Optimization of an Alternative Culture Medium for Phycocyanin Production from Arthrospira platensis under Laboratory Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material and Inoculum Preparation
2.2. Experimental Design
2.3. Biomass Quantification
2.4. Quantification of Phycocyanin Concentration
2.5. Quantification of the Concentration of Nitrates and Ammonia Salts
2.6. Statistical Analysis
3. Results and Discussion
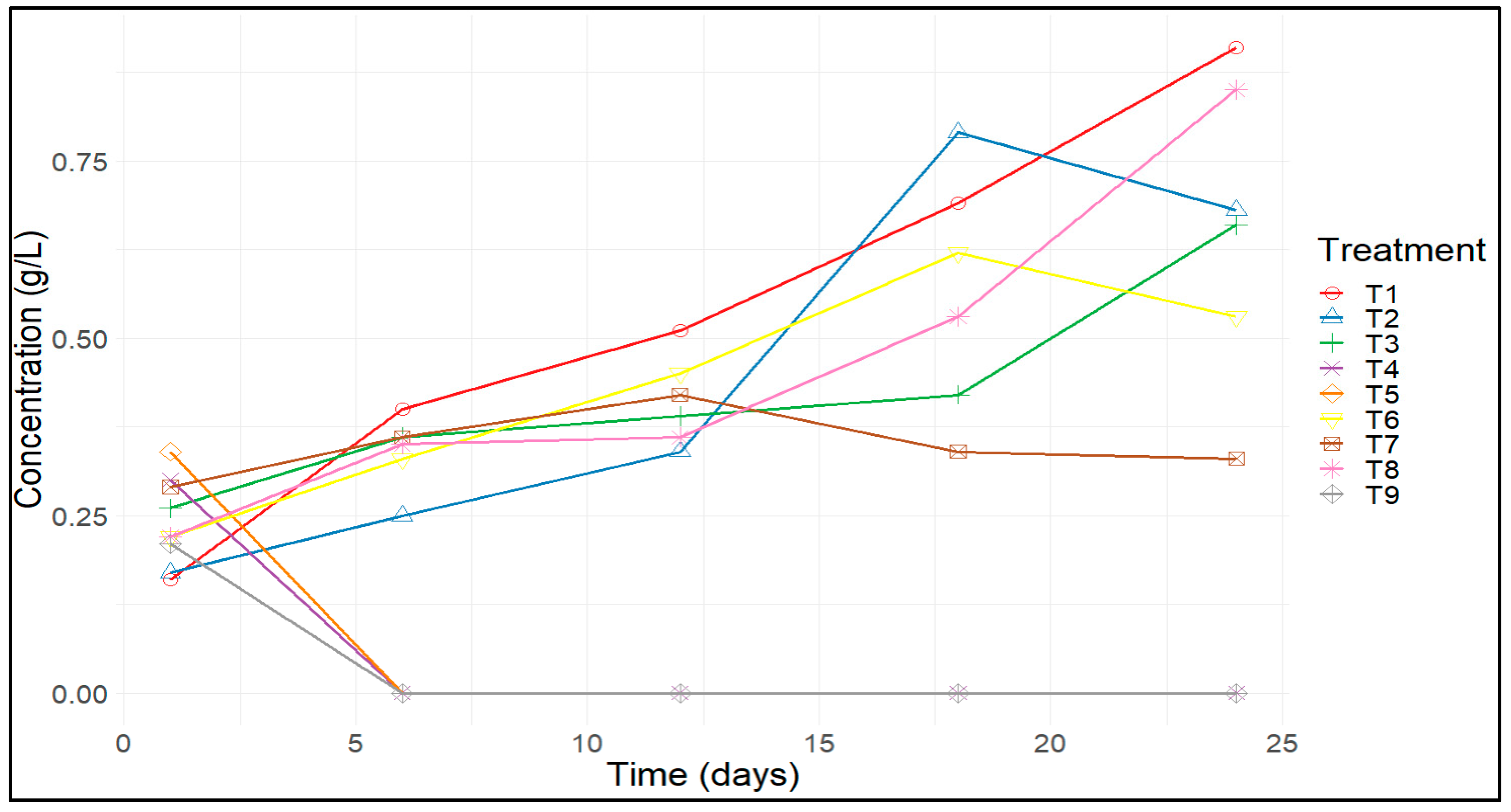
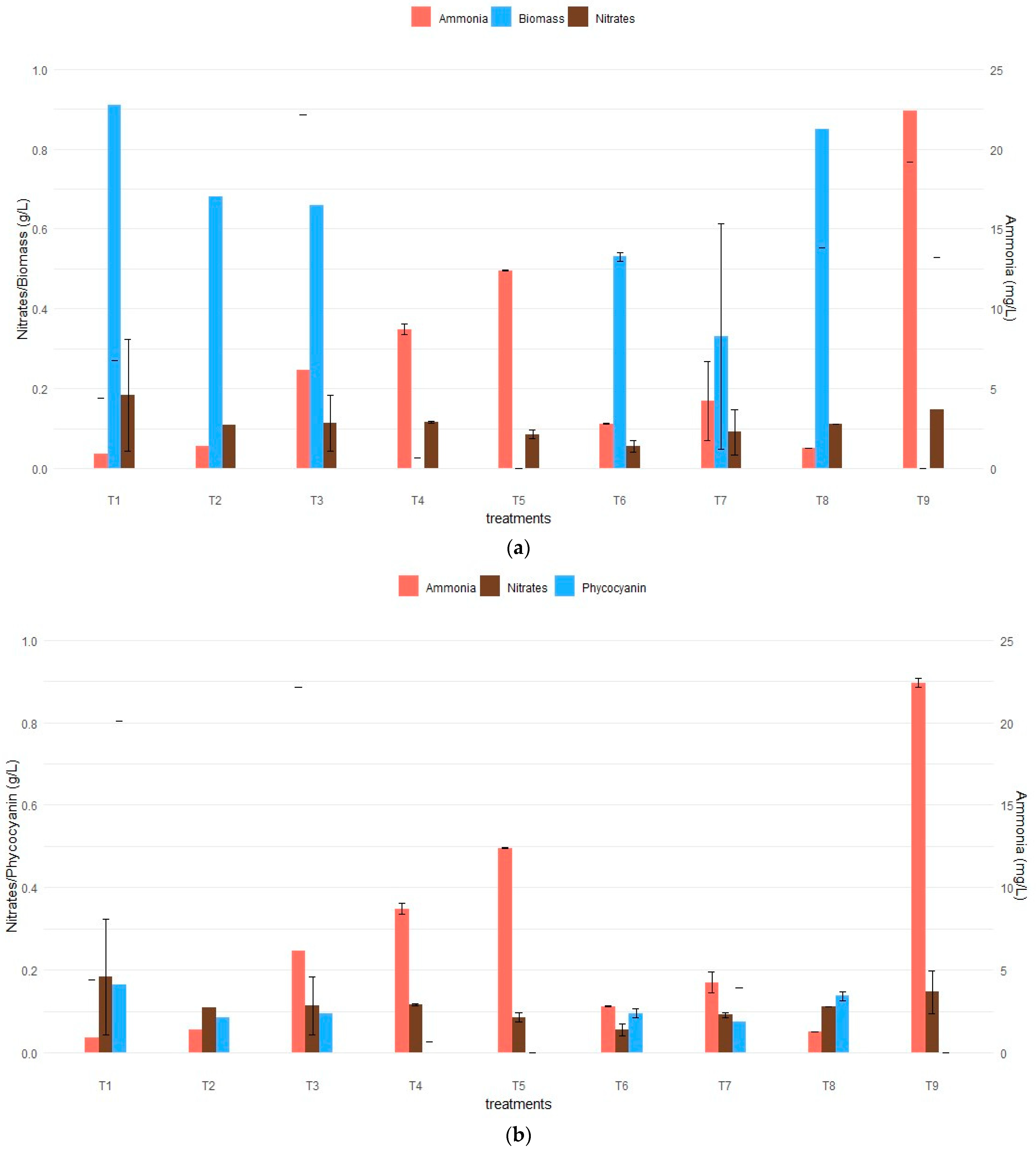
3.1. Effects of KNO3 and Urea on the Biomass Formation and Phycocyanin Production of A. platensis
3.2. Model Adjustment and Analysis of Variance (ANOVA)
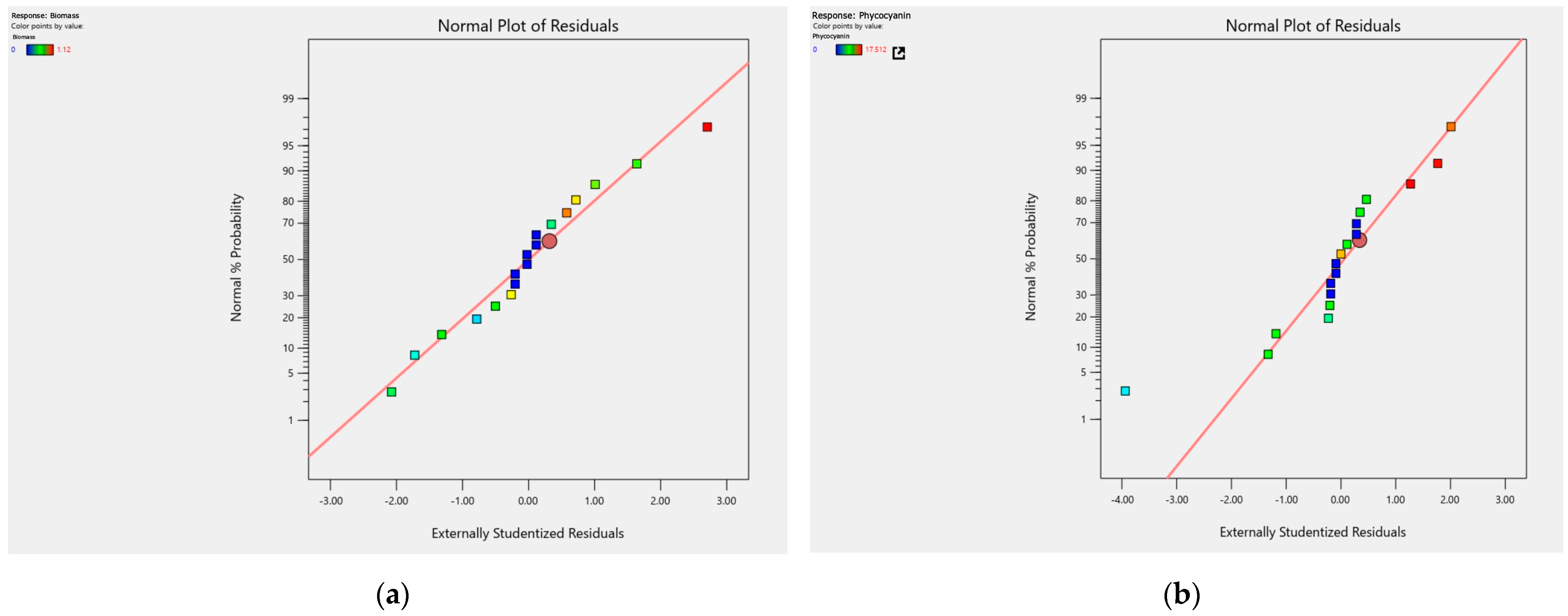
3.3. Adjustment Quality
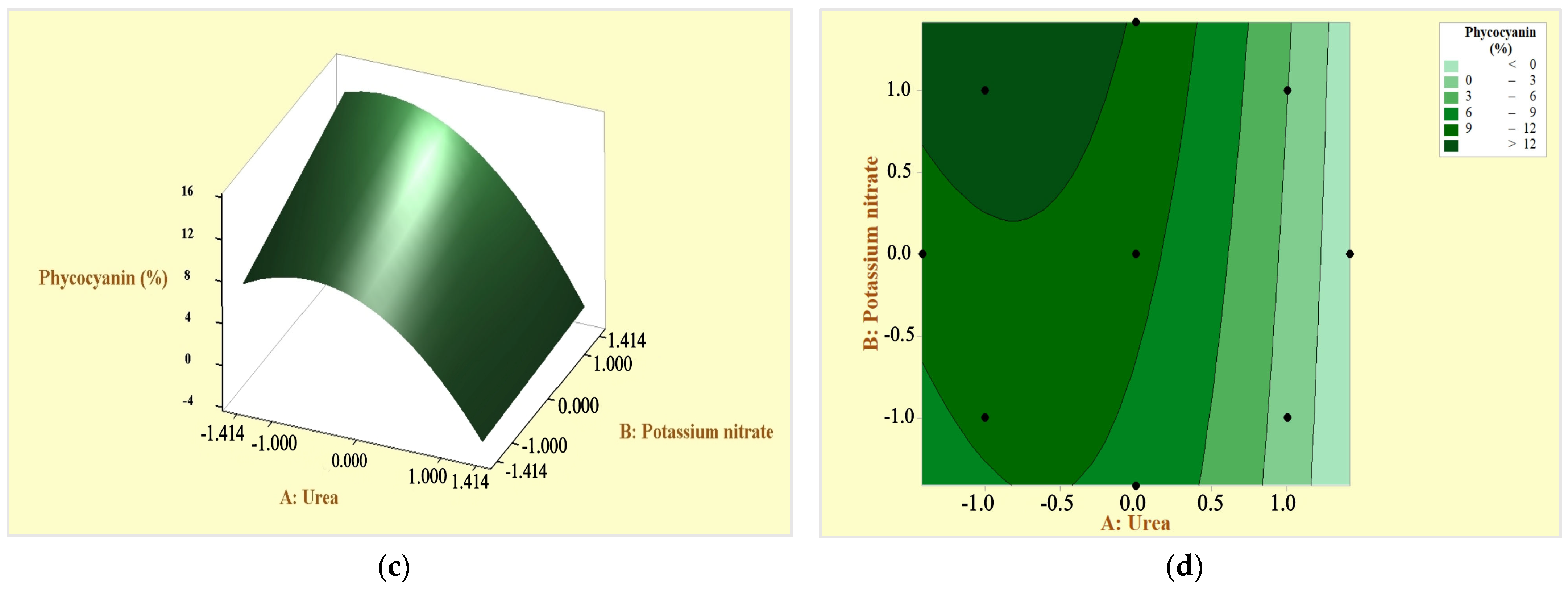
3.4. Response Surfaces and Contour Plots for Optimizing Response Variables: Arthrospira platensis Biomass and Phycocyanin
3.5. Optimization of Nitrogen Sources in Maximizing Response Variables
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef] [PubMed]
- Elain, A.; Nkounkou, C.; Le Fellic, M.; Donnart, K. Green extraction of polysaccharides from Arthrospira platensis using high pressure homogenization. J. Appl. Phycol. 2020, 32, 1719–1727. [Google Scholar] [CrossRef]
- Andrade, L.; De Andrade, C.J.; Dias, M.; Nascimento, C.; Mendes, M. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process. Technol. 2018, 6, 00144. [Google Scholar] [CrossRef]
- Otero, C.; Verdasco-Martín, C.M. Preparation and Characterization of a Multicomponent Arthrospira platensis Biomass Hydrolysate with Superior Anti-Hypertensive, Anti-Hyperlipidemic and Antioxidant Activities via Selective Proteolysis. Mar. Drugs 2023, 21, 255. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.; Guevara, M.; Gómez, B.; Arredondo-Vega, B.; Cortez, R.; Licet, B.; Romero, L.; Guevara, M.; Gómez, B.; Arredondo-Vega, B.; et al. Producción de pigmentos procedentes de Arthrospira maxima cultivada en fotobiorreactores. Rev. Colomb. Biotecnol. 2017, 19, 108–114. [Google Scholar] [CrossRef]
- El Baky, H.H.A.; El Baroty, G.S.; Mostafa, E.M. Optimization Growth of Spirulina (Arthrospira) Platensis in Photobioreactor Under Varied Nitrogen Concentration for Maximized Biomass, Carotenoids and Lipid Contents. Recent Pat. Food Nutr. Agric. 2020, 11, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.; Mehrzad, J.; Najafi, M.F.; Zhiani, R.; Shamsian, S.A.A. Nitrate and ammonia: Two key nitrogen sources for biomass and phycocyanin production by Arthrospira (Spirulina) platensis. J. Appl. Phycol. 2022, 34, 2271–2281. [Google Scholar] [CrossRef]
- Shanthi, G.; Premalatha, M.; Anantharaman, N. Effects of l-amino acids as organic nitrogen source on the growth rate, biochemical composition and polyphenol content of Spirulina platensis. Algal Res. 2018, 35, 471–478. [Google Scholar] [CrossRef]
- Sukumaran, P.; Nulit, R.; Halimoon, N.; Simoh, S.; Omar, H.; Ismail, A. Formulation of cost-effective medium using urea as a nitrogen source for Arthrospira platensis cultivation under real environment. Annu. Res. Rev. Biol. 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Cruz-Martínez, C.; Jesus, C.; Matsudo, M.; Danesi, E.; Sato, S.; Carvalho, J.C. Growth and composition of arthrospira (spirulina) platensis in a tubular photobioreactor using ammonium nitrate as the nitrogen source in a fed-batch process. Braz. J. Chem. Eng. 2015, 32, 347–356. [Google Scholar] [CrossRef]
- Carvalho, J.C.M.; Francisco, F.R.; Almeida, K.A.; Sato, S.; Converti, A. Cultivation of Arthrospira (Spirulina) Platensis (Cyanophyceae) By Fed-Batch Addition of Ammonium Chloride at Exponentially Increasing Feeding Rates. J. Phycol. 2004, 40, 589–597. [Google Scholar] [CrossRef]
- Mashor, N.; Yazam, M.S.M.; Naqqiuddin, M.A.; Omar, H.; Ismail, A. Cultivation of A. platensis based on the optimum concentrations of urea and triple super phosphate, ammonium sulfate and phosphoric acid, ammonium nitrate and triple super phosphate. In Proceedings of the Seminar Ekologi Malaysia, Putrajaya, Malaysia, 19 October 2015. [Google Scholar]
- Deschoenmaeker, F.; Bayon-Vicente, G.; Sachdeva, N.; Depraetere, O.; Cabrera Pino, J.C.; Leroy, B.; Muylaert, K.; Wattiez, R. Impact of different nitrogen sources on the growth of Arthrospira sp. PCC 8005 under batch and continuous cultivation—A biochemical, transcriptomic and proteomic profile. Bioresour. Technol. 2017, 237, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Avila-Leon, I.; Chuei Matsudo, M.; Sato, S.; de Carvalho, J.C.M. Arthrospira platensis biomass with high protein content cultivated in continuous process using urea as nitrogen source. J. Appl. Microbiol. 2012, 112, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Pulido, H.G.; De la Vara Salazar, R.; González, P.G.; Martínez, C.T.; Pérez, M.d.C.T. Análisis y Diseño de Experimentos; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Cosenza, Z.; Astudillo, R.; Frazier, P.I.; Baar, K.; Block, D.E. Multi-information source Bayesian optimization of culture media for cellular agriculture. Biotechnol. Bioeng. 2022, 119, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Gómez, G.; Batista, C. Optimizacion de medios de cultivos para microorganismos, una valiosa estrategia para la produccion de biopreparados de interes agricola. Cultiv. Trop. 2006, 27, 17–25. [Google Scholar]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- HACH COMPANY. Nitrate, Cadmium Reduction Method. Colorímetro DR900|Hach Mexico. 2019. Available online: https://latam.hach.com/colorimetros/colorimetro-dr900/family-downloads?productCategoryId=54617006119 (accessed on 27 September 2023).
- HACH COMPANY. Nitrogen, Ammonia: Salicylate Method. Colorímetro DR900|Hach Mexico|Hach. 2015. Available online: https://latam.hach.com/colorimetros/colorimetro-dr900/family-downloads?productCategoryId=54617006119 (accessed on 27 September 2023).
- Kasina, M.M.; Joseph, K.; John, M. Application of Central Composite Design to Optimize Spawns Propagation. Open J. Optim. 2020, 9, 47–70. [Google Scholar] [CrossRef]
- Gómez, C.; Guzmán-Carrasco, A.; Lafarga, T.; Acién-Fernández, F.G. Optimization of a New Culture Medium for the Large-Scale Production of Protein-Rich Arthrospira platensis (Oscillatoriales, Cyanophyceae). J. Phycol. 2021, 57, 636–644. [Google Scholar] [CrossRef]
- Da Silva de Castro, G.F.P.; Rizzo, R.F.; Passos, T.S.; Santos, B.N.C.D.; da Silva Dias, D.; Domingues, J.R.; de Lima Araújo, K.G. Biomass production by Arthrospira platensis under different culture conditions. Food Sci. Technol. 2015, 35, 18–24. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Morsi, H.; Hassan, L. Growth Enhancement of Spirulina platensis through Optimization of Media and Nitrogen Sources. Egypt. J. Bot. 2021, 61, 61–69. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zhou, T.; Cao, L.; Cai, Y.; Wang, Y.; Cui, X.; Yan, H.; Ruan, R.; Zhang, Q. Effects of Culture Conditions on the Performance of Arthrospira platensis and Its Production of Exopolysaccharides. Foods 2022, 11, 2020. [Google Scholar] [CrossRef]
- Mateucci, R. Efecto de Diferentes Fuentes de Nitrógeno y de Fósforo Sobre la Producción y la Composición de la Biomasa Microalgal de Arthrospira (Spirulina) Plantesis LMPA55. 2018. Available online: http://ria.utn.edu.ar/xmlui/handle/20.500.12272/3902 (accessed on 27 September 2023).
- Madkour, F.F.; Kamil, A.E.-W.; Nasr, H.S. Production and nutritive value of Spirulina platensis in reduced cost media. Egypt. J. Aquat. Res. 2012, 38, 51–57. [Google Scholar] [CrossRef]
- Torre, P.; Sassano, C.E.N.; Sato, S.; Converti, A.; Gioielli, L.A.; Carvalho, J.C.M. Fed-batch addition of urea for Spirulina platensis cultivation: Thermodynamics and material and energy balances. Enzym. Microb. Technol. 2003, 33, 698–707. [Google Scholar] [CrossRef]
- Vieira, D.C.M.; Matsudo, M.C.; Sato, S.; Converti, A.; de Carvalho, J.C.M. Simultaneous use of urea and potassium nitrate for Arthrospira (Spirulina) platensis cultivation. Biotechnol. J. 2012, 7, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Lwin, H.W.; Siangdung, W.; Ngoenphoemsup, S.; Taweesang, P.; Paithoonrangsarid, K. Beneficial effect of urea utilization in response to high temperature in Arthrospira platensis C1. Algal Res. 2023, 74, 103229. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Ammonia inhibition on Arthrospira platensis in relation to the initial biomass density and pH. Bioresour. Technol. 2014, 166, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Mulokozi, D.P.L.; Mtolera, M.S.P.; Mmochi, A.J. Biomass production and growth performance of Momela Lake’s spirulina (Arthrospira fusiformis) cultured under urea and N: P: K fertilizers as cheaper nitrogen sources. Int. J. Biol. Chem. Sci. 2019, 13, 861–869. [Google Scholar] [CrossRef]
- Khazi, M.I.; Demirel, Z.; Dalay, M.C. Enhancement of biomass and phycocyanin content of Spirulina platensis. Front. Biosci.-Elit. 2018, 10, 276–286. [Google Scholar] [CrossRef]
- Zhou, T.; Reji, R.; Kairon, R.S.; Chiam, K.H. A review of algorithmic approaches for cell culture media optimization. Front. Bioeng. Biotechnol. 2023, 11, 1195294. [Google Scholar] [CrossRef]
- Ruliaty, L.; Amalia, I.R.; Sari, R.I.; Aulia, R. Different Nitrogen Sources to Improve the Quality of Spirulina platensis Culture in Mass Scale. IOP Conf. Ser. Earth Environ. Sci. 2022, 1118, 012015. [Google Scholar] [CrossRef]
- Smalheiser, N.R. (Ed.) Chapter 11—ANOVA. In How to Make your Experiments Robust and Reproducible; Academic Press: Cambridge, MA, USA, 2017; pp. 149–155. [Google Scholar] [CrossRef]
- Mohr, D.L.; Wilson, W.J.; Freund, R.J. (Eds.) Chapter 2—Probability and Sampling Distributions. In Statistical Methods, 4th ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 65–122. [Google Scholar] [CrossRef]
- Boylan, G.L.; Cho, B.R. The Normal Probability Plot as a Tool for Understanding Data: A Shape Analysis from the Perspective of Skewness, Kurtosis, and Variability. Qual. Reliab. Eng. Int. 2012, 28, 249–264. [Google Scholar] [CrossRef]
- Mares Castro, A.; Domínguez Domínguez, J. Evaluación estadística de índices de desempeño para el proceso de división de rollos de EVA. Ing. Investig. Tecnol. 2022, 23, 1–15. [Google Scholar] [CrossRef]
- Rudnykh, S.I.; Ríos, V.I.L.; Rudnykh, S.I.; Ríos, V.I.L. Elección de la Función de Deseabilidad para Diseños Óptimos bajo Restricciones. Rev. EIA 2018, 15, 13–24. [Google Scholar] [CrossRef]


| Factors | Levels | ||||
|---|---|---|---|---|---|
| −1.4142 | −1 | 0 | 1 | 1.4142 | |
| KNO3 (g L−1) | 0.5 | 1.5 | 2.5 | 3.5 | 4.5 |
| Urea (g L−1) | 0.01 | 0.08 | 0.15 | 0.23 | 0.31 |
| Treatments | Coded Units | Original Units | Biomass (g L−1) | Phycocyanin (%) | ||
|---|---|---|---|---|---|---|
| KNO3 | Urea | KNO3 | Urea | |||
| 1 | 1.4142 | 0 | 4.5 | 0.15 | 0.91 | 15.87 |
| 2 | 0 | 0 | 2.5 | 0.15 | 0.68 | 8.68 |
| 3 | 1 | −1 | 3.5 | 0.08 | 0.66 | 9.83 |
| 4 | 0 | 1.4142 | 2.5 | 0.31 | 0.00 | 0.00 |
| 5 | −1 | 1 | 1.5 | 0.23 | 0.00 | 0.00 |
| 6 | −1.4142 | 0 | 0.5 | 0.15 | 0.53 | 10.44 |
| 7 | −1 | −1 | 1.5 | 0.08 | 0.33 | 7.12 |
| 8 | 0 | −1.4142 | 2.5 | 0.01 | 0.85 | 13.65 |
| 9 | 1 | 1 | 3.5 | 0.23 | 0.00 | 0.00 |
| Response | Model | F-Value | p-Value | p-Value Lack of Adjustment | R2 | R2adj |
|---|---|---|---|---|---|---|
| Biomass | Reduced quadratic | 11.34 | 0.0005 | 0.6211 | 0.7907 | 0.7210 |
| Phycocyanin | Reduced quadratic | 10.78 | 0.0006 | 0.7146 | 0.7822 | 0.7096 |
| Factors: Coded Values | Actual Optimal Values | Optimization of Results | General Desirability | |||
|---|---|---|---|---|---|---|
| Urea | KNO3 | Urea (g L−1) | KNO3 (g L−1) | Biomass | Phycocyanin | |
| −0.755 | 0.51 | 3.1 | 0.098 | 0.6211 | 0.7907 | 0.843 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire Balseca, D.A.; Castro Reyes, K.S.; Maldonado Rodríguez, M.E. Optimization of an Alternative Culture Medium for Phycocyanin Production from Arthrospira platensis under Laboratory Conditions. Microorganisms 2024, 12, 363. https://doi.org/10.3390/microorganisms12020363
Freire Balseca DA, Castro Reyes KS, Maldonado Rodríguez ME. Optimization of an Alternative Culture Medium for Phycocyanin Production from Arthrospira platensis under Laboratory Conditions. Microorganisms. 2024; 12(2):363. https://doi.org/10.3390/microorganisms12020363
Chicago/Turabian StyleFreire Balseca, Daniel Alberto, Kimberly Susana Castro Reyes, and María Elena Maldonado Rodríguez. 2024. "Optimization of an Alternative Culture Medium for Phycocyanin Production from Arthrospira platensis under Laboratory Conditions" Microorganisms 12, no. 2: 363. https://doi.org/10.3390/microorganisms12020363
APA StyleFreire Balseca, D. A., Castro Reyes, K. S., & Maldonado Rodríguez, M. E. (2024). Optimization of an Alternative Culture Medium for Phycocyanin Production from Arthrospira platensis under Laboratory Conditions. Microorganisms, 12(2), 363. https://doi.org/10.3390/microorganisms12020363








