Exploring the Bioactive Potential of Pisolithus (Basidiomycota): Comprehensive Insights into Antimicrobial, Anticancer, and Antioxidant Properties for Innovative Applications
Abstract
1. Introduction
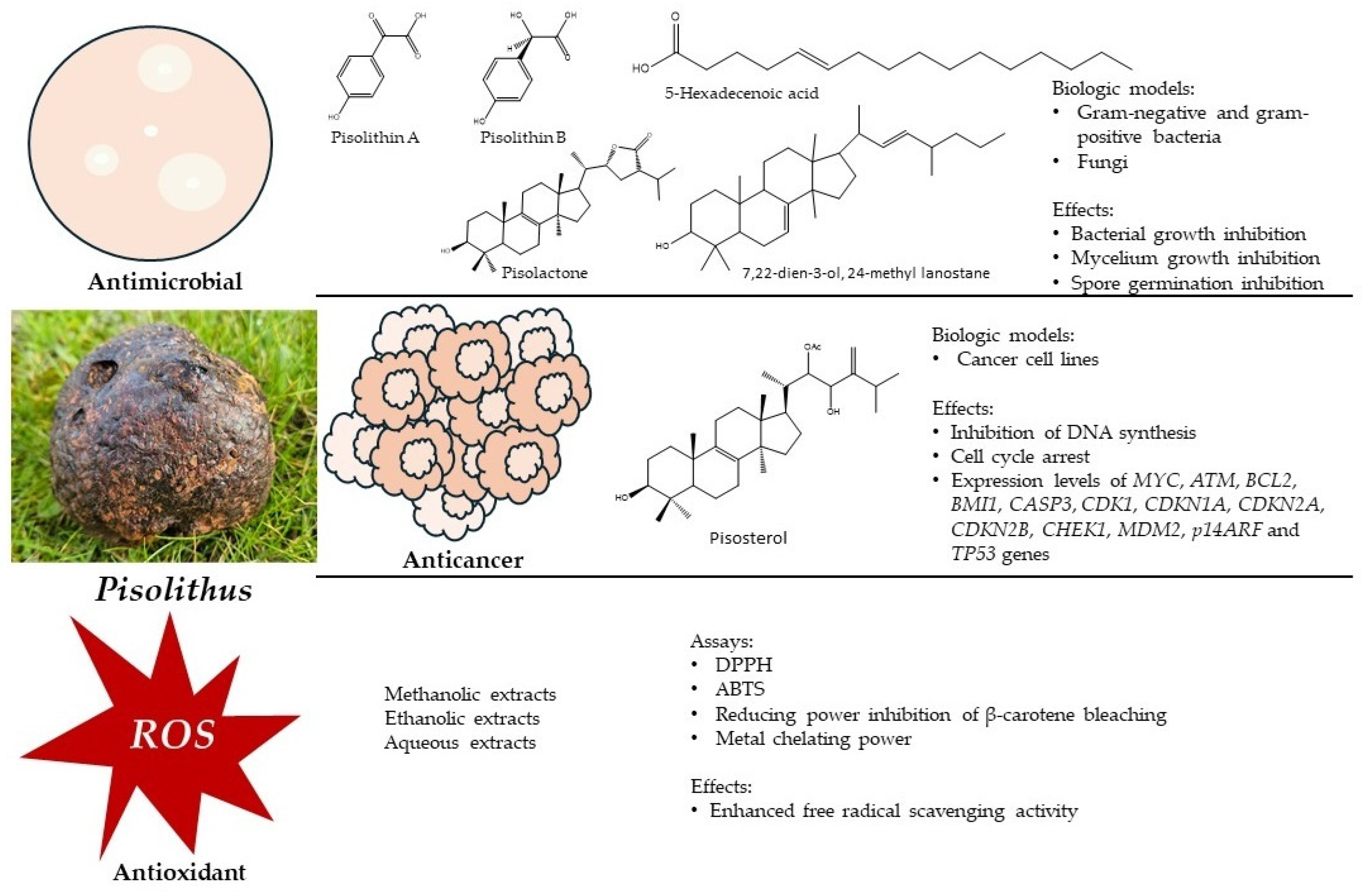
2. The Bioactive Potential of Pisolithus
2.1. Antimicrobial Activity

| Extract/Compound | Species | Fungal Structure | Assay | Antimicrobial Activity | Reference |
|---|---|---|---|---|---|
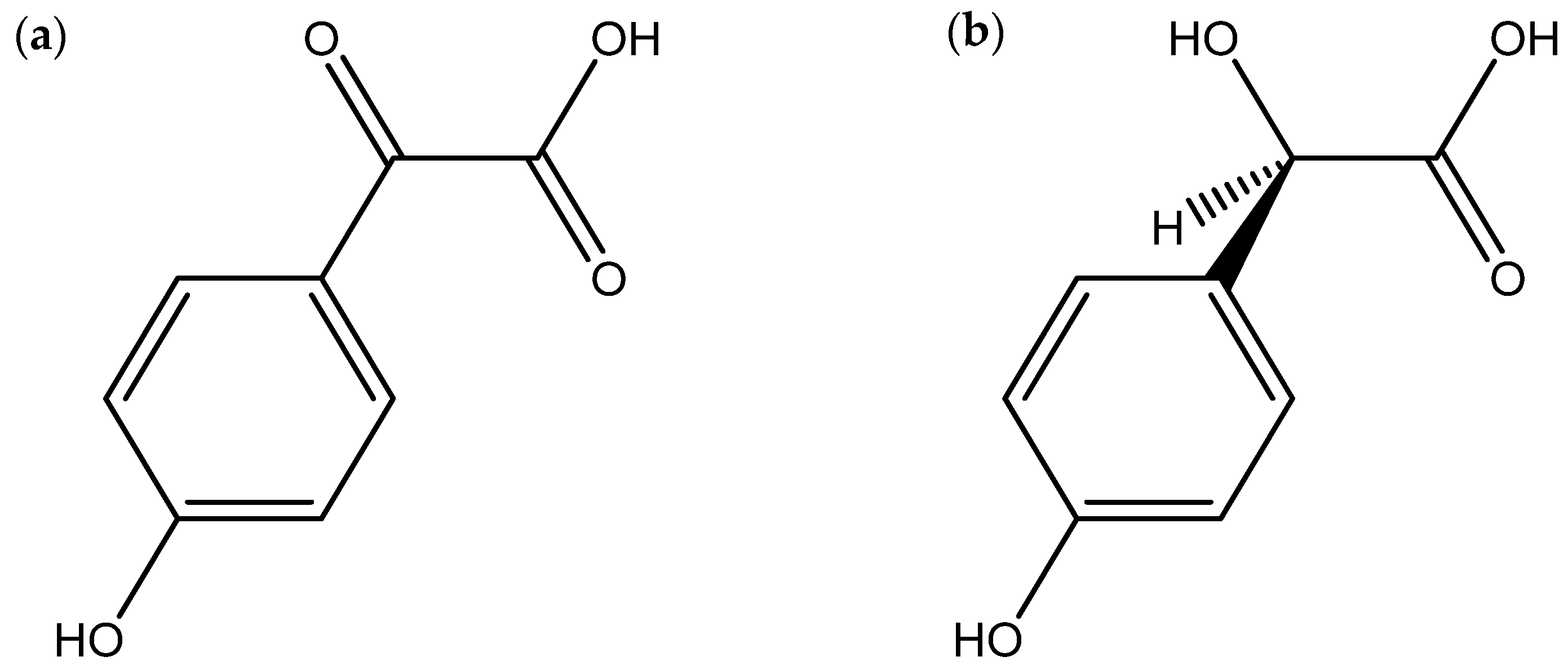
| Pisolithin A Pisolithin B (phenolic compounds) | P. arhizus | Mycelium | Spore germination Hyphal growth measured by protein estimation | Activity against phytopathogenic fungi, phytopathogenic oomycetes, and dermopathogenic fungi | [43,44] |
| Pisolactone (triterpenoid) | P. tinctorius | Basidiocarp | Broth microdilution | Activity against Gram-negative and Gram-positive bacteria Antimycobacterial Antifungal | [50] |
| 7,22-dien-3-ol, 24-methyl lanostane (triterpenoid) | P. tinctorius | Basidiocarp | Broth microdilution | Activity against Gram-negative and Gram-positive bacteria Antifungal | [50] |
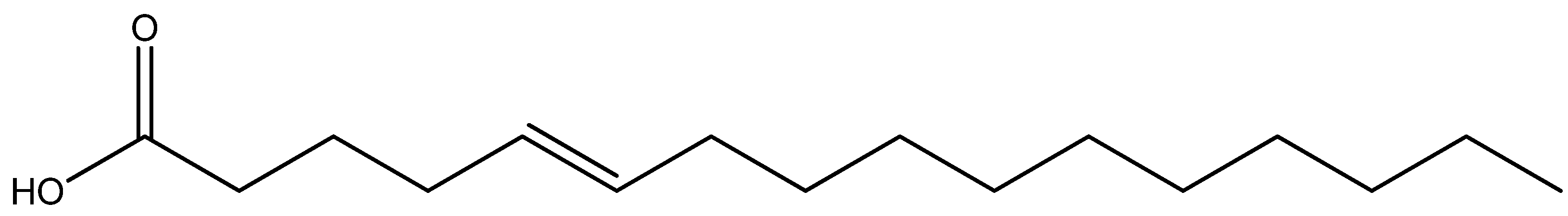
| 5-hexadecenoic acid (unsaturated fatty acid) | P. tinctorius | Basidiocarp | Broth microdilution | Activity against Gram-negative and Gram-positive bacteria Antifungal | [50] |
| Ceramide P56 (ceramide) | P. tinctorius | Basidiocarp | Broth microdilution | Activity against Gram-negative and Gram-positive bacteria Antimycobacterial Antifungal | [50] |
| Crude extracts/fractions | P. albus | Basidiocarp | Agar well diffusion | Activity against methicillin-resistant Staphylococcus aureus | [46] |
| Methanolic extracts/fractions | P. microcarpus | Mycelium | Disk diffusion | Activity against Pseudomonas aeruginosa and S. aureus | [47] |
| Ethanolic extracts | P. albus | Basidiocarp | Disk diffusion | Activity against Gram-negative and Gram-positive bacteria | [48] |
| Ethyl acetate extracts | P. tinctorius | Basidiocarp and spores | Agar well diffusion | Activity against phytopathogenic fungi | [51] |
| Ethyl acetate extracts | P. tinctorius | Mycelium filtrate | Mycelium growth in solid medium | Activity against phytopathogenic fungi | [52] |
| Hydroethanolic extracts | P. tinctorius | Basidiocarp | Broth microdilution | Activity against multidrug-resistant Gram-negative and Gram-positive bacteria | [49] |
2.2. Anticancer Activity
| Biological Model | Assay | Mechanism/Effect | Reference |
|---|---|---|---|
| CEM, HL-60, B16, HCT-8, MCF-7, PC-3, SF-268 cell lines | MTT | Cytotoxicity | [59] |
| HL-60 cell line | MTT | Cytotoxicity | [62] |
| Trypan blue exclusion | Viability | ||
| α-Naphthyl acetate esterase activity | Cell differentiation | ||
| NBT | |||
| BrdU incorporation | Inhibition of DNA synthesis | ||
| Differential fluorescent staining with acridine/orange ethidium bromide | Apoptosis | ||
| Mitotic index | Cell cycle arrest | [63] | |
| Chromosome analysis | Homogeneously staining region (HSR) 8q24 aberration | ||
| U343, AHOL1 cell lines | Cytogenetic characterization: metaphases stained with Giemsa solution and banded with trypsin-Giemsa Mitotic index FISH analysis | 8q24.12–q24.13 chromosome aberrations Cytotoxicity Expression of C-MYC gene | [76] |
| U343, AHOL1, U-87 MG, 1321N1 cell lines | MTT | Cytotoxicity | [65] |
| Trypan blue exclusion | Viability | ||
| Flow cytometry | Cell cycle arrest | ||
| Staining with Annexin V-FITC/PI | Apoptosis | ||
| qPCR and western blotting | Expression levels of MYC, ATM, BCL2, BMI1, CASP3, CDK1, CDKN1A, CDKN2A, CDKN2B, CHEK1, MDM2, p14ARF and TP53 genes | ||
| Swiss female mice | Histopathology and morphologic observations | Induction of cellular and nuclear pleomorphism; coagulative type necrosis | [66] |
| MG63, T47D, RKO cell lines | MTT | Cytotoxicity | [75] |
2.3. Antioxidant Activity
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Painuli, S.; Semwal, P.; Egbuna, C. Mushroom: Nutraceutical, mineral, proximate constituents and bioactive component. In Functional Foods and Nutraceuticals; Egbuna, C., Dable Tupas, G., Eds.; Springer: Cham, Switzerland, 2020; pp. 307–336. [Google Scholar]
- Gupta, A.; Meshram, V.; Gupta, M.; Goyal, S.; Qureshi, K.A.; Jaremko, M.; Shukla, K.K. Fungal endophytes: Microfactories of novel bioactive compounds with therapeutic interventions; A comprehensive review on the biotechnological developments in the field of fungal endophytic biology over the last decade. Biomolecules 2023, 13, 1038. [Google Scholar] [CrossRef] [PubMed]
- Ghobad-Nejhad, M.; Dima, B.; Cui, B.-K.; Si, J. Editorial: Basidiomycete fungi: From biosystematics and biodiversity to biotechnology. Front. Microbiol. 2023, 14, 1128319. [Google Scholar] [CrossRef]
- He, M.-Q.; Zhao, R.-L.; Liu, D.-M.; Denchev, T.T.; Begerow, D.; Yurkov, A.; Kemler, M.; Millanes, A.M.; Wedin, M.; McTaggart, A.R.; et al. Species diversity of Basidiomycota. Fungal Divers. 2022, 114, 281–325. [Google Scholar] [CrossRef]
- Taylor, T.N.; Krings, M.; Taylor, E.L. 9–Basidiomycota. In Fossil Fungi; Taylor, T.N., Krings, M., Taylor, E.L., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 173–199. [Google Scholar]
- Sande, D.; Oliveira, G.P.; Moura, M.A.F.E.; Martins, B.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Hobbs, C. The health and clinical benefits of medicinal fungi. In Biochemical Engineering and Biotechnology of Medicinal Mushrooms. Advances in Biochemical Engineering/Biotechnology; Berovic, M., Zhong, J.J., Eds.; Springer: Cham, Switzerland, 2023; Volume 184, pp. 285–356. [Google Scholar]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production–A review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Garcia, J.; Costa, V.M.; Carvalho, A.; Baptista, P.; de Pinho, P.G.; de Lourdes Bastos, M.; Carvalho, F. Amanita phalloides poisoning: Mechanisms of toxicity and treatment. Food Chem. Toxicol. 2015, 86, 41–55. [Google Scholar] [CrossRef]
- Wennig, R.; Eyer, F.; Schaper, A.; Zilker, T.; Andresen-Streichert, H. Mushroom poisoning. Dtsch. Arztebl. Int. 2020, 117, 701–708. [Google Scholar] [CrossRef]
- Krishnakumar, N.M.; Ceasar, S.A. Wild edible and medicinal mushrooms used by the tribes in the State of Kerala, India: A review. Int. J. Med. Mushrooms 2022, 24, 63–72. [Google Scholar] [CrossRef]
- Gomes, D.C.V.; de Alencar, M.V.O.B.; dos Reis, A.C.; de Lima, R.M.T.; Santos, J.V.O.; da Mata, A.M.O.F.; Dias, A.C.S.; Junior, J.S.C.; de Medeiros, M.D.G.F.; Paz, M.F.C.J.; et al. Antioxidant, anti-inflammatory and cytotoxic/antitumoral bioactives from the phylum Basidiomycota and their possible mechanisms of action. Biomed. Pharmacother. 2019, 112, 108643. [Google Scholar]
- Varghese, R.; Dalvi, Y.B.; Lamrood, P.Y.; Shinde, B.P.; Nair, C.K.K. Historical and current perspectives on therapeutic potential of higher basidiomycetes: An overview. 3 Biotech 2019, 9, 362. [Google Scholar] [CrossRef]
- Chopra, H.; Mishra, A.K.; Baig, A.A.; Mohanta, T.K.; Mohanta, Y.K.; Baek, K.-H. Narrative review: Bioactive potential of various mushrooms as the treasure of versatile therapeutic natural product. J. Fungi 2021, 7, 728. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Preto, M.; Grosso, C.; Vieira, M.; Delerue-Matos, C.; Vasconcelos, V.; Reis, M.; Barros, L.; Martins, R. Tracing the path between mushrooms and Alzheimer’s disease—A literature review. Molecules 2023, 28, 5614. [Google Scholar] [CrossRef] [PubMed]
- Cairney, J.W.G. Pisolithus–death of the pan-global super fungus. New Phytol. 2002, 153, 199–201. [Google Scholar] [CrossRef]
- Plett, J.M.; Miyauchi, S.; Morin, E.; Plett, K.; Wong-Bajracharya, J.; Pereira, M.F.; Kuo, A.; Henrissat, B.; Drula, E.; Wojtalewicz, D.; et al. Speciation underpinned by unexpected molecular diversity in the mycorrhizal fungal genus Pisolithus. Mol. Biol. Evol. 2023, 40, msad045. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.H. Tree host range and world distribution of the ectomycorrhizal fungus Pisolithus tinctorius. Can. J. Microbiol. 1977, 23, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Cairney, J.; Chambers, S. Interactions between Pisolithus tinctorius and its hosts: A review of current knowledge. Mycorrhiza 1997, 7, 117–131. [Google Scholar] [CrossRef]
- Ramos, M.A.; Sousa, N.R.; Franco, A.R.; Costa, V.; Oliveira, R.S.; Castro, P.M.L. Effect of diflubenzuron on the development of Pinus pinaster seedlings inoculated with the ectomycorrhizal fungus Pisolithus tinctorius. Environ. Sci. Pollut. Res. 2013, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.R.; Franco, A.R.; Ramos, M.A.; Oliveira, R.S.; Castro, P.M.L. Reforestation of burned stands: The effect of ectomycorrhizal fungi on Pinus pinaster establishment. Soil Biol. Biochem. 2011, 43, 2115–2120. [Google Scholar] [CrossRef]
- Sousa, N.R.; Franco, A.R.; Oliveira, R.S.; Castro, P.M.L. Ectomycorrhizal fungi as an alternative to the use of chemical fertilisers in nursery production of Pinus pinaster. J. Environ. Manage 2012, 95, S269–S274. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.R.; Sousa, N.R.; Ramos, M.A.; Oliveira, R.S.; Castro, P.M.L. Diversity and persistence of ectomycorrhizal fungi and their effect on nursery-inoculated Pinus pinaster in a post-fire plantation in Northern Portugal. Microb. Ecol. 2014, 68, 761–772. [Google Scholar] [CrossRef]
- Sousa, N.; Ramos, M.; Franco, A.; Oliveira, R.S.; Castro, P.M.L. Mycorrhizal symbiosis affected by different genotypes of Pinus pinaster. Plant Soil 2012, 359, 245–253. [Google Scholar] [CrossRef]
- Upadhayay, A.; Ling, J.; Pal, D.; Xie, Y.; Ping, F.-F.; Kumar, A. Resistance-proof antimicrobial drug discovery to combat global antimicrobial resistance threat. Drug Resist. Updates 2023, 66, 100890. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.K.; Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Wilson, M.; Wilson, P.J.K. Microbes and infectious diseases. In Close Encounters of the Microbial Kind; Wilson, M., Wilson, P.J.K., Eds.; Springer: Cham, Switzerland, 2021; pp. 3–48. [Google Scholar]
- Cella, E.; Giovanetti, M.; Benedetti, F.; Scarpa, F.; Johnston, C.; Borsetti, A.; Ceccarelli, G.; Azarian, T.; Zella, D.; Ciccozzi, M. Joining forces against antibiotic resistance: The one health solution. Pathogens 2023, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Mcelwain, T.F.; Thumbi, S.M. Animal pathogens and their impact on animal health, the economy, food security, food safety and public health. Rev. Sci. Tech. 2017, 36, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, R.W.; Mustafa, M.; Charles, K.; Wagara, I.W.; Kappel, N. Selected emerging and reemerging plant pathogens affecting the food basket: A threat to food security. J. Agric. Food Res. 2023, 14, 100827. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Hai, P.; Gao, Y.; Yang, L.; Chen, N.; Jia, H.; Wang, M.; Li, H.; Jiang, W.; Yang, J.; Li, R. Two new compounds from the endophytic fungi of Dryopteris crassirhizoma and their antimicrobial activities. Molecules 2023, 28, 8043. [Google Scholar] [CrossRef]
- Chakraverty, R.; Samanta, K.; Mandal, P.; Karmakar, S.; Karmakar, S. Chapter 18—Mechanisms of action of antibacterial agents (AMA). In How Synthetic Drugs Work; Kazmi, I., Karmakar, S., Shaharyar, M.A., Afzal, M., Al-Abbasi, F.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 421–429. [Google Scholar]
- Kope, H.H.; Fortin, J.A. Antifungal activity in culture filtrates of the ectomycorrhizal fungus Pisolithus tinctorius. Can. J. Bot. 1990, 68, 1254–1259. [Google Scholar] [CrossRef]
- Kope, H.H.; Tsantrizos, Y.S.; Fortin, J.A.; Ogilvie, K.K. p-Hydroxybenzoylformic acid and (R)-(-)-p-hydroxymandelic acid, two antifungal compounds isolated from the liquid culture of the ectomycorrhizal fungus Pisolithus arhizus. Can. J. Microbiol. 1991, 37, 258–264. [Google Scholar] [CrossRef]
- Tsantrizos, Y.S.; Kope, H.H.; Fortin, J.A.; Ogilvie, K.K. Antifungal antibiotics from Pisolithus tinctorius. Phytochemistry 1991, 30, 1113–1118. [Google Scholar] [CrossRef]
- Shrestha, V.G.; Shrestha, K.; Wallander, H. Antagonistic study of ectomycorrhizal fungi isolated from Baluwa forest (Central Nepal) against with pathogenic fungi and bacteria. Sci. World 2005, 3, 49–52. [Google Scholar]
- Ameri, A.; Ghadge, C.; Vaidya, J.G.; Deokule, S.S. Anti-Staphylococcus aureus activity of Pisolithus albus from Pune, India. J. Med. Plant Res. 2011, 5, 527–532. [Google Scholar]
- Mamede, A.C.P.B. Atividade Biológica do Fungo Pisolithus microcarpus. Master Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2015. [Google Scholar]
- Khadhri, A.; Aouadhi, C.; Aschi-Smiti, S. Screening of bioactive compounds of medicinal mushrooms collected on Tunisian territory. Int. J. Med. Mushrooms 2017, 19, 127–135. [Google Scholar] [CrossRef]
- Martins, T.; Machado-Carvalho, L.; Aires, A.; Saavedra, M.J.; Marques, G. Antioxidant, antimicrobial and cosmeceutical potential of wild mushroom extracts. Appl. Microbiol. 2023, 3, 562–579. [Google Scholar] [CrossRef]
- Carmo, G. Isolamento, Determinação Estrutural e Atividades Biológicas de Metabolitos dos Fungos Phellinotus piptadeniae e Pisolitus tinctorius. Ph.D. Thesis, Universidade Federal de Santa Maria, Santa Maria, Brazil, 2019. [Google Scholar]
- Ganesh, P.; Krishnamoorthy, A.S.; Sangeetha, C.; Nakkeeran, S.; Thiribhuvanamala, G.; Akshaya, S.B. Exploration of biomolecules from Pisolithus tinctorius (Pers.) against major soil-borne plant pathogens. Ann. Phytomed. 2021, 10, 311–318. [Google Scholar] [CrossRef]
- Ganeshkumar, P.; Krishnamoorthy, A.S.; Sangeetha, C.; Nakkeeran, S.; Sivakumar, U.; Thiribhuvanamala, G. Antimicrobial metabolites from ectomycorrhizal fungus, Pisolithus tinctorius (Pers.) Coker against soil borne plant pathogens. Madras Agric. J. 2021, 108, 234–241. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- WHO. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Mittra, I.; Pal, K.; Pancholi, N.; Shaikh, A.; Rane, B.; Tidke, P.; Kirolikar, S.; Khare, N.K.; Agrawal, K.; Nagare, H.; et al. Prevention of chemotherapy toxicity by agents that neutralize or degrade cell-free chromatin. Ann. Oncol. 2017, 28, 2119–2127. [Google Scholar] [CrossRef]
- Pathak, K.; Pathak, M.P.; Saikia, R.; Gogoi, U.; Sahariah, J.J.; Zothantluanga, J.H.; Samanta, A.; Das, A. Cancer chemotherapy via natural bioactive compounds. Curr. Drug Discov. Technol. 2022, 19, e310322202888. [Google Scholar] [CrossRef]
- Sengupta, M.; Guha, A.; Bhowmik, R.; Kazmi, I.; Hosawi, S.B.I.; Al-Abbasi, F.; Kaleem, M. Chapter 21—Insight into the molecular mechanism of action of anticancer drugs. In How Synthetic Drugs Work; Kazmi, I., Karmakar, S., Shaharyar, M.A., Afzal, M., Al-Abbasi, F.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 477–502. [Google Scholar]
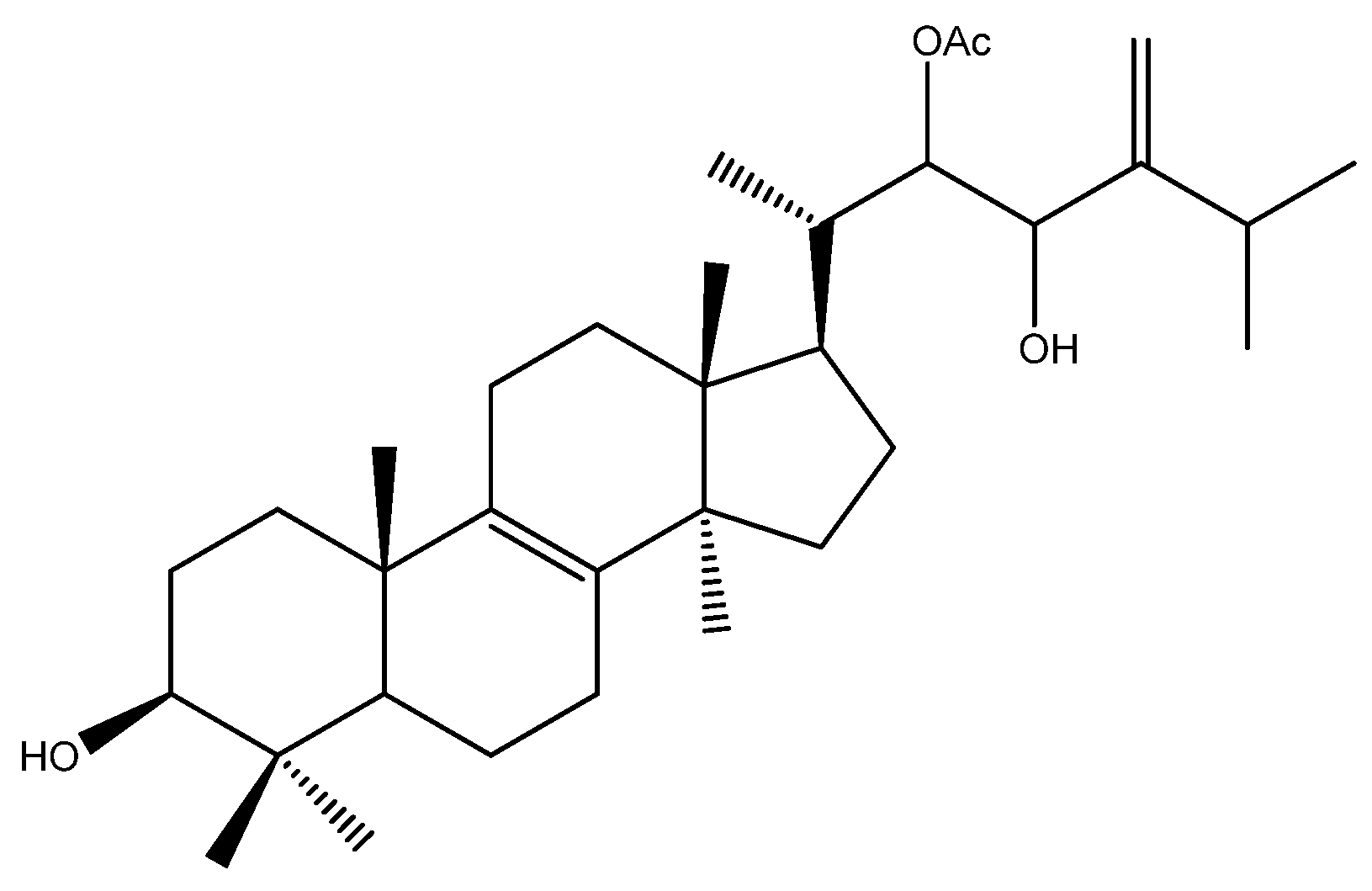
- Gill, M.; Kiefel, M.J.; Skelton, B.W.; White, A.H. The structure and absolute stereochemistry of pisosterol, the principal triterpenoid from fruitbodies of the fungus Pisolithus tinctorius. Aust. J. Chem. 1989, 42, 995–1001. [Google Scholar] [CrossRef]
- Montenegro, R.C.; Jimenez, P.C.; Farias, R.A.F.; Andrade-Neto, M.; Bezerra, F.S.; Moraes, M.E.A.; de Moraes, M.O.; Pessoa, C.; Costa-Lotufo, L.V. Cytotoxic activity of pisosterol, a triterpene isolated from Pisolithus tinctorius (Mich.: Pers.) Coker & Couch, 1928. Z. Naturforsch. C 2004, 59, 519–522. [Google Scholar]
- Adonin, L.; Drozdov, A.; Barlev, N.A. Sea urchin as a universal model for studies of gene networks. Front. Genet. 2021, 11, 627259. [Google Scholar] [CrossRef]
- Eurtivong, C.; Semenov, V.; Semenova, M.; Konyushkin, L.; Atamanenko, O.; Reynisson, J.; Kiselyov, A. 3-Amino-thieno [2,3-b]pyridines as microtubule-destabilising agents: Molecular modelling and biological evaluation in the sea urchin embryo and human cancer cells. Bioorg. Med. Chem. 2017, 25, 658–664. [Google Scholar] [CrossRef]
- Montenegro, R.C.; Vasconcellos, M.C.; Bezerra, F.S.; Andrade-Neto, M.; Pessoa, C.; Moraes, M.O.; Costa-Lotufo, L.V. Pisosterol induces monocytic differentiation in HL-60 cells. Toxicol. In Vitro 2007, 21, 795–800. [Google Scholar] [CrossRef]
- Burbano, R.R.; Lima, P.D.L.; Bahia, M.O.; Khayat, A.S.; Silva, T.C.R.; Bezerra, F.S.; Neto, M.A.; de Moraes, M.O.; Montenegro, R.C.; Costa-Lotufo, L.V.; et al. Cell cycle arrest induced by pisosterol in HL60 cells with gene amplification. Cell Biol. Toxicol. 2009, 25, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.L.; Lima, P.D.; Khayat, A.S.; Bahia, M.O.; Bezerra, F.S.; Andrade-Neto, M.; Montenegro, R.C.; Pessoa, C.; Costa-Lotufo, L.V.; Moraes, M.O.; et al. Inhibitory effect of pisosterol on human glioblastoma cell lines with C-MYC amplification. J. Appl. Toxicol. 2011, 31, 554–560. [Google Scholar] [CrossRef]
- Ferreira, W.A.S.; Burbano, R.R.; Pessoa, C.O.; Harada, M.L.; Borges, B.N.; de Oliveira, E.H.C. Pisosterol induces G2/M cell cycle arrest and apoptosis via the ATM/ATR signaling pathway in human glioma cells. Anticancer Agents Med. Chem. 2020, 20, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, R.C.; Farias, R.A.F.; Pereira, M.R.P.; Alves, A.P.N.N.; Bezerra, F.S.; Andrade-Neto, M.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor activity of pisosterol in mice bearing with S180 tumor. Biol. Pharm. Bull. 2008, 31, 454–457. [Google Scholar] [CrossRef]
- Abreu, P.M. Produtos Naturais do Fungo Pisolithus Tinctorius. Ph.D. Thesis, Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa, Lisbon, Portugal, 1987. [Google Scholar]
- Narayanan, A.; Baskaran, S.A.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Anticarcinogenic Properties of nedium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef]
- Lappano, R.; Sebastiani, A.; Cirillo, F.; Rigiracciolo, D.C.; Galli, G.R.; Curcio, R.; Malaguarnera, R.; Belfiore, A.; Cappello, A.R.; Maggiolini, M. The lauric acid-activated signaling prompts apoptosis in cancer cells. Cell Death Discov. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Sheela, D.L.; Narayanankutty, A.; Nazeem, P.A.; Raghavamenon, A.C.; Muthangaparambil, S.R. Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor downregulation: An in silico and in vitro study. Hum. Exp. Toxicol. 2019, 38, 753–761. [Google Scholar] [CrossRef]
- Fujimoto, H.; Nakayama, M.; Nakayama, Y.; Yamazaki, M. Isolation and characterization of immunosuppressive components of three mushrooms, Pisolithus tinctorius, Microporus flabelliformis and Lenzites betulina. Chem. Pharm. Bull. 1994, 42, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Nocera, R.; Franceschelli, S.; Tedesco, C.; De Riccardis, F.; Braca, A.; De Tommasi, N.; Donadio, G. Cytotoxic triterpenoids from the ectomycorrhizal fungus Pisolithus arhizus. Phytochemistry 2023, 209, 113635. [Google Scholar] [CrossRef] [PubMed]
- Baumert, A.; Schumann, B.; Porzel, A.; Schmidt, J.; Strack, D. Triterpenoids from Pisolithus tinctorius isolates and ectomycorrhizas. Phytochemistry 1997, 45, 499–504. [Google Scholar] [CrossRef]
- Alves, R.; Preto, M.; Vasconcelos, V.; Oliveira, R.S.; Martins, R. Cytotoxicity induced by extracts of Pisolithus tinctorius spores on human cancer and normal cell lines—Evaluation of the anticancer potential. J. Toxicol. Environ. Health A 2015, 78, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Siddiqui, M.; Abdellatif, B.; Liskova, A.; Kubatka, P.; Büsselberg, D. Natural compounds in glioblastoma therapy: Preclinical insights, mechanistic pathways, and outlook. Cancers 2021, 13, 2317. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Thomas Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. [Google Scholar]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Kibel, A.; Lukinac, A.M.; Dambic, V.; Juric, I.; Selthofer-Relatic, K. Oxidative stress in ischemic heart disease. Oxid. Med. Cell. Longev. 2020, 2020, 6627144. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Wang, L.; Wang, G.; Wang, Z.; Dong, X.; Wen, B.; Zhang, Z. The pathogenesis of diabetes mellitus by oxidative stress and inflammation: Its inhibition by berberine. Front. Pharmacol. 2018, 9, 782. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Forcados, G.E.; James, D.B.; Sallau, A.B.; Muhammad, A.; Mabeta, P. Oxidative stress and carcinogenesis: Potential of phytochemicals in breast cancer therapy. Nutr. Cancer 2017, 69, 365–374. [Google Scholar] [CrossRef]
- Strzelczyk, J.; Wiczkowski, A. Oxidative damage and carcinogenesis. Contemp. Oncol. 2012, 16, 230–233. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 Indonesian indigenous herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Pringle, N.; Van de Venter, M.; Boukes, G.J.; Koekemoer, T. Therapeutic potential of selected South African macrofungi in diabetic wound healing: An in vitro evaluation. S. Afr. J. Bot. 2021, 138, 337–347. [Google Scholar] [CrossRef]
- Campi, M.; Mancuello, C.; Ferreira, F.; Maubet, Y.; Cristaldo, E.; Benítez, D. Preliminary evaluation of phenolic compounds, antioxidant activity and bioactive compounds in some species of basidiomycetes fungi from Paraguay. Steviana 2019, 11, 26–41. [Google Scholar] [CrossRef]
- Reis, F.S.; Ferreira, I.C.F.R.; Barros, L.; Martins, A. A comparative study of tocopherols composition and antioxidant properties of in vivo and in vitro ectomycorrhizal fungi. LWT-Food Sci. Technol. 2011, 44, 820–824. [Google Scholar] [CrossRef][Green Version]
- Camara, A.G.N. Pigmentos Produzidos pelo Fungo Basidiomiceto Pisolithus tinctorius: Desreplicação, Isolamento e Estudo do Potencial Biotecnológico. Master Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2023. [Google Scholar]
- Sevindik, M.; Ajaz, M.; Özdemir, B.; Akata, I.; Selamoglu, Z. Oxidant/antioxidant potentials and heavy metal levels of Pisolithus arhizus and its effects on cardiovascular diseases. Indian J. Nat. Prod. Resour. 2021, 12, 600–604. [Google Scholar]
- Onbaşlı, D.; Yuvali, G.; Aslım, B. Medicinal potential of ectomycorrhizal mushroom Pisolithus arhizus extracts from Turkey. Fresenius Environ. Bull. 2020, 29, 9455–9464. [Google Scholar]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Wimmerová, M.; Wimmer, Z. Saponins of selected triterpenoids as potential therapeutic agents: A review. Pharmaceuticals 2023, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kinghorn, A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-planktonic and anti-biofilm properties of pentacyclic triterpenes—Asiatic acid and ursolic acid as promising antibacterial future pharmaceuticals. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Darshani, P.; Sarma, S.S.; Srivastava, A.K.; Baishya, R.; Kumar, D. Anti-viral triterpenes: A review. Phytochem. Rev. 2022, 21, 1761–1842. [Google Scholar] [CrossRef]
- Hardianto, A.; Mardetia, S.S.; Destiarani, W.; Budiman, Y.P.; Kurnia, D.; Mayanti, T. Unveiling the anti-cancer potential of onoceranoid triterpenes from Lansium domesticum Corr. cv. kokosan: An in silico study against estrogen receptor alpha. Int. J. Mol. Sci. 2023, 24, 15033. [Google Scholar]
| Extract | Species | Fungal Structure | Assay | Reference |
|---|---|---|---|---|
| Hydroethanolic | P. tinctorius | Basidiocarp | DPPH radical scavenging activity ABTS radical scavenging activity FRAP | [49] |
| Ethanolic Aqueous | P. tinctorius | Basidiocarp | DPPH radical scavenging activity FRAP | [87] |
| Ethanolic | P. arhizus | Basidiocarp | DPPH radical absorbance | [88] |
| Methanolic | P. arhizus | Basidiocarp Mycelium | DPPH radical scavenging activity Reducing power Inhibition of β-carotene bleaching | [89] |
| Hydroethanolic Methanolic | P. tinctorius | Basidiocarp | DPPH radical absorbance | [90] |
| Butanolic Ethyl acetate | P. microcarpus | Mycelium | DPPH radical scavenging activity | [47] |
| Ethanolic | P. arhizus | Basidiocarp | Total antioxidant status (TAS) Total oxidant status (TOS) Oxidative stress index (OSI)t | [91] |
| Ethanolic | P. albus | Basidiocarp | DPPH radical scavenging activity Reducing power of iron iron-chelating power | [48] |
| Methanolic Ethanolic | P. arhizus | Basidiocarp | DPPH radical scavenging activity Metal chelating power | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, R.S.; Preto, M.; Santos, G.; Silva, A.M.; Vasconcelos, V.; Martins, R. Exploring the Bioactive Potential of Pisolithus (Basidiomycota): Comprehensive Insights into Antimicrobial, Anticancer, and Antioxidant Properties for Innovative Applications. Microorganisms 2024, 12, 450. https://doi.org/10.3390/microorganisms12030450
Oliveira RS, Preto M, Santos G, Silva AM, Vasconcelos V, Martins R. Exploring the Bioactive Potential of Pisolithus (Basidiomycota): Comprehensive Insights into Antimicrobial, Anticancer, and Antioxidant Properties for Innovative Applications. Microorganisms. 2024; 12(3):450. https://doi.org/10.3390/microorganisms12030450
Chicago/Turabian StyleOliveira, Rui S., Marco Preto, Germana Santos, Ana Margarida Silva, Vitor Vasconcelos, and Rosário Martins. 2024. "Exploring the Bioactive Potential of Pisolithus (Basidiomycota): Comprehensive Insights into Antimicrobial, Anticancer, and Antioxidant Properties for Innovative Applications" Microorganisms 12, no. 3: 450. https://doi.org/10.3390/microorganisms12030450
APA StyleOliveira, R. S., Preto, M., Santos, G., Silva, A. M., Vasconcelos, V., & Martins, R. (2024). Exploring the Bioactive Potential of Pisolithus (Basidiomycota): Comprehensive Insights into Antimicrobial, Anticancer, and Antioxidant Properties for Innovative Applications. Microorganisms, 12(3), 450. https://doi.org/10.3390/microorganisms12030450











