Seroprevalence of the Hepatitis E Virus in Indigenous and Non-Indigenous Communities from the Brazilian Amazon Basin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Population
2.2. Blood Sample Collection
2.3. Serological Analysis
2.4. Ethical Approval
2.5. Statistical Analysis
3. Results
3.1. Anti-HEV Detection
3.2. Odds Ratio and Adjusted Odds Ratio
3.3. Multivariate Logistic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purdy, M.A.; Drexler, J.F.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.M.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef]
- Prpić, J.; Baymakova, M. Hepatitis E Virus (HEV) Infection among Humans and Animals: Epidemiology, Clinical Characteristics, Treatment, and Prevention. Pathogens 2023, 12, 931. [Google Scholar] [CrossRef]
- Pavio, N.; Doceul, V.; Bagdassarian, E.; Johne, R. Recent knowledge on hepatitis E virus in Suidae reservoirs and transmission routes to human. Vet. Res. 2017, 48, 78. [Google Scholar] [CrossRef]
- Sato, Y.; Sato, H.; Naka, K.; Furuya, S.; Tsukiji, H.; Kitagawa, K.; Sonoda, Y.; Usui, T.; Sakamoto, H.; Yoshino, S.; et al. A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: Identification of boar HEV strains of genotypes 3 and 4 and unrecognized genotypes. Arch. Virol. 2011, 156, 1345–1358. [Google Scholar] [CrossRef]
- Lee, G.H.; Tan, B.H.; Teo, E.C.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic Infection with Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Tsang, A.K.; Joseph, M.; Wong, E.Y.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R.; et al. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, 1044–1048. [Google Scholar] [CrossRef]
- Porea, D.; Raileanu, C.; Crivei, L.A.; Gotu, V.; Savuta, G.; Pavio, N. First Detection of Hepatitis E Virus (Rocahepevirus ratti Genotype C1) in Synanthropic Norway Rats (Rattus norvegicus) in Romania. Viruses 2023, 15, 1337. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 16 July 2023).
- Kumar, S.; Subhadra, S.; Singh, B.; Panda, B.K. Hepatitis E virus: The current scenario. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2013, 17, e228–e233. [Google Scholar] [CrossRef]
- Takakusagi, S.; Kakizaki, S.; Takagi, H. The Diagnosis, Pathophysiology, and Treatment of Chronic Hepatitis E Virus Infection—A Condition Affecting Immunocompromised Patients. Microorganisms 2023, 11, 1303. [Google Scholar] [CrossRef]
- de Oliveira, J.M.; dos Santos, D.R.L.; Pinto, M.A. Hepatitis E Virus Research in Brazil: Looking Back and Forwards. Viruses 2023, 15, 548. [Google Scholar] [CrossRef]
- Pisano, M.B.; Martinez-Wassaf, M.G.; Mirazo, S.; Fantilli, A.; Arbiza, J.; Debes, J.D.; Ré, V.E. Hepatitis E virus in South America: The current scenario. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 1536–1546. [Google Scholar] [CrossRef]
- Mirazo, S.; Mainardi, V.; Ramos, N.; Gerona, S.; Rocca, A.; Arbiza, J. Indigenous Hepatitis E Virus Genotype 1 Infection, Uruguay. Emerg. Infect. Dis. 2014, 20, 171–173. [Google Scholar] [CrossRef]
- García, C.G.; Sánchez, D.; Villalba, M.C.; Pujol, F.H.; de Los Ángeles Rodríguez Lay, L.; Pinto, B.; Chacón, E.P.; Guzmán, M.G. Molecular characterization of hepatitis E virus in patients with acute hepatitis in Venezuela. J. Med. Virol. 2012, 84, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Mehmood, B.F.; Sohal, A.; Roytman, M. Hepatitis E infection: A review. World J. Virol. 2023, 12, 262–271. [Google Scholar] [CrossRef]
- Aslan, A.T.; Balaban, H.Y. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J. Gastroenterol. 2020, 26, 5543–5560. [Google Scholar] [CrossRef]
- Pujol, F.H.; Favorov, M.O.; Marcano, T.; Esté, J.A.; Magris, M.; Liprandi, F.; Khudyakov, Y.E.; Khudyakova, N.S.; Fields, H.A. Prevalence of antibodies against hepatitis e virus among urban and rural populations in Venezuela. J. Med. Virol. 1994, 42, 234–236. [Google Scholar] [CrossRef]
- Ibarra, H.; Riedemann, S.; Reinhardt, G.; Frieck, P.; Siegel, F.; Toledo, C.; Calvo, M.; Froösner, G. Prevalence of hepatitis E virus antibodies in blood donors and other population groups in southern Chile. Rev. Medica Chile 1997, 125, 275–278. [Google Scholar]
- León, P.; Venegas, E.; Bengoechea, L.; Rojas, E.; López, J.A.; Elola, C.; Echevarría, J.M. Prevalence of infections by hepatitis B, C, D and E viruses in Bolivia. Rev. Panam. Salud Publica Pan Am. J. Public Health 1999, 5, 144–151. [Google Scholar]
- Trinta, K.S.; Liberto, M.I.M.; Paula, V.S.d.; Yoshida, C.F.T.; Gaspar, A.M.C. Hepatitis E virus infection in selected Brazilian populations. Memórias Inst. Oswaldo Cruz 2001, 96, 25–29. [Google Scholar] [CrossRef][Green Version]
- Freitas, N.R.D.; Teles, S.A.; Caetano, K.A.A.; Matos, M.A.D.; Carneiro, M.A.D.S.; Gardinali, N.R.; Pinto, M.A.; Martins, R.M.B. Hepatitis E seroprevalence and associated factors in rural settlers in Central Brazil. Rev. Soc. Bras. Med. Trop. 2017, 50, 675–679. [Google Scholar] [CrossRef]
- Caetano, K.A.A.; Bergamaschi, F.P.R.; Carneiro, M.A.S.; Pinheiro, R.S.; Araújo, L.A.; Matos, M.A.; Carvalho, P.; de Souza, M.M.; de Matos, M.A.D.; Del-Rios, N.H.A.; et al. Hepatotropic viruses (hepatitis A, B, C, D and E) in a rural Brazilian population: Prevalence, genotypes, risk factors and vaccination. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 91–98. [Google Scholar] [CrossRef]
- Souto, F.J.; Fontes, C.J. Prevalence of IgG-class antibodies against hepatitis E virus in a community of the southern Amazon: A randomized survey. Ann. Trop. Med. Parasitol. 1998, 92, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Vitral, C.L.; da Silva-Nunes, M.; Pinto, M.A.; de Oliveira, J.M.; Gaspar, A.M.; Pereira, R.C.; Ferreira, M.U. Hepatitis A and E seroprevalence and associated risk factors: A community-based cross-sectional survey in rural Amazonia. BMC Infect. Dis. 2014, 14, 458. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.; Mesquita, J.R.; Dutra, V.; Nascimento, M.S.J. Systematic Review of Hepatitis E Virus in Brazil: A One-Health Approach of the Human-Animal-Environment Triad. Animals 2021, 11, 2290. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Alencar, F.E.; Cerutti, C., Jr.; Milhous, W.K.; Andrade, A.L.; Oliveira, R.; Kanesa-Thasan, N.; MaCarthy, P.O.; Hoke, C.H., Jr. Short report: Hepatitis E infection in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 1995, 52, 347–348. [Google Scholar] [CrossRef]
- Assis, S.B.; Souto, F.J.; Fontes, C.J.; Gaspar, A.M. Prevalence of hepatitis A and E virus infection in school children of an Amazonian municipality in Mato Grosso State. Rev. Soc. Bras. Med. Trop. 2002, 35, 155–158. [Google Scholar] [CrossRef]
- Paula, V.S.d.; Arruda, M.E.; Vitral, C.L.; Gaspar, A.M.C. Seroprevalence of viral hepatitis in riverine communities from the Western Region of the Brazilian Amazon Basin. Memórias Inst. Oswaldo Cruz 2001, 96, 1123–1128. [Google Scholar] [CrossRef][Green Version]
- Pandolfi, R.; Ramos de Almeida, D.; Alves Pinto, M.; Kreutz, L.C.; Frandoloso, R. In house ELISA based on recombinant ORF2 protein underline high prevalence of IgG anti-hepatitis E virus amongst blood donors in south Brazil. PLoS ONE 2017, 12, e0176409. [Google Scholar] [CrossRef]
- Zorzetto, R.; Klein, R.L.; Erpen, L.M.S.; Klein, B.D.; Giacobbo, I.; da Silveira, R.A.; Frandoloso, R.; Kreutz, L.C. Unusual high prevalence of antibodies to hepatitis E virus in South Brazil. FEMS Microbiol. Lett. 2021, 368, fnab076. [Google Scholar] [CrossRef]
- Villar, L.M.; Milagres, F.A.P.; Marques, J.T.; de Paula, V.S. Hepatitis E prevalence in indigenous communities from Western Brazilian Amazon. Liver Int. Off. J. Int. Assoc. Study Liver 2021, 41, 235–236. [Google Scholar] [CrossRef]
- Remondegui, C.; Ceballos, S.; Arce, L.P.; Pintado, E.; Vidaurre, R.; Nitschko, H.; Osterman, A.; Vizoso Pinto, M.G. Serologic evidence of the circulation of the hepatitis E virus and the prevalence of antibodies against hepatitis A in an indigenous population in northern Argentina. Rev. Argent. Microbiol. 2021, 53, 314–324. [Google Scholar] [CrossRef]
- Mac Donald-Ottevanger, M.S.; Prins, M.; van Dissel, J.; Rier, N.; Reimerink, J.; Zijlmans, W.; Vreden, S.G.S.; Boyd, A. Ethnic differences in hepatitis A and E virus seroprevalence in patients attending the Emergency Department, Paramaribo, Suriname. Trans. R. Soc. Trop. Med. Hyg. 2023, 117, 197–204. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Gastroenterology & Hepatology. Hepatitis E: A neglected virus. Editorial. Lancet Gastroenterol. Hepatol. 2016, 1, 261. [Google Scholar] [CrossRef] [PubMed]
- Azman, A.S.; Ciglenecki, I.; Wamala, J.F.; Lynch, J.; Aggarwal, R.; Rahman, M.; Wong, S.; Serafini, M.; Moussa, A.M.; Dalton, H.R.; et al. Hepatitis E should be considered a neglected tropical disease. PLoS Negl. Trop. Dis. 2019, 13, e0007453. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.P.A.; Sánchez-Arcila, J.C.; Peres, L.; de Sousa, P.S.F.; dos Santos Alvarenga, M.A.; Castro-Alves, J.; de Fatima Ferreira-da-Cruz, M.; Maia-Herzog, M.; Oliveira-Ferreira, J. Malarial and intestinal parasitic co-infections in indigenous populations of the Brazilian Amazon rainforest. J. Infect. Public Health 2023, 16, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arcila, J.C.; de França, M.M.; Pereira, V.A.; Vasconcelos, M.P.; Têva, A.; Perce-da-Silva Dde, S.; Neto, J.R.; Aprígio, C.J.; Lima-Junior Jda, C.; Rodrigues, M.M.; et al. The influence of intestinal parasites on Plasmodium vivax-specific antibody responses to MSP-119 and AMA-1 in rural populations of the Brazilian Amazon. Malar. J. 2015, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Melgaço, J.G.; Gardinali, N.R.; de Mello, V.D.M.; Leal, M.; Lewis-Ximenez, L.L.; Pinto, M.A. Hepatitis E: Update on Prevention and Control. BioMed Res. Int. 2018, 2018, 5769201. [Google Scholar] [CrossRef]
- Ciglenecki, I. Hepatitis E: Urgent action needed. Lancet Gastroenterol. Hepatol. 2017, 2, 154. [Google Scholar] [CrossRef]
- Tengan, F.M.; Figueiredo, G.M.; Nunes, A.K.S.; Manchiero, C.; Dantas, B.P.; Magri, M.C.; Prata, T.V.G.; Nascimento, M.; Mazza, C.C.; Abdala, E.; et al. Seroprevalence of hepatitis E in adults in Brazil: A systematic review and meta-analysis. Infect. Dis. Poverty 2019, 8, 3. [Google Scholar] [CrossRef]
- Huang, F.F.; Haqshenas, G.; Shivaprasad, H.L.; Guenette, D.K.; Woolcock, P.R.; Larsen, C.T.; Pierson, F.W.; Elvinger, F.; Toth, T.E.; Meng, X.J. Heterogeneity and seroprevalence of a newly identified avian hepatitis e virus from chickens in the United States. J. Clin. Microbiol. 2002, 40, 4197–4202. [Google Scholar] [CrossRef]
- Priemer, G.; Cierniak, F.; Wolf, C.; Ulrich, R.G.; Groschup, M.H.; Eiden, M. Co-Circulation of Different Hepatitis E Virus Genotype 3 Subtypes in Pigs and Wild Boar in North-East Germany, 2019. Pathogens 2022, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.; Ge, S.X.; Li, Y.P.; Zheng, Y.J.; Nong, Y.; Guo, Q.S.; Zhang, J.; Ng, M.H.; Xia, N.S. Seroprevalence of hepatitis E virus infection, rural southern People’s Republic of China. Emerg. Infect. Dis. 2006, 12, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.R.; Vitral, C.L.; de Paula, V.S.; Marchevsky, R.S.; Lopes, J.F.; Gaspar, A.M.; Saddi, T.M.; Júnior, N.C.; Guimarães Fde, R.; Júnior, J.G.; et al. Serological and molecular evidence of hepatitis E virus in swine in Brazil. Vet. J. 2009, 182, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.P.; Alias, H.; Choy, S.H.; Goh, X.T.; Lee, S.C.; Lim, Y.A.L.; Kee, B.P.; Chua, K.H.; Kamaruzaman, A.; Zheng, Z.; et al. The study of seroprevalence of hepatitis E virus and an investigation into the lifestyle behaviours of the aborigines in Malaysia. Zoonoses Public Health 2020, 67, 263–270. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, E.A.D.C.; de Oliveira, J.M.; Haddad, S.K.; da Roza, D.L.; Bottino, F.O.; Faria, S.; Bellíssimo-Rodrigues, F.; Passos, A.D.C. Declining prevalence of hepatitis A and silent circulation of hepatitis E virus infection in southeastern Brazil. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 101, 17–23. [Google Scholar] [CrossRef]
- Kmush, B.; Wierzba, T.; Krain, L.; Nelson, K.; Labrique, A.B. Epidemiology of hepatitis E in low- and middle-income countries of Asia and Africa. Semin. Liver Dis. 2013, 33, 15–29. [Google Scholar] [CrossRef]
- Bricks, G.; Senise, J.F.; Pott Junior, H.; Grandi, G.; Passarini, A.; Caldeira, D.B.; Carnaúba Junior, D.; Moraes, H.A.B.d.; Granato, C.F.H.; Castelo, A. Seroprevalence of hepatitis E virus in chronic hepatitis C in Brazil. Braz. J. Infect. Dis. 2018, 22, 85–91. [Google Scholar] [CrossRef]
- Bendall, R.; Ellis, V.; Ijaz, S.; Ali, R.; Dalton, H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J. Med. Virol. 2010, 82, 799–805. [Google Scholar] [CrossRef]

| Urban | Rural | Indigenous | ||||
|---|---|---|---|---|---|---|
| n = 101 | n = 282 | n = 428 | ||||
| Pos/n (%) | p | Pos/n (%) | p | Pos/n (%) | p | |
| HEV | 3/101 (3.0) | 40/282 (14.2) | 12/428 (2.8) | |||
| Sex | 0.575 | 0.33 | 0.009 | |||
| Female | 2/42 (4.7) | 20/130 (15.38) | 2/246 (0.8) | |||
| Male | 1/59 (1.7) | 18/149 (12.0) | 10/180 (5.5) | |||
| Missing | - | 3 | 2 | |||
| Age (years) | 0.397 | 0.011 | 0.162 | |||
| [0–15] | 0/4 (0) | 6/76 (7.8) | 2/95 (2.1) | |||
| [16–30] | 0/46 (0) | 9/64 (14.06) | 3/149(2.0) | |||
| [31–45] | 2/30 (6.7) | 15/88 (17.0) | 1/89 (1.1) | |||
| 45+ | 1/21 (4.8) | 8/51 (15.7) | 6/89 (6.7) | |||
| Missing | - | 3 | 6 | |||
| Place of birth | 1 | 0.37 | ||||
| Midwest | 0/2 (0.0) | 1/19 (5.2) | - | - | ||
| Northeast | 0/7 (0.0) | 2/24 (8.3) | - | - | ||
| North | 3/80 (3.7) | 23/173 (13.3) | 12/428 (2.8) | |||
| Southeast | 0/5 (0.0) | 6/27 (22.2) | - | |||
| South | 0/4 (0.0) | 5/33 (15.1) | - | |||
| Missing | 3 | 6 | - | |||
| Indigenous Village | 0.066 | |||||
| Alapusi | - | - | 2/76 (2.6) | |||
| Castanha/Ahima | - | - | 0/126 (0.0) | |||
| Gasolina | - | - | 6/97 (6.2) | |||
| Taibrapa | - | - | 4/117 (3.4) | |||
| Occupation | 0.47 | 0.335 | ||||
| Farmer | 0/4 (0.0) | 10/83 (12.0) | - | |||
| Health worker | 2/20 (10.0) | 1/7 (14.2) | - | |||
| Homemaker | 0/7 (0.0) | 11/56 (19.6) | - | |||
| Other | 1/42 (2.4) | 1/24 (4.2) | 12/428 (2.8) | |||
| Missing | 28 | 112 | - |
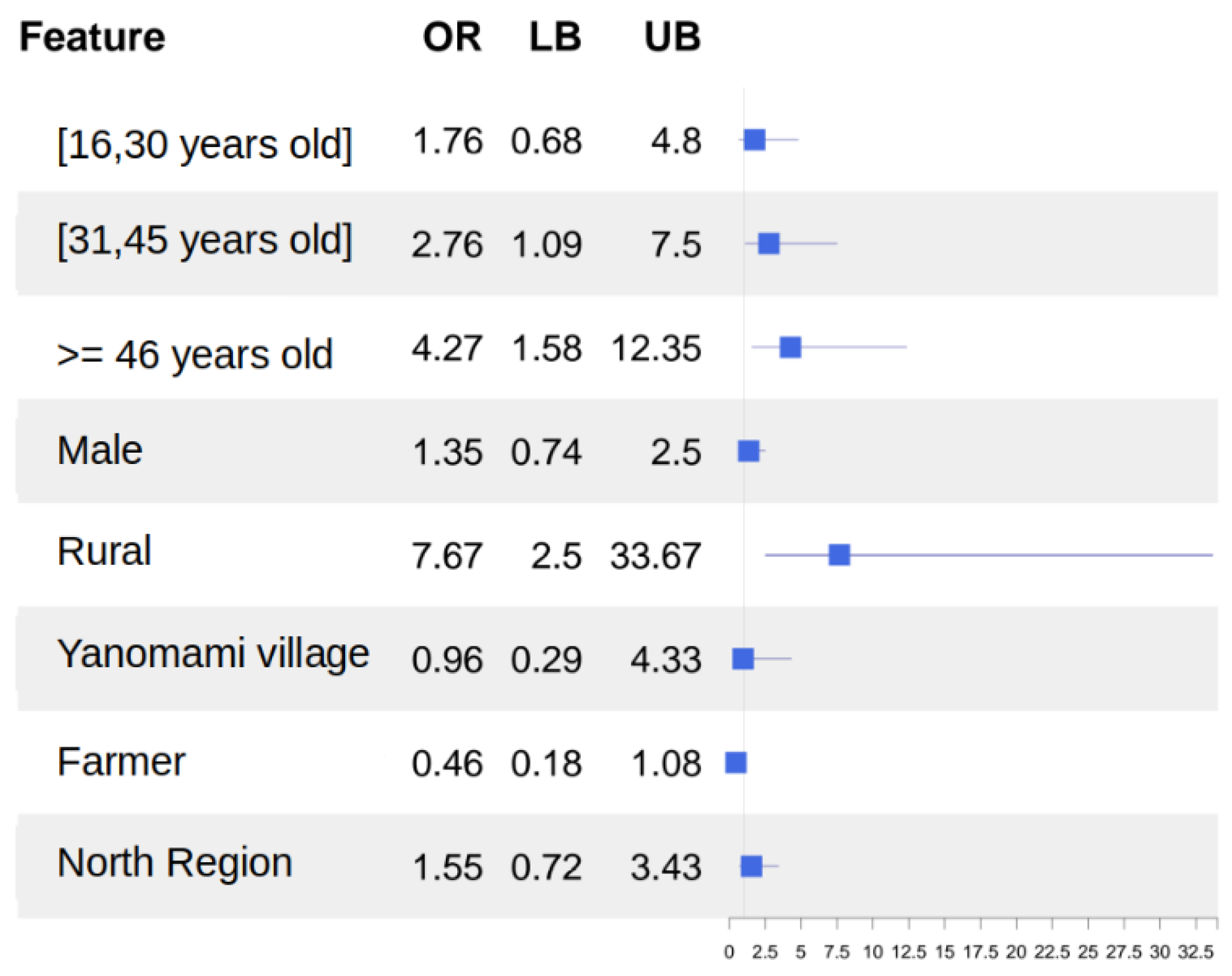
| Anti-HEV Negative | Anti-HEV Positive | OR | p-Value | |
|---|---|---|---|---|
| Age (years) | 0.103 | |||
| [0–15] | 167 (22.3%) | 8 (15.1%) | Ref. | |
| [16–30] | 247 (33.0%) | 12 (22.6%) | 1.01 [0.40; 2.66] | |
| [31–45] | 189 (25.2%) | 18 (34.0%) | 1.96 [0.85; 4.95] | |
| 45+ | 146 (19.5%) | 15 (28.3%) | 2.12 [0.89; 5.47] | |
| Sex | 0.396 | |||
| Female | 394 (52.3%) | 24 (45.3%) | Ref. | |
| Male | 359 (47.7%) | 29 (54.7%) | 1.32 [0.76; 2.34] | |
| Area of residence | <0.001 | |||
| Urban | 98 (13.0%) | 3 (5.45%) | Ref. | |
| Rural | 242 (32.0%) | 40 (72.7%) | 5.14 [1.81; 22.4] | |
| Yanomami territory | 416 (55.0%) | 12 (21.8%) | 0.91 [0.28; 4.24] | |
| Occupation | 0.10 | |||
| Non-Farmer | 679 (89.8%) | 45 (81.8%) | Ref. | |
| Farmer | 77 (10.2%) | 10 (18.2%) | 1.98 [0.90; 3.95] | |
| Place of birth | 0.023 | |||
| Non-North region | 107 (14.3%) | 14 (26.9%) | Ref. | |
| North Region | 643 (85.7%) | 38 (73.1%) | 0.45 [0.24; 0.89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, M.P.A.; de Oliveira, J.M.; Sánchez-Arcila, J.C.; Faria, S.C.; Rodrigues, M.M.; Perce-da-Silva, D.; Rezende-Neto, J.; Pinto, M.A.; Maia-Herzog, M.; Banic, D.M.; et al. Seroprevalence of the Hepatitis E Virus in Indigenous and Non-Indigenous Communities from the Brazilian Amazon Basin. Microorganisms 2024, 12, 365. https://doi.org/10.3390/microorganisms12020365
Vasconcelos MPA, de Oliveira JM, Sánchez-Arcila JC, Faria SC, Rodrigues MM, Perce-da-Silva D, Rezende-Neto J, Pinto MA, Maia-Herzog M, Banic DM, et al. Seroprevalence of the Hepatitis E Virus in Indigenous and Non-Indigenous Communities from the Brazilian Amazon Basin. Microorganisms. 2024; 12(2):365. https://doi.org/10.3390/microorganisms12020365
Chicago/Turabian StyleVasconcelos, Mariana Pinheiro Alves, Jaqueline Mendes de Oliveira, Juan Camilo Sánchez-Arcila, Sarah Castro Faria, Moreno Magalhães Rodrigues, Daiana Perce-da-Silva, Joffre Rezende-Neto, Marcelo Alves Pinto, Marilza Maia-Herzog, Dalma Maria Banic, and et al. 2024. "Seroprevalence of the Hepatitis E Virus in Indigenous and Non-Indigenous Communities from the Brazilian Amazon Basin" Microorganisms 12, no. 2: 365. https://doi.org/10.3390/microorganisms12020365
APA StyleVasconcelos, M. P. A., de Oliveira, J. M., Sánchez-Arcila, J. C., Faria, S. C., Rodrigues, M. M., Perce-da-Silva, D., Rezende-Neto, J., Pinto, M. A., Maia-Herzog, M., Banic, D. M., & Oliveira-Ferreira, J. (2024). Seroprevalence of the Hepatitis E Virus in Indigenous and Non-Indigenous Communities from the Brazilian Amazon Basin. Microorganisms, 12(2), 365. https://doi.org/10.3390/microorganisms12020365






