the Activity of Conventional Antibiotics: Bioactivities and Phytochemistry. Microorganisms. 2024; 12(3):498. https://doi.org/10.3390/microorganisms12030498
Chicago/Turabian StyleZai, Muhammad Jawad, Matthew James Cheesman, and Ian Edwin Cock. 2024. "Selected Australian Terminalia Species Extracts Inhibit β-Lactam Drug-Resistant Bacteria Growth and Potentiate the Activity of Conventional Antibiotics: Bioactivities and Phytochemistry" Microorganisms 12, no. 3: 498. https://doi.org/10.3390/microorganisms12030498
APA StyleZai, M. J., Cheesman, M. J., & Cock, I. E. (2024). Selected Australian Terminalia Species Extracts Inhibit β-Lactam Drug-Resistant Bacteria Growth and Potentiate the Activity of Conventional Antibiotics: Bioactivities and Phytochemistry. Microorganisms, 12(3), 498. https://doi.org/10.3390/microorganisms12030498







