Abstract
Terminalia ferdinandiana Exell, Terminalia grandiflora Benth., Terminalia microcarpa Decne., and Terminalia muelleri Benth. (family: Combretaceae) belong to the genus Terminalia. Plants of this genus have been extensively used as traditional medicines to treat a variety of illnesses, including pathogen infections. However, we were unable to find any studies that have investigated the antibacterial activity of T. microcarpa. Similarly, whilst some preliminary studies have examined the antimicrobial properties of T. muelleri and T. grandiflora, they did not test the extracts against antibiotic-resistant pathogens. This study screens the antimicrobial activity of T. grandiflora, T. microcarpa, and T. muelleri and compares it to that of T. ferdinandiana extracts prepared from both the fruit and leaves against a range of pathogens, including multi-antibiotic-resistant strains. Solvents with varying polarities were used to extract different phytochemical constituents from the leaves of T. grandiflora, T. microcarpa, and T. muelleri and from the fruit and leaves of T. ferdinandiana. The aqueous and methanolic extracts each displayed significant antimicrobial activity when tested against the bacterial pathogens, including against the multidrug-resistant strains. When these extracts were tested in combination with selected antibiotics, some extracts potentiated the antimicrobial activity. This study identifies twelve synergistic, fifty-eight additive, and sixty non-interactive combinations, as well as thirty antagonistic effects. The extracts were evaluated for toxicity using the Artemia franciscana nauplii lethality assay (ALA) and were each classified as non-toxic, with the exception of the methanolic and aqueous T. ferdinandiana fruit extracts and the aqueous and ethyl acetate T. ferdinandiana leaf extracts. Metabolomic analysis using liquid chromatography–mass spectrometry (LC-MS) highlighted several flavonoids and tannins that may contribute to the antimicrobial activities reported herein. The potential antibacterial mechanism(s) of the T. ferdinandiana extracts are discussed in this study.
1. Introduction
Bacterial resistance to antibiotic therapies (AMR) is a serious threat to public health. Indeed, a 2016 AMR review authorized by the British Government claimed that if left unaddressed, AMR may result in 10 million mortalities annually by 2050 [1]. As such, the World Health Organization (WHO) has classified AMR as perhaps the most urgent threat to human health and recommended increased medical research and the adoption of a global plan to mitigate the effects of AMR [2]. Another study reported that AMR currently takes more lives than any other infectious disease, including malaria and human immunodeficiency virus (HIV) [3]. One in eight deaths worldwide is due to bacterial infections, making this the second leading cause of death after heart-related disease [4]. In 2019, AMR-associated deaths were estimated to be 4.95 million [3]. The six bacterial pathogens most frequently associated with the development of antibiotic resistance (Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumannii, and Streptococcus pneumoniae) caused approximately 3.57 million deaths in 2019 [3]. The number of deaths attributable to methicillin-resistant Staphylococcus aureus (MRSA) in that year was over 100,000 [3], while multidrug-resistant (MDR) tuberculosis; third-generation cephalosporin-resistant strains of K. pneumonia and E. coli; carbapenem-resistant strains of K. pneumonia and A. baumannii; and fluoroquinolone-resistant E. coli each caused 50,000–100,000 deaths in 2019 [3].
There has been a significant increase in research to develop novel treatments against MDR and extensively resistant pathogens. One of the suggested strategies involves the combination of potentiating molecules with ineffective (or reduced efficacy) antibiotics to restore/improve antimicrobial activity [5,6]. Some phytochemicals exhibit potent antimicrobial activities alone or when tested in combination with some clinical antibiotics, against a wide range of pathogens [7,8]. The genus Terminalia consists of 200–250 species, many of which have traditional uses in the treatment of a myriad of diseases and illnesses [9]. This includes (but is not limited to) treating bacterial infections, skin diseases, heart diseases, gastric ulcers, headaches, and diarrhea [10]. Phytochemical investigations into several Terminalia species have identified several classes of phytochemicals, including triterpenoids and their glycoside derivatives, tannins, flavonoids, and other phenolic compounds [11]. Terminalia ferdinandiana Exell. is an endemic Australian species that has an exceptionally high antioxidant content [12]. Indeed, the ascorbic acid levels of the fruit of this plant are substantially higher than those quantified for any plant globally [13]. Notably, previous studies have shown that T. ferdinandiana extracts prepared using both fruit and leaf materials inhibit MRSA growth, and it was postulated that some notable phytochemicals identified in these extracts may contribute to the therapeutic potential of Terminalia spp. [14]. Despite these previous studies, the therapeutic potential of Terminalia grandiflora Benth., Terminalia microcarpa Decne., and Terminalia muelleri Benth. remain largely unexplored.
This study assesses the antimicrobial activity of T. grandiflora, T. microcarpa, and T. muelleri and compares their activity with that of T. ferdinandiana fruit and leaf extracts. Solvents of different polarities, such as methanol, water, and ethyl acetate, were used to extract active phytochemical constituents. This study focuses on β-lactam antibiotic resistance. The bacterial species included in this study were E. coli and ESBL E. coli; K. pneumoniae and ESBL K. pneumoniae; and S. aureus and MRSA. Notably, whilst the bacteria studied herein are classified on the basis of their β-lactam resistance, they also exhibit resistance to several other classes of antibiotics [14]. Additionally, the antibacterial activity of T. grandiflora, T. microcarpa, T. muelleri, and T. ferdinandiana extracts was also tested in combination with conventional antibiotics to determine whether these extracts can potentiate the activity of antibiotics when used in combination, thereby repurposing those antibiotics for clinical use. Compounds were separated using high-performance liquid chromatography–mass spectrometry (HPLC-MS) for the most promising extracts. The toxicity of each extract was evaluated using Artemia franciscana nauplii lethality toxicity assays (ALAs).
2. Materials and Methods
2.1. Materials
All chemicals and reagents used in this study were purchased from Ajax Fine-Chemicals Ltd., Taren Point, NSW, Australia, and were of an Analytical Research (AR) grade unless otherwise stated. Bacterial growth media was prepared using Mueller–Hinton broth and agar powders (Oxoid Ltd., Thebarton, SA, Australia) according to the manufacturer’s instructions. All other reagents were purchased from Sigma-Aldrich (Coopers Plains, QLD, Australia) unless otherwise stated.
2.2. Plant Collection and Extraction
The leaves of T. grandiflora, T. microcarpa, and T. muelleri were supplied by Dr Phurpa Wangchuk from the James Cook University Cairns campus. The fruit and leaves of T. ferdinandiana were provided by David Boehme from Wild Harvest, Northern Territory, Australia. All leaf and fruit materials were ground into a fine powder after drying in a Sunbeam food dehydrator. Voucher specimens of each plant are stored individually at Griffith University, Nathan, QLD, Australia, and are summarized in Table 1. Extraction was performed by adding 50 mL of either sterile deionized water, methanol, or ethyl acetate to the individual Falcon tubes, each containing one gram of dried and powdered plant material. The extraction of the plant material was performed via maceration for at 23 °C for 24 h. The extracts were filtered through Whatman No. 54 filter paper to remove the particulates from the extracts and dried in a vacuum oven. The mass of dried extract was measured to determine extraction yields. DMSO (100 µL) was used to partially dissolve the extract pellet, and the total volume was increased to 10 mL using sterile deionized water. The extracts were filtered using a syringe-driven filter (0.22 µm; Millipore Australia Ltd., North Ryde, NSW, Australia) and stored at 4 °C until further use.

Table 1.
Source and voucher numbers for plant specimens used in this study.
2.3. Antibacterial Studies
2.3.1. Bacterial Strains Screened
This study examined the effects of the extracts against β-lactam-susceptible bacterial strains and against their antibiotic sensitive counterparts. References of ESBL β-lactam-resistant Klebsiella pneumoniae (ATCC 700603) and methicillin-resistant strain Staphylococcus aureus (MRSA) (ATCC 43300) were purchased from the American Type Culture Collection (ATCC). An ESBL-resistant Escherichia coli clinical isolate was supplied by the Gold Coast University Hospital, Australia. The antibiotic susceptibility of these strains towards multiple antibiotics, including β-lactam, has previously been verified in our laboratory [14,15]. As a comparison, antibiotic-susceptible strains of E. coli (ATCC 25922), K. pneumoniae (ATCC 31488), and S. aureus (ATCC 25923) were used in this study.
2.3.2. Growth of Bacterial Cultures
Bacterial stock cultures were initially streaked onto the Mueller–Hinton agar plates and incubated at 37 °C for 24 h to obtain pure cultures. A single bacterial colony was then transferred into freshly prepared Mueller–Hinton broth (50 mL) and incubated at 37 °C until the bacteria attained a log growth phase, with the exception of MRSA, which was incubated at 35 °C. The purity of each culture was confirmed by re-streaking the bacterial culture on Mueller–Hinton agar plates.
2.3.3. Disc Diffusion Assay and Liquid Microdilution Assay
Standardized Kirby-Bauer disc diffusion and liquid-phase microdilution assays were performed to evaluate the antimicrobial susceptibility and to quantify the minimum inhibitory concentration (MIC) of each bacterial strain [15].
2.4. Examination of Combinational Effects and Identifying Optimal Ratios
The combinational effects between the plant extracts and selected antibiotics were initially examined at 1:1 ratios. MICs were evaluated as described in Section 2.3.3. Fractional inhibitory concentration (FIC) values were calculated using the formula below:
FIC (extract) = (MIC of extract in combination with antibiotic)/MIC of extract alone.
FIC (antibiotic) = (MIC of antibiotic in combination with extract)/MIC of antibiotic alone.
∑FIC = FIC (extract) + FIC (antibiotic).
∑FIC values ≤ 0.5 were classed as synergistic, >0.5–≤1.0 were termed as additive, >1.0–≤4.0 were designated as non-interactive, and values > 4.0 were categorized as antagonistic.
Synergistic combinations were subsequently examined across a range of ratios to highlight ratio(s) that produced synergistic interactions. A modified version of the protocol described in Section 2.3.3 was used. Ratios ranged from 90% antibiotic to 10%, using 10% decreasing increments (corresponding to 10% to 90% extract). The assay was performed in two independent experiments, and FIC values were calculated. Isobolograms were plotted and used to determine the synergistic ratio(s) of extract and antibiotic.
2.5. Non-Targeted LC-MS Conditions for Quantitative Analysis
Studies to identify phytochemicals in the most promising extracts were undertaken using liquid chromatography–mass spectrometry (HPLC-MS) methods previously developed by our group [15]. Putative compound identification was performed through molecular annotation against mzCloud, ChemSpider, mzVault, CyanoMetDB, and Global Natural Product Social Molecular Networking (GNPS) databases, as well as through comparison with published data.
2.6. Toxicity Studies
The toxicity of the plant extracts was evaluated using Artemia franciscana nauplii lethality assays (ALAs) using standard methods [15].
3. Results
3.1. Antimicrobial Susceptibility Studies
Powdered leaves of T. grandiflora, T. microcarpa, and T. muelleri, as well as the fruit and leaves of T. ferdinandiana, were extracted using solvents of different polarities and then dried and resuspended in 10 mL of 1% DMSO, resulting in the yields reported in Table 2. The antibacterial activity of each extract was first examined against E. coli and ESBL E. coli (Figure 1); K. pneumoniae and ESBL K. pneumoniae (Figure 2); and S. aureus and MRSA (Figure 3) in disc diffusion assays and was measured as zones of inhibition (ZOIs). The disc diffusion assays were performed to provide an estimate of bacterial infections on the solid surface. Liquid microdilution assays (Table 2) were used to quantify the growth inhibitory activity of the extracts by determining minimum inhibitory concentrations (MICs). The ethyl acetate Terminalia ferdinandiana leaf extract was the most potent inhibitor of ESBL E. coli growth (MIC = 37.5 µg/mL) and had similar activity against both antibiotic-sensitive K. pneumoniae and the ESBL K. pneumoniae (MIC = 75 µg/mL). The methanol and water extracts of T. grandiflora, T. microcarpa, and T. muelleri were also active, albeit with substantially lower activity. In contrast, none of the ethyl acetate extracts prepared using T. grandiflora, T. microcarpa, or T. muelleri leaves showed activity against any bacterial species.

Table 2.
Yield (mg/mL) and MIC values (µg/mL) of plant extracts and conventional antibiotics against the bacteria tested in this study.
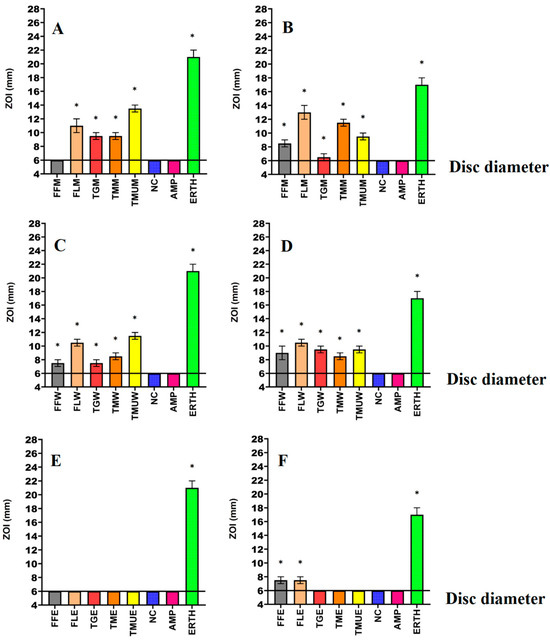
Figure 1.
Antimicrobial effects of the (A) methanol extracts against E. coli, (B) methanol extracts against ESBL E. coli, (C) water extracts against E. coli, (D) water extracts against ESBL E. coli, (E) ethyl acetate extracts against E. coli, (F) ethyl acetate extracts against ESBL E. coli. FF = Terminalia ferdinandiana fruit; FL = Terminalia ferdinandiana leaf; TG = Terminalia grandiflora; TM = Terminalia microcarpa; TMU= Terminalia muelleri; M = methanol extract; W = water extract; E = ethyl acetate extract; Positive controls = ampicillin (AMP; 2 µg) and erythromycin (ERTH; 10 µg). Negative control (NC) = water. Results are expressed as mean zones of inhibition of three independent replicates ± SEM (n = 3). * indicates that the results are significantly different to the negative control (p < 0.01).
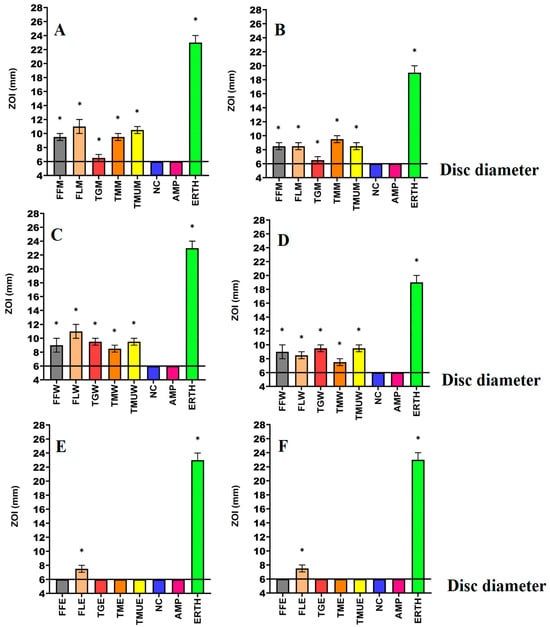
Figure 2.
Antimicrobial effects of the (A) methanol extracts against K. pneumoniae, (B) methanol extracts against ESBL K. pneumoniae, (C) water extracts against K. pneumoniae, (D) water extracts against ESBL K. pneumoniae, (E) ethyl acetate extracts against K. pneumoniae, (F) ethyl acetate extracts against ESBL K. pneumonia. FF = Terminalia ferdinandiana fruit; FL = Terminalia ferdinandiana leaf; TG = Terminalia grandiflora; TM = Terminalia microcarpa; TMU= Terminalia muelleri; M = methanol extract; W = water extract; E = ethyl acetate extract. Positive controls = ampicillin (AMP; 2 µg) and erythromycin (ERTH; 10 µg). Negative control (NC) = water. Results are expressed as mean zones of inhibition of three independent replicates ± SEM (n = 3). * indicates that the results are significantly different to the negative control (p < 0.01).
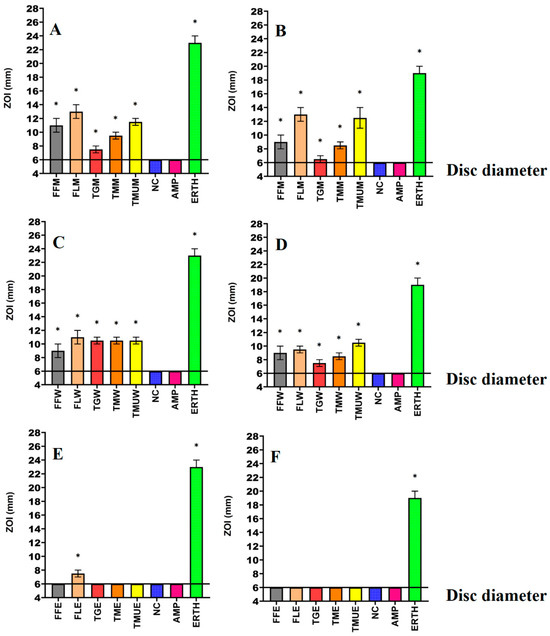
Figure 3.
Antimicrobial effects of the (A) methanol extract against S. aureus, (B) methanol extracts against MRSA, (C) water extracts against S. aureus, (D) water extracts against MRSA, (E) ethyl acetate extracts against S. aureus, (F) ethyl acetate extracts against MRSA. FF = Terminalia ferdinandiana fruit; FL = Terminalia ferdinandiana leaf; TG = Terminalia grandiflora; TM = Terminalia microcarpa, TMU= Terminalia muelleri; M = methanol extract; W = water extract; E = ethyl acetate extract. Positive controls = ampicillin (AMP; 2 µg) and erythromycin (ERTH; 10 µg). Negative control (NC) = water. Results are expressed as mean zones of inhibition of three independent replicates ± SEM (n = 3). * indicates that the results are significantly different to the negative control (p < 0.01).
3.2. Fractional Inhibitory Concentration
Methanol, water, and ethyl acetate extracts of T. ferdinandiana, T. grandiflora, T. microcarpa, and T. muelleri leaves, as well as T. ferdinandiana fruit, were combined with a range of common clinical antibiotics and tested to determine the effects of the extracts on antibiotic potency. Multiple classes of interactions were observed for combinations tested against antibiotic-sensitive and antibiotic-resistant strains of E. coli, S. aureus, and K. pneumoniae (Table 3). There were twelve synergistic interactions, fifty-eight combinations were determined to be additive, sixty non-interactive combinations were noted, and thirty combinations were antagonistic. As the antagonistic combinations have less efficacy compared to using either component separately, these combinations should be avoided against those bacteria.

Table 3.
∑ FIC values for interactions between plant extracts and antibiotics.
3.3. Synergistic Interaction of Extract Antibiotic at Different Ratios
Three synergistic interactions were noted against E. coli (Figure 4). Two further synergistic interactions were noted against S. aureus, as well as two against MRSA (Figure 5). Additionally, four synergistic interactions were noted against K. pneumoniae and one against ESBL K. pneumoniae (Figure 6). Therefore, these combinations were also tested at various ratios and graphed as isobolograms to identify the ratios that produced synergistic interactions.
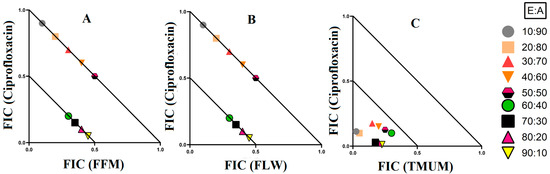
Figure 4.
Isobologram analysis of the E. coli against (A) FFM in combination with ciprofloxacin, (B) FLW in combination with ciprofloxacin, (C) TMUM in combination with ciprofloxacin. FF = Terminalia ferdinandiana fruit; FL = Terminalia ferdinandiana leaf; TMU = Terminalia muelleri leaf extracts. M = methanol; W = water. FIC values are displayed as the means of two independent repeats (n = 2). Ratio = % extract: % antibiotic. Values below the 0.5/0.5 line represent synergy; the segment between the 0.5/0.5 and 1/1 lines represents additive interactions. Only the synergistic and additive ratios are displayed in these graphs.
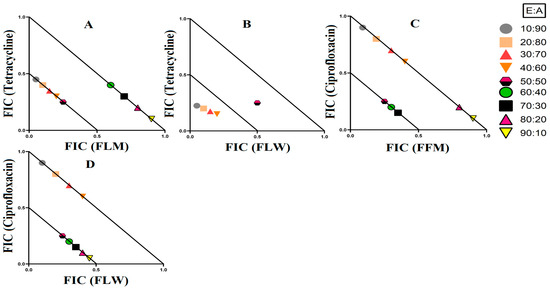
Figure 5.
Isobologram analysis of the S. aureus against (A) FLW in combination with tetracycline, (B) FLW in combination with tetracycline, as well as MRSA tested against (C) FFM in combination with ciprofloxacin, (D) FLW in combination with ciprofloxacin. FF = Terminalia ferdinandiana fruit; FL = Terminalia ferdinandiana leaf; M = methanol; W = water. FIC values are displayed as the means of two independent repeats (n = 2). Ratio = % extract: % antibiotic. Values below the 0.5/0.5 line represent synergy; the segment between the 0.5/0.5 and 1/1 lines represents additive interactions. Only the synergistic and additive ratios are displayed in these graphs.
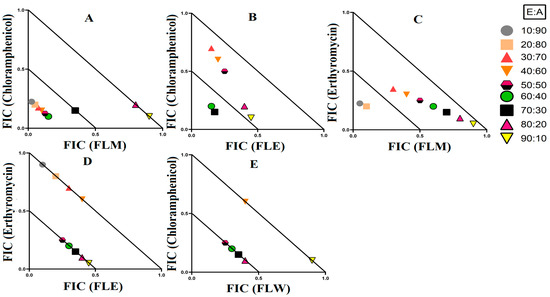
Figure 6.
Isobologram analysis of the K. pneumoniae against (A) FLM in combination with chloramphenicol, (B) FLE in combination with chloramphenicol, (C) FLM in combination with erythromycin, (D) FLE in combination with erythromycin, as well as isobologram of ESBL K. pneumonia against (E) FLW in combination with ciprofloxacin. FL = Terminalia ferdinandiana leaf; M = methanol; W = water; E = ethyl acetate. FIC values are displayed as the means of two independent repeats (n = 2). Ratio = % extract: % antibiotic. Values below the 0.5/0.5 line represent synergy; the segment between the 0.5/0.5 and 1/1 lines represents additive interactions. Only the synergistic and additive ratios are displayed in these graphs.
3.3.1. Extract and Antibiotic Synergistic Interactions against E. coli
There were three synergistic interactions noted against E. coli, all of which were in combinations containing ciprofloxacin as the antibiotic component. When T. ferdinandiana fruit methanol extracts were combined with ciprofloxacin, it exhibited synergistic inhibition in ratios containing 60–90% extract, while ratios containing 10–50% extracts produced additive effects (Figure 4A). The combination of T. ferdinandiana leaf water extract with ciprofloxacin produced synergistic interaction in ratios containing 60–90% extract and additive effects in ratios that were made up of 10–50% extracts (Figure 4B). Interestingly, the combination containing the T. muelleri methanol extract produced a synergistic effect at all ratios (10–90% extract; Figure 4C). Importantly, ratios with indifferent effects have no benefits compared to the use of individual components. Therefore, ratios producing indifferent effects are not shown in the isobolograms.
3.3.2. Extract and Antibiotic Synergistic Interactions against S. aureus and MRSA
Four extract and antibiotic combinations produced synergistic effects against the bacterial pairing of S. aureus and MRSA. Combinations containing either T. ferdinandiana leaf methanol extract or T. ferdinandiana water extract in combination with tetracycline produced synergistic effects against S. aureus. Terminalia ferdinandiana leaf methanol extract in combination with tetracycline produced synergy in ratios containing 10–50% extract (Figure 5A), whilst the combination of T. ferdinandiana leaf water extract and tetracycline yielded synergistic interactions in ratios containing 10–40% extract (Figure 5B). Two synergistic interactions were recorded against MRSA, both of which contained ciprofloxacin. Combining T. ferdinandiana fruit methanol extract with ciprofloxacin produced synergistic inhibition at ratios containing 50–70% extract (Figure 5C), while T. ferdinandiana leaf water extract in combination with ciprofloxacin induced synergy at ratios comprising 50–90% extract (Figure 5D).
3.3.3. Extract and Antibiotic Interactions against K. pneumoniae and ESBL K. pneumoniae
Four synergistic interactions were recorded against K. pneumoniae and one against ESBL K. pneumoniae. The combination of T. ferdinandiana leaf methanol extracts with chloramphenicol produced synergy against K. pneumoniae at ratios containing 10–60% extract (Figure 6A). However, only two ratios (containing 60% and 70% extract) were synergistic when T. ferdinandiana leaf ethyl acetate was combined with chloramphenicol and tested against K. pneumoniae (Figure 6B). Similarly, two ratios (10% and 20% extract) were synergistically active against K. pneumoniae when T. ferdinandiana leaf methanol extracts were combined with erythromycin (Figure 6C). Terminalia ferdinandiana leaf ethyl acetate extract produced synergy in combination with erythromycin against K. pneumoniae at ratios comprising 50–90% extract (Figure 6D). Synergistic interactions against ESBL K. pneumoniae were recorded at ratios containing 50–80% T. ferdinandiana leaf water extract combined with chloramphenicol (Figure 6E).
3.4. Identification of Compounds in the FLM and FLW Extracts
The aqueous and methanolic T. ferdinandiana leaf extracts showed the greatest antibacterial activity in the disc diffusion assays, as well as in the liquid dilution assays. Therefore, these extracts were subjected to phytochemical separation and identification studies. Optimized HPLC-MS parameters that were previously established by our group for [15] were employed to develop a metabolomic fingerprint of the aqueous and methanolic T. ferdinandiana leaf extracts, focusing on the flavonoids and tannins. Total compound chromatograms (TCCs) recorded in the positive ionization mode are shown in Figure 7A and Figure 7B for T. ferdinandiana leaf methanol and water extracts, respectively.
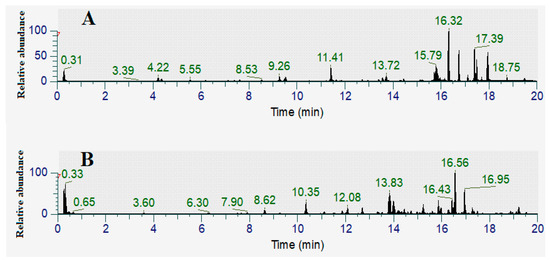
Figure 7.
Total compound LC-MS chromatograms in positive ionization mode of (A) FLM (Terminalia ferdinandiana leaf methanol extract), (B) FLW (Terminalia ferdinandiana leaf water extract).
In this study, we focused on the identification of flavonoids and tannins. Flavonoids are well known as an antimicrobial agent and with an increasing incidence of highly antibiotic-resistant infections, flavonoids have potential to be substituted for antibiotics [16]. Similarly, studies have also reported the use of extracts rich in tannins for treating ailments such as bacterial infections [17]. A range of flavonoids and tannins were identified in this study (Table 4). Flavonoids identified in the T. ferdinandiana leaf methanol extracts include vitexin, robinetin, quercitin-3β-D-glucoside, quercetin 3-O-rhamnoside-7-O-glucoside, orientin, hispidulin 7-glucuronide, hibiscetin 3-glucoside, 6-hydroxyluteolin 6-glucuronide, 4-(3,4-dihydroxyphenyl)-7-hydroxy-5-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2H-chromen-2-one, 3,7-dihydroxy-4,5-dimethoxy-8-prenylflavan 7-O-beta-D-glucopyranoside, 4-(3,4-dihydroxyphenyl)-7-hydroxy-5-{[2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl]-2H-chromen-2-one, 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-8-{[(2R,3R,4S,5S,6R)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one, 1,5-Anhydro-1-[5,7-dihydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-oxo-4H-chromen-8-yl] hexitol, and (1ξ)-1,5-Anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-D-galactitol. Orientin and 1,5-Anhydro-1-[5,7-dihydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl] hexitol were also identified in the T. ferdinandiana leaf water extract, in addition to vitexin 2″-p-hydroxybenzoate, 5,7,2′,5′-tetrahydroxy-6-methoxyflavanone, and (2S,3R,4R,5S,6S)-2-{[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl]oxy}-3,5-dihydroxy-6-methyloxan-4-yl 3,4,5-trihydroxy-benzoate. In contrast, the tannin ellagic acid was identified in both the T. ferdinandiana leaf methanol and water extracts, whilst pyrogallol was only identified in the water extract.

Table 4.
Qualitative analysis of LC-MS of FLM and FLW.
3.5. Quantification of Toxicity
The toxicity of the extracts was determined using ALA toxicity assays. Extract dilutions that produce ≤50% mortality were deemed to be non-toxic at those concentrations. With the exception of the T. ferdinandiana fruit methanol, T. ferdinandiana fruit water, T. ferdinandiana leaf ethyl acetate, and T. ferdinandiana leaf water extracts, all other extracts were non-toxic at 1000 µg/mL. The toxic extracts were further diluted to allow for the determination of the LC50. The LC50 for T. ferdinandiana fruit methanol, T. ferdinandiana fruit water, and T. ferdinandiana leaf water was calculated to be 250 µg/mL, whilst the LC50 of T. ferdinandiana leaf ethyl acetate was 500 µg/mL. These extracts were therefore classified as toxic. The potassium dichromate positive control (2 mg/mL) induced 100% mortality following 24 h of exposure, whilst the seawater negative control induced 0% mortality.
4. Discussion
Solvent extraction is commonly used for the isolation of phytoconstituents present in plant materials [18]. The yield of the extracts and the resulting bioactive compounds present in the plant extracts are strongly dependent on the nature of the extraction solvent, due to the presence of compounds of varied chemical characteristics that may or may not be soluble in a particular solvent. The methanolic and aqueous extracts tested in this study inhibited all of the bacterial strains tested including the antibiotic-resistant strains in the disc diffusion susceptibility assay, (Figure 1, Figure 2 and Figure 3) as well as in the liquid dilution assay (Table 2). In contrast, the ethyl acetate extracts prepared from all plant materials completely lacked inhibitory activity in the liquid dilution assay, except the FLE extract, which was highly effective against ESBL E. coli (37.5 µg/mL), K. pneumoniae (75 µg/mL), and ESBL K. pneumoniae (75 µg/mL). These differences may be due to the phytochemical components extracted from the leaves and fruit of plants using different solvents, with varying polarities. Higher-polarity solvents generally extract a wider variety of phytochemicals and are in greater abundance compared to solvents that are lower in polarity [19]. Additionally, phytochemicals that are larger in molecular size and/or lower in polarity may not easily diffuse through agar gels, potentially affecting the observed antibacterial efficacy of the extracts in solid-phase assays such as the disc diffusion assay [19]. The compositional differences between the extracts may explain the variation in antimicrobial activity between the solid and liquid phase assays and between the different solvent extracts. Additionally, volatile phytochemicals evaporate from the surface of agar gels, thereby decreasing their concentration in the assay and therefore decreasing the observed effectiveness [20]. Polar compounds are generally more soluble in aqueous solutions and, as a result, diffuse more rapidly through agar gels. In contrast, less water-soluble compounds may diffuse less rapidly and concentrate around the disc, which may result in underestimating the MIC values of the extracts using disc diffusion methods [21]. Broth microdilution assays are less susceptible to the impact of the compound size and polarity and are generally deemed to be better than the disc diffusion assay for quantifying antibacterial activity.
We were unable to find previous studies that examined the antimicrobial activity of T. grandiflora and T. microcarpa against MRSA and ESBL antibiotic-resistant pathogens. Interestingly, the T. grandiflora water extract (TGW) had moderate activity against E. coli and ESBL E. coli (1600 µg/mL), whilst the T. muelleri methanol extract also had moderate growth inhibitory activity against E. coli (1300 µg/mL) and ESBL E. coli (2600 µg/mL) (Table 2). Notably, a previous study reported the antibacterial activity for an ethyl acetate extract of T. muelleri against E. coli and S. aureus using disc diffusion assays [22]. However, that study used a fractionated ethyl acetate extract, which was tested at a single high concentration (10 mg/mL). That study did not determine MIC values, and therefore it is not possible to compare the activity with other studies. Notably, the Terminalia muelleri ethyl acetate extract tested in our study lacked antibacterial activity in the disc diffusion assay. However, our study tested crude extract (3.6 mg/mL), without the fractionation and concentration steps reported in the earlier study. Therefore, the ethyl acetate extract tested in our study was of a substantially lower concentration than in the previous study. In contrast, the Terminalia muelleri aqueous and methanolic extracts tested herein inhibited the growth of both antibiotic-sensitive and antibiotic-resistant strains in the liquid dilution assays, with MICs as low as 1375 µg/mL against S. aureus (Table 2).
Previous studies have reported antimicrobial activity for T. ferdinandiana fruit methanolic extracts, as well as the aqueous, methanolic, and ethyl acetate leaf extracts of this species [14]. Terminalia ferdinandiana fruit and leaf extracts inhibited bacterial growth in our study (Table 2). The ethyl acetate leaf extract was particularly potent against ESBL E. coli (37.5 µg/mL), as well as the antibiotic-sensitive and antibiotic-resistant strains of K. pneumoniae (75 µg/mL). The plant extracts in our study may function via a mechanism(s) that is/are distinct from those of the β-lactam antibiotics to which these strains have developed resistance. Alternatively, the phytochemical constituents present in these extracts may block bacterial antibiotic-resistance mechanisms, allowing the inhibitory components to function with increased potency. This is encouraging, as the resistant bacterial strains investigated in our study have substantially reduced susceptibilities to antibiotics from diverse classes, including aminoglycosides, β-lactams, fluoroquinolones, macrolides, sulfonamides, and tetracycline. Additionally, these extracts were effective against both Gram-positive and Gram-negative pathogens, highlighting their potential for broad-spectrum antibiotic therapy. Future studies to screen these extracts against a more comprehensive range of bacteria, including further MDR strains, are planned to comprehensively assess their potential as antibiotic therapy components.
Synergistic combinations containing plant extracts or pure compounds are an emerging area of interest for medical research to combat bacteria that are resistant to conventional antibiotics [23]. In this study, we observed twelve synergistic, fifty-eight additive, sixty non-interactive, and thirty antagonistic interactions. Synergistic combinations significantly increase the antimicrobial effectiveness of the antibiotics compared to additive interactions and therefore have substantial potential for the development of novel and effective antibiotic therapies. Synergistic interactions were observed when T. ferdinandiana fruit methanol, T. ferdinandiana leaf water, and T. muelleri methanol extracts were combined with ciprofloxacin and tested against E. coli (Figure 4). Synergy was also observed when T. ferdinandiana leaf methanol and T. ferdinandiana leaf water extracts were combined with tetracycline and tested against S. aureus, whilst combining T. ferdinandiana fruit methanol and T. ferdinandiana leaf water with ciprofloxacin produced synergy against MRSA (Figure 5). Combining T. ferdinandiana leaf methanol and T. ferdinandiana leaf ethyl acetate with chloramphenicol or combining T. ferdinandiana leaf methanol and T. ferdinandiana leaf ethyl acetate with erythromycin results in synergistic inhibition of K. pneumoniae. Additionally, the combination of T. ferdinandiana leaf water and chloramphenicol was synergistic against ESBL K. pneumoniae (Figure 6). The plant extracts tested in this study may contain components that block the resistance mechanism of ciprofloxacin, tetracycline, chloramphenicol, and erythromycin, although this needs to be verified in future studies. In contrast, non-interactive combinations neither increase nor decrease the antimicrobial effects compared to the extract or antibiotic when tested alone, indicating that they are safe for simultaneous use, despite providing no additional benefits over using individual components alone. Antagonistic interactions reduce the antimicrobial activity of combinations and hence should be avoided. Notably, all the extracts except T. ferdinandiana fruit methanol, T. ferdinandiana fruit water, T. ferdinandiana leaf ethyl acetate, and T. ferdinandiana leaf water were determined to be non-toxic in the ALA toxicity assay.
Phytochemical evaluations of several Terminalia spp. in previous studies have highlighted several interesting compounds, including tannins such as chebulic acid, ellagic acid, and gallic acid, as well as multiple flavonoids [24]. Several tannins and flavonoids were also identified in our study in the aqueous and methanolic T. ferdinandiana leaf extracts (Table 4). In particular, the flavonoids vitexin (Figure 8A), robinetin (Figure 8B), quercitin-3β-D-glucoside (Figure 8C), quercetin 3-O-rhamnoside-7-O-glucoside (Figure 8D), orientin (Figure 8E), hispidulin 7-glucuronide (Figure 8F), and (1ξ)-1,5-anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-D-galactitol (Figure 8G), as well as the tannins ellagic acid (7H) and pyrogallol (7I), were identified herein. Interestingly, flavonoids produce antimicrobial activities via several mechanisms, including alterations in cytoplasmic membrane function, suppressing nucleic acid synthesis, and via modulation of energy metabolism [16,25,26,27]. Flavonoids also reduce biofilm formation and adhesion, membrane permeability, and the number of porins in the cell membrane, as well as pathogenicity, which all are important factors for bacterial growth and survival [16,26,27]. Interestingly, flavonoids can also reverse antibiotic resistance in some bacteria and may also improve the effectiveness of other antibiotic components [26,27]. Hence, flavonoid-based medications may have potential for the treatment of antibiotic-resistant infections.
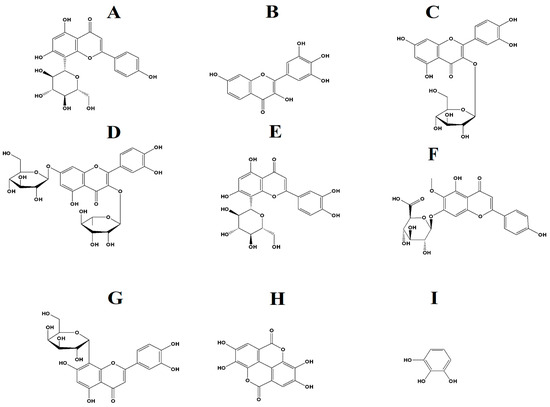
Figure 8.
(A) vitexin, (B) robinetin, (C) quercitin-3β-D-glucoside, (D) quercetin 3-O-rhamnoside-7-O-glucoside, (E) orientin, (F) hispidulin 7-glucuronide, (G) (1ξ)-1,5-Anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-D-galactitol, (H) ellagic acid, (I) pyrogallol.
Two flavonoids, kaempferol 3-O-α-l-(2″-Z-p-coumaroyl-4‴-E-p-coumaroyl)-rhamnoside and kaempferol 3-O-α-l-(2‴,4‴-di-E-p-coumaroyl)-rhamnoside, which was isolated from Laurus nobilis L. leaves, have been shown to possess anti-MRSA activity [28]. Both compounds out-performed conventional antibiotics, including ciprofloxacin, erythromycin, norfloxacin, oxacillin, and tetracycline, against MRSA, with MIC values of 0.5–2.0 µg/mL [28]. Flavonoids isolated from a Paulownia tomentosa Steud. fruit ethanolic extract also possess antimicrobial activity and inhibit the growth of Bacillus cereus, Bacillus subtilis, Enterococcus faecalis, Listeria monocytogenes, S. aureus, and Staphylococcus epidermidis [29]. Flavonoids may exert antimicrobial activity by inhibiting the activity of the enzyme DNA gyrase, which is required to relieve torsional strain in the double-stranded DNA helix during bacterial replication [30]. The flavonoids quercetin and hispidulin inhibit the activity of Mycobacterium tuberculosis (MIC = 50 µg/mL and MIC = 100 µg/mL, respectively) [31]. Notably, the phytochemical analysis reported in our study identified quercetin derivatives (quercetin-3β-D-glucoside; quercetin 3-O-rhamnoside-7-O-glucoside), as well as a hispidulin glycoside (hispidulin 7-glucuronide) (Table 4). These compounds may contribute to the antibacterial activity of these extracts, although this needs to be verified.
Tannins also possess an antimicrobial activity as they can pass through the cell wall of the bacteria and interfere with the cell metabolism, thereby causing their destruction [17]. Many chronic and persistent bacterial infections are linked to the formation of biofilms, with more than 60% of all microbial infections involving the formation of biofilms [32]. Biofilm forming bacteria are often difficult to treat as they can tolerate immune defenses, biocides, antibiotics, and hydrodynamic shear forces [33]. Ellagic acid, also identified in this study (Table 4), inhibits the biofilm formation of E. coli by 22–26% [33]. Furthermore, the combination of ellagic acid and thioridazine (an efflux pump inhibitor) can reduce the formation of biofilm by E. coli by between 73% and 89% [33]. Pyrogallol, also identified in our study (Table 4), has also previously been reported to have antibacterial activity, with an MIC in the range of 32–64 µg/mL against Vibrio parahaemolyticus [34]. Additionally, 5 mM and 10 mM concentrations of pyrogallol were reported to inhibit the growth of Pseudomonas pyocyanea, Pseudomonas putida, and Corynebacterium xerosis in disc diffusion assays [35]. However, the antimicrobial activity of pyrogallol against MRSA and ESBL-producing bacterial strains has not yet been verified.
The flavonoids and tannins identified in this study may possess inherent antimicrobial activities by themselves, and/or they may potentiate the activity of other phytochemicals present in the extract. However, further studies are required to examine their effects and their antimicrobial mechanisms. Studies should also explore the potential of these compounds for new antibiotic therapies or as potentiators of current antibiotics. The extracts tested herein were effective against both the antibiotic-sensitive strains and their antibiotic-resistant counterparts, a desirable trait for new and effective antibiotics. Notably, some compounds were unable to be identified using LC-MS metabolomic profiling, and these compounds may contribute to the antibacterial activity noted in our study (either directly or as potentiators). Furthermore, volatile and low-polarity compounds may also have evaporated during the assay and/or extraction process. Hence, further phytochemical studies should utilize different methods such as GC-MS for a complete evaluation of the extract composition. Artemia nauplii toxicity assays were used to evaluate the toxicity of the extracts, with the majority of the extracts determined to be non-toxic. However, further toxicity evaluations of the extracts against an extensive panel of human cell lines are needed to confirm their low toxicity and hence their safety for medicinal use.
5. Conclusions
The urgent need to address antimicrobial resistance has led to a significant increase in the number of studies testing natural products and extracts as potential sources to develop new antibiotics or to increase the efficacy of current clinical antibiotics. The plant extracts examined in our study inhibited ESBL and MRSA bacterial growth as effectively as they inhibited the antibiotic-susceptible strains. This indicates that the phytochemicals present in these extracts may have novel and/or uncharacterized antibacterial mechanisms. Future studies should investigate the antimicrobial properties of these compounds and their ability to potentiate the activity of conventional antibiotics.
Author Contributions
Conceptualization: M.J.Z., M.J.C. and I.E.C.; methodology: M.J.Z., M.J.C. and I.E.C.; validation, formal analysis, data curation: M.J.Z.; writing—original draft preparation: M.J.Z.; writing—review and editing, supervision: M.J.C. and I.E.C.; project administration and funding acquisition: I.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We are thankful to David Boehme from Wild Harvest Northern Territory for supplying T. ferdinandiana fruit and leaves and to Phurpha Wangchuk from James Cook University, Australia, for supplying T. grandiflora, T. microcarpa, and T. muelleri.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Brown, D. Antibiotic resistance breakers: Can repurposed drugs fill the antibiotic discovery void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef]
- Rana, R.; Sharma, R.; Kumar, A. Repurposing of existing statin drugs for treatment of microbial infections: How much promising? Infect. Disord.-Drug Targets 2019, 19, 224–237. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, B.S.F.; Khameneh, B.; Ostad, M.R.Z.; Hosseinzadeh, H. In vitro evaluation of antibacterial activity of verbascoside, lemon verbena extract and caffeine in combination with gentamicin against drug-resistant Staphylococcus aureus and Escherichia coli clinical isolates. Avicenna J. Phytomed. 2018, 8, 246. [Google Scholar]
- McGaw, L.; Rabe, T.; Sparg, S.; Jäger, A.; Eloff, J.; Van Staden, J. An investigation on the biological activity of Combretum species. J. Ethnopharmacol. 2001, 75, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.; Katerere, D.; McGaw, L. The biological activity and chemistry of the southern African Combretaceae. J. Ethnopharmacol. 2008, 119, 686–699. [Google Scholar] [CrossRef]
- Garcez, F.R.; Garcez, W.S.; Miguel, D.L.; Serea, A.A.; Prado, F.C. Chemical constituents from Terminalia glabrescens. J. Braz. Chem. Soc. 2003, 14, 461–465. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Woods, B.E. A Study of the Intra-Specific Variations and Commercial Potential of Terminalia ferdinandiana (Exell) (the Kakadu Plum). Master’s Thesis, Charles Darwin University, Casuarina, Australia, 1995. [Google Scholar]
- Cheesman, M.J.; White, A.; Matthews, B.; Cock, I.E. Terminalia ferdinandiana fruit and leaf extracts inhibit methicillin-resistant Staphylococcus aureus growth. Planta Med. 2019, 85, 1253–1262. [Google Scholar] [CrossRef]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Terminalia petiolaris A. Cunn ex Benth. extracts have antibacterial activity and potentiate conventional antibiotics against β-lactam-drug-resistant bacteria. Antibiotics 2023, 12, 1643. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Eloff, J. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies—Methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Anam, K.; Suganda, A.; Sukandar, E.; Kardono, L. Antibacterial agents of Terminalia muelleri Benth. leaves. Res. J. Med. Plant 2010, 4, 197–205. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57. [Google Scholar] [PubMed]
- Fahmy, N.; Al-Sayed, E.; Singab, A. Genus Terminalia: A phytochemical and biological review. Med. Aromat. Plants 2015, 4, 1–22. [Google Scholar]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Otsuka, N.; Liu, M.-H.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Anti-methicillin resistant Staphylococcus aureus (MRSA) compounds isolated from Laurus nobilis. Biol. Pharm. Bull. 2008, 31, 1794–1797. [Google Scholar] [CrossRef]
- Šmejkal, K.; Chudík, S.; Kloucek, P.; Marek, R.; Cvacka, J.; Urbanová, M.; Julínek, O.; Kokoška, L.; Šlapetová, T.; Holubová, P. Antibacterial C-geranylflavonoids from Paulownia tomentosa fruits. J. Nat. Prod. 2008, 71, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure–activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Thakur, J.; Prakash, O.; Khan, F.; Saikia, D.; Gupta, M.M. Screening of flavonoids for antitubercular activity and their structure–activity relationships. Med. Chem. Res. 2013, 22, 2706–2716. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Hancock, V.; Dahl, M.; Vejborg, R.M.; Klemm, P. Dietary plant components ellagic acid and tannic acid inhibit Escherichia coli biofilm formation. J. Med. Microbiol. 2010, 59, 496–498. [Google Scholar] [CrossRef]
- Tinh, T.H.; Nuidate, T.; Vuddhakul, V.; Rodkhum, C. Antibacterial activity of pyrogallol, a polyphenol compound against Vibrio parahaemolyticus isolated from the central region of Thailand. Procedia Chem. 2016, 18, 162–168. [Google Scholar] [CrossRef]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforschung C 2006, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).