Progress in Research on the Gut Microflora of the Red Panda (Ailurus fulgens)
Abstract
1. Introduction
2. Methodology Used in Research on the Gut Microflora of the Red Panda
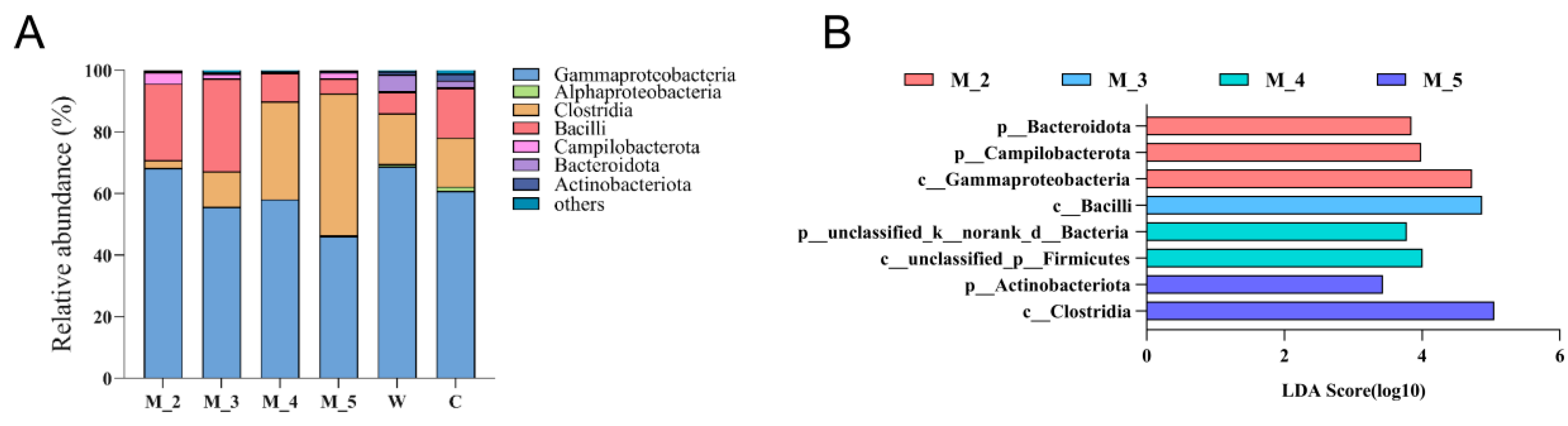
3. Composition of the Red Panda Gut Microflora
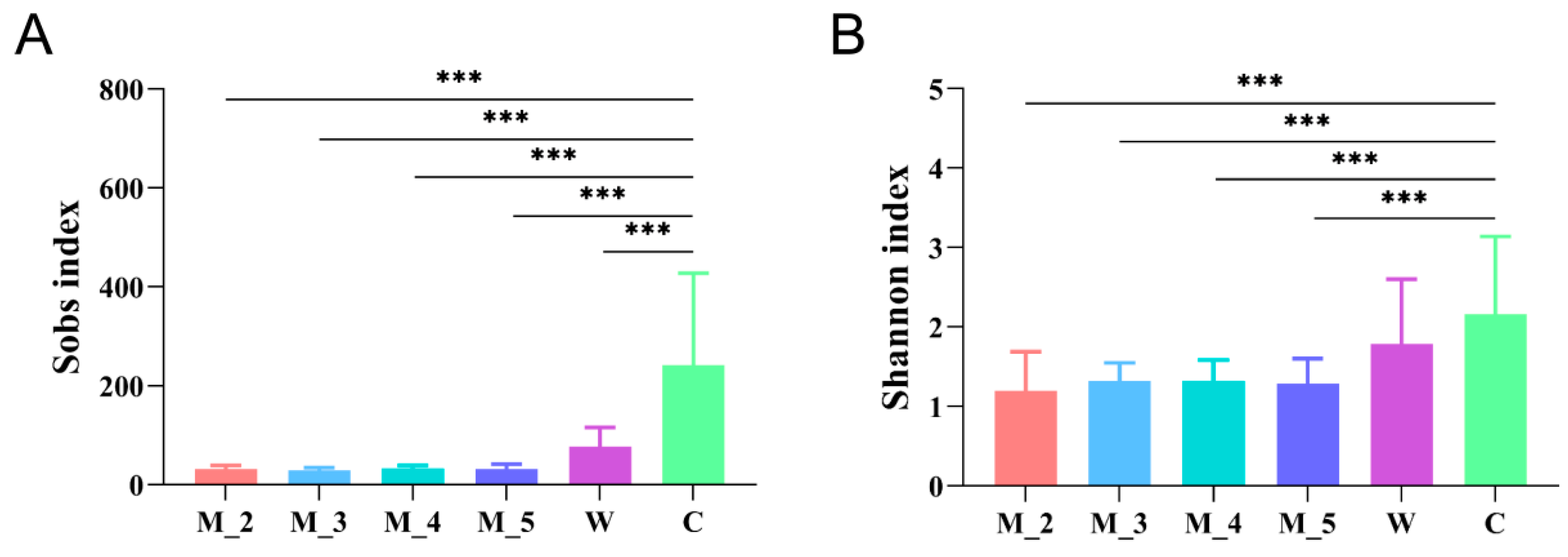
4. Alpha Diversity of Red Panda Gut Microflora
5. Beta Diversity of the Red Panda Gut Microflora
6. Functions of the Red Panda Gut Microflora
7. Pathogenic Microflora in the Red Panda Gut
8. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Hooper, L.; Macpherson, A.; Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef]
- Relman, D.A. The human microbiome: Ecosystem resilience and health. Nutr. Rev. 2012, 70 (Suppl. 1), S2–S9. [Google Scholar] [CrossRef]
- Russell, D.A.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Metabolic activities and probiotic potential of bifidobacteria. Int. J. Food. Microbiol. 2011, 149, 88–105. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell. Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; González, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet Drives Convergence in Gut Microbiome Functions Across Mammalian Phylogeny and Within Humans. Science 2011, 332, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, A.; Kiliś-Pstrusińska, K. Gut Microbiota Alterations and Primary Glomerulonephritis in Children: A Review. Int. J. Mol. Sci. 2023, 24, 574. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Van Belle, S.; Di Fiore, A.; Estrada, A.; Stumpf, R.; White, B.; Nelson, K.E.; Knight, R.; Leigh, S.R. Patterns in Gut Microbiota Similarity Associated with Degree of Sociality among Sex Classes of a Neotropical Primate. Microb. Ecol. 2017, 74, 250–258. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Ding, Y.; Hu, Y.; Nie, Y.; Wei, W.; Ma, S.; Yan, L.; Zhu, L.; Wei, F. Seasonal variation in nutrient utilization shapes gut microbiome structure and function in wild giant pandas. Proc. R. Soc. Lond. B Biol. Sci. 2017, 284, 20170955. [Google Scholar] [CrossRef]
- Stumpf, R.M.; Gomez, A.; Amato, K.R.; Yeoman, C.J.; Polk, J.D.; Wilson, B.A.; Nelson, K.E.; White, B.A.; Leigh, S.R. Microbiomes, metagenomics, and primate conservation: New strategies, tools, and applications. Biol. Conserv. 2016, 199, 56–66. [Google Scholar] [CrossRef]
- Wiebler, J.M.; Kohl, K.D.; Lee, R.E.; Costanzo, J.P. Urea hydrolysis by gut bacteria in a hibernating frog: Evidence for urea-nitrogen recycling in Amphibia. Proc. R. Soc. Lond. B Biol. Sci. 2018, 285, 20180241. [Google Scholar] [CrossRef]
- Guo, W.; Mishra, S.; Zhao, J.; Tang, J.; Zeng, B.; Kong, F.; Ning, R.; Li, M.; Zhang, H.; Zeng, Y.; et al. Metagenomic Study Suggests That the Gut Microbiota of the Giant Panda (Ailuropoda melanoleuca) May Not Be Specialized for Fiber Fermentation. Front. Microbiol. 2018, 9, 229. [Google Scholar] [CrossRef]
- Kartzinel, T.R.; Hsing, J.C.; Musili, P.M.; Brown, B.R.P.; Pringle, R.M. Covariation of diet and gut microbiome in African megafauna. Proc. Natl. Acad. Sci. USA 2019, 116, 23588–23593. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, Z.; Yao, R.; Xu, L.; Chen, H.; Gu, X.; Wu, T.; Yang, X. Potential Mechanism of Detoxification of Cyanide Compounds by Gut Microbiomes of Bamboo-Eating Pandas. mSphere 2018, 3, 00229-18. [Google Scholar] [CrossRef]
- Demeyer, D.; Van Nevel, C. Influence of substrate and microbial interaction on efficiency of rumen microbial growth. Reprod. Nutr. Dev. 1986, 261B, 161–179. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Wang, G.; Zhang, Q.; Guan, D. research progress of gut microflora of leeches. Anhui Agric. Sci. 2015, 43, 164–166. (In Chinese) [Google Scholar] [CrossRef]
- Dou, H.; Guo, W.; Wang, T.; Li, L. Progress in research methods of intestinal flora. Chin. J. Microecol. 2014, 1, 119–121. (In Chinese) [Google Scholar] [CrossRef]
- Liu, H. Applied Microbial Molecular Ecology Method to Study the Bacterial Composition and its Variation Law in Shrimp Intestines. Ph.D. Thesis, Chinese Academy of Sciences (Institute of Oceanography), Qingdao, China, 2010. (In Chinese). [Google Scholar]
- Wang, Y.; Liu, Y.; Chen, S.; Liu, H.; Zhang, X. Isolation and Identification of a Pasteurella multocida. Chin. J. Vet. Med. 2014, 50, 68–69. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Zhang, Z.; Dong, H.; Li, Z. Research progress of shrimp intestinal microflora. South. China Fish. Sci. 2015, 11, 114–119. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, R.; Chen, M. Advances in research methods of intestinal microbiome. J. Biomed. Eng 2015, 32, 1150–1154. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Sun, X.; Ni, J. overview of research methods of termite gut microflora. Biot. Resour. 2017, 4, 247–255. (In Chinese) [Google Scholar] [CrossRef]
- Crowe, O.; Williams, C.; Reaves, P.; Dill-McFarland, K.; Kouba, A.; Willard, S.; Sparks, D.; Suen, G.; Brown, A. Gastrointestinal Microbiome of the Vulnerable Red Panda; American Society for Microbiology-Southeastern Branch: New Orleans, LA, USA, 2013. [Google Scholar]
- Williams, C.L.; Sparks, D.L.; Kouba, A.J.; Willard, S.T.; Dill-Mcfarland, K. Giant and red pandas utilize distinct microbial communities for their bamboo diet degradation. In Proceedings of the 248th American Chemical Society, San Francisco, CA, USA, 12–14 August 2014. [Google Scholar]
- Kong, F.; Zhao, J.; Han, S.; Zeng, B.; Yang, J.; Si, X.; Yang, B.; Yang, M.; Xu, H.; Li, Y. Characterization of the Gut Microbiota in the Red Panda (Ailurus fulgens). PLoS ONE 2014, 9, e87885. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, X.; Hu, Y.; Wu, Q.; Nie, Y.; Dong, J.; Ding, Y.; Yan, L.; Wei, F. Diet drives convergent evolution of gut microbiomes in bamboo-eating species. Sci. China. Life. Sci. 2021, 64, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, W.; Han, S.; Kong, F.; Wang, C.; Li, D.; Zhang, H.; Yang, M.; Xu, H.; Zeng, B.; et al. The evolution of the gut microbiota in the giant and the red pandas. Sci. Rep. 2015, 5, 10185. [Google Scholar] [CrossRef]
- McKenney, E.A.; Maslanka, M.; Rodrigo, A.; Yoder, A.D. Bamboo Specialists from Two Mammalian Orders (Primates, Carnivora) Share a High Number of Low-Abundance Gut Microbes. Microb. Ecol. 2018, 76, 272–284. [Google Scholar] [CrossRef]
- Williams, C.L.; Dill-McFarland, K.A.; Sparks, D.L.; Kouba, A.J.; Willard, S.T.; Suen, G.; Brown, A.E. Dietary changes during weaning shape the gut microbiota of red pandas (Ailurus fulgens). Conserv. Physiol. 2018, 6, cox075. [Google Scholar] [CrossRef]
- Long, J. Seasonal Changes of Gut Microorganisms in Captive Giant and Red Pandas (Ailurus styani) and Their Relationship with Leaf-Peripheral Microorganisms. Master’s Thesis, China West Normal University, Nanchong, China, 2022. (In Chinese). [Google Scholar]
- Kang, L. Effects of Seasonal Changes in Food Composition on Gut Microorganisms in Wild Red Pandas (Ailurus styani). Master’s Thesis, China West Normal University, Nanchong, China, 2023. (In Chinese). [Google Scholar]
- Wang, J. Changes in Intestinal Microbial Diversity and Function in the Red Panda (Ailurus styani) during Growth and Development. Master’s Thesis, China West Normal University, Nanchong, China, 2023. (In Chinese). [Google Scholar]
- Zeng, Y.; Deng, J.B.; Niu, L.L.; Pu, Y.; Li, Y.; Hook, K.; Zeng, D.; Ni, X.Q. Analysis of gastrointestinal flora of red panda (Ailurus styani) using real-time fluorescence quantitative PCR. J. Hunan Agric. Univ. 2018, 44, 638–644. (In Chinese) [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell. Host. Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Dis. Primers 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Q.; Dai, J.; Zhang, S.; Wei, F. Evidence of cellulose metabolism by the giant panda gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 17714–17719. [Google Scholar] [CrossRef]
- Patterson-Kane, J.C.; Gibbons, L.M.; Jefferies, R.; Morgan, E.R.; Wenzlow, N.; Redrobe, S.P. Pneumonia from Angiostrongylus Vasorum Infection in a Red Panda (Ailurus fulgens). J. Vet. Diagn. Invest. 2009, 21, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Socolar, J.B.; Gilroy, J.J.; Kunin, W.E.; Edwards, D.P. How Should Beta-Diversity Inform Biodiversity Conservation? Trends. Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef]
- Shi, Y.; Kellingray, L.; Le Gall, G.; Zhao, J.; Zhang, H.; Narbad, A.; Zhai, Q.; Chen, W. The divergent restoration effects of Lactobacillus strains in antibiotic-induced dysbiosis. J. Funct. Foods 2018, 51, 142–152. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Q.; Ma, S.; Ma, T.; Shan, L.; Wang, X.; Nie, Y.; Ning, Z.; Yan, L.; Xiu, Y.; et al. Comparative genomics reveals convergent evolution between the bamboo-eating giant and red pandas. Proc. Natl. Acad. Sci. USA 2017, 114, 1081–1086. [Google Scholar] [CrossRef]
- Thapa, A.; Hu, Y.; Wei, F. The endangered red panda (Ailurus fulgens): Ecology and conservation approaches across the entire range. Biol. Conserv. 2018, 220, 112–121. [Google Scholar] [CrossRef]
- Xia, W.; Liu, G.; Wang, D.; Chen, H.; Zhu, L.; Li, D. Functional convergence of Yunnan snub-nosed monkey and bamboo-eating panda gut microbiomes revealing the driving by dietary flexibility on mammal gut microbiome. Comput. Struct. Biotechnol. J. 2022, 20, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, F.W.; Hu, J.C.; Feng, J.J.; Yang, G. A preliminary study on habitat selection by the red panda (Ailurus styani) in Mabian. J. Vet. Sci. 1998, 1, 16–21. (In Chinese) [Google Scholar] [CrossRef]
- Lama, B. Status and distribution of Red Panda (Ailurus fulgens) in Simsime community forest of Papung VDC of Taplejung district, Nepal. Nepal. Banko 2019, 29, 25–32. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Zhang, D.; Su, X.; Yue, C.; Ayala, J.E.; Yan, X.; Hou, R.; Li, L.; Xie, Y.; et al. Mortality analysis of captive red panda cubs within Chengdu, China. BMC. Vet. Res. 2022, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wen, J.; Gong, Y.; Wang, C.; Deng, L.; Huang, J.; Ren, L.; Yan, Q. Examination and drug sensitivity test for Klebsiella pneumoniae infection in red pandas (Ailurus styani). Chin. J. Zoonoses 2017, 33, 271–275. (In Chinese) [Google Scholar] [CrossRef]
- Xu, S. Isolation and characterization of the Fuzhou strain of canine microvirus from the red panda (Ailurus styani). Adv. Anim. Med. 2016, 37, 124–127. (In Chinese) [Google Scholar] [CrossRef]
- Geng, Y.; Shen, F.; Wu, W.; Zhang, L.; Luo, L.; Fan, Z.; Hou, R.; Yue, B.; Zhang, X. First demonstration of giant panda’s immune response to canine distemper vaccine. Dev. Comp. Immunol. 2020, 102, 103489. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef]
- Wei, F.; Wang, X.; Wu, Q. The giant panda gut microbiome. Trends. Microbiol. 2015, 23, 450–452. [Google Scholar] [CrossRef]
- Huang, G.; Qi, D.; Yang, Z.; Hou, R.; Shi, W.; Zhao, F.; Li, Z.; Yan, L.; Wei, F. Gut microbiome as a key monitoring indicator for reintroductions of captive animals. Conserv. Biol. 2024, 38, e14173. [Google Scholar] [CrossRef]

| Manufacturer/ Sequencing Platform | Objective Sequences | Captive/Wild | Sample Number: Captive/Wild | References |
|---|---|---|---|---|
| Roche/454 GS Junior | Hypervariable regions of the bacterial 16S rRNA gene | Captive | 4 | Crowe et al. (2013) [27] |
| Captive | 2 | Williams et al. (2014) [28] | ||
| Roche/454 GS-FLX | V1–V3 hypervariable regions of the bacterial 16S rRNA gene | Captive/Wild | 16/6 | Kong et al. (2014) [29] |
| Captive | 6 | Li et al. (2015) [31] | ||
| Captive/Wild | 4/4 | Huang et al. (2020) [30] | ||
| Thermo Fisher/Ion PGM | V2, V3, V4, V6, V8 and V9 hypervariable regions of the bacterial 16S rRNA gene | Captive | 2 | McKenney et al. (2018) [32] |
| Illumina/MiSeq | V3–V4 hypervariable regions of the bacterial 16S rRNA gene | Captive | 15 | Williams et al. (2018) [33] |
| Illumina/Miseq PE300 | V3–V4 hypervariable regions of the bacterial 16S rRNA gene | Captive | 116 | Long et al. (2022) [34] |
| Captive/Wild | 157/16 | Wang (2023) [36] | ||
| V1–V3 hypervariable regions of the bacterial 16S rRNA gene | Wild | 103 | Kang (2023) [35] | |
| Illumina/HiSeq 2500 | V4 hypervariable region of the bacterial 16S rRNA gene | Captive | 1 (stomach, duodenum, jejunum, ileum, colon and rectum of one dead individual), 1 (faecal sample) | Zeng et al. (2018) [37] |
| Illumina/HiSeq 2000 | Metagenome | Captive/Wild | 4/4 | Huang et al. (2020) [30] |
| Illumina/HiSeq 2500 | Wild | 6 | Zhu et al. (2018) [18] | |
| Illumina/Novaseq 6000 | Wild | 10 | Kang (2023) [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, Z.; Wang, L.; Zhang, Q.; Kang, L.; Wang, J.; Long, J.; Hong, M. Progress in Research on the Gut Microflora of the Red Panda (Ailurus fulgens). Microorganisms 2024, 12, 478. https://doi.org/10.3390/microorganisms12030478
Zhao X, Zhang Z, Wang L, Zhang Q, Kang L, Wang J, Long J, Hong M. Progress in Research on the Gut Microflora of the Red Panda (Ailurus fulgens). Microorganisms. 2024; 12(3):478. https://doi.org/10.3390/microorganisms12030478
Chicago/Turabian StyleZhao, Xing, Zejun Zhang, Le Wang, Qian Zhang, Liwen Kang, Jia Wang, Juejie Long, and Mingsheng Hong. 2024. "Progress in Research on the Gut Microflora of the Red Panda (Ailurus fulgens)" Microorganisms 12, no. 3: 478. https://doi.org/10.3390/microorganisms12030478
APA StyleZhao, X., Zhang, Z., Wang, L., Zhang, Q., Kang, L., Wang, J., Long, J., & Hong, M. (2024). Progress in Research on the Gut Microflora of the Red Panda (Ailurus fulgens). Microorganisms, 12(3), 478. https://doi.org/10.3390/microorganisms12030478







