Epidemiology of Enterotoxigenic Escherichia coli among Children and Adults Seeking Care at Hospitals in Two Geographically Distinct Rural Areas in Bangladesh
Abstract
1. Introduction
2. Materials and Methods
2.1. Surveillance
2.2. Detection of ETEC from Stool Samples
2.3. Detection of CFs
2.4. Serotyping
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results
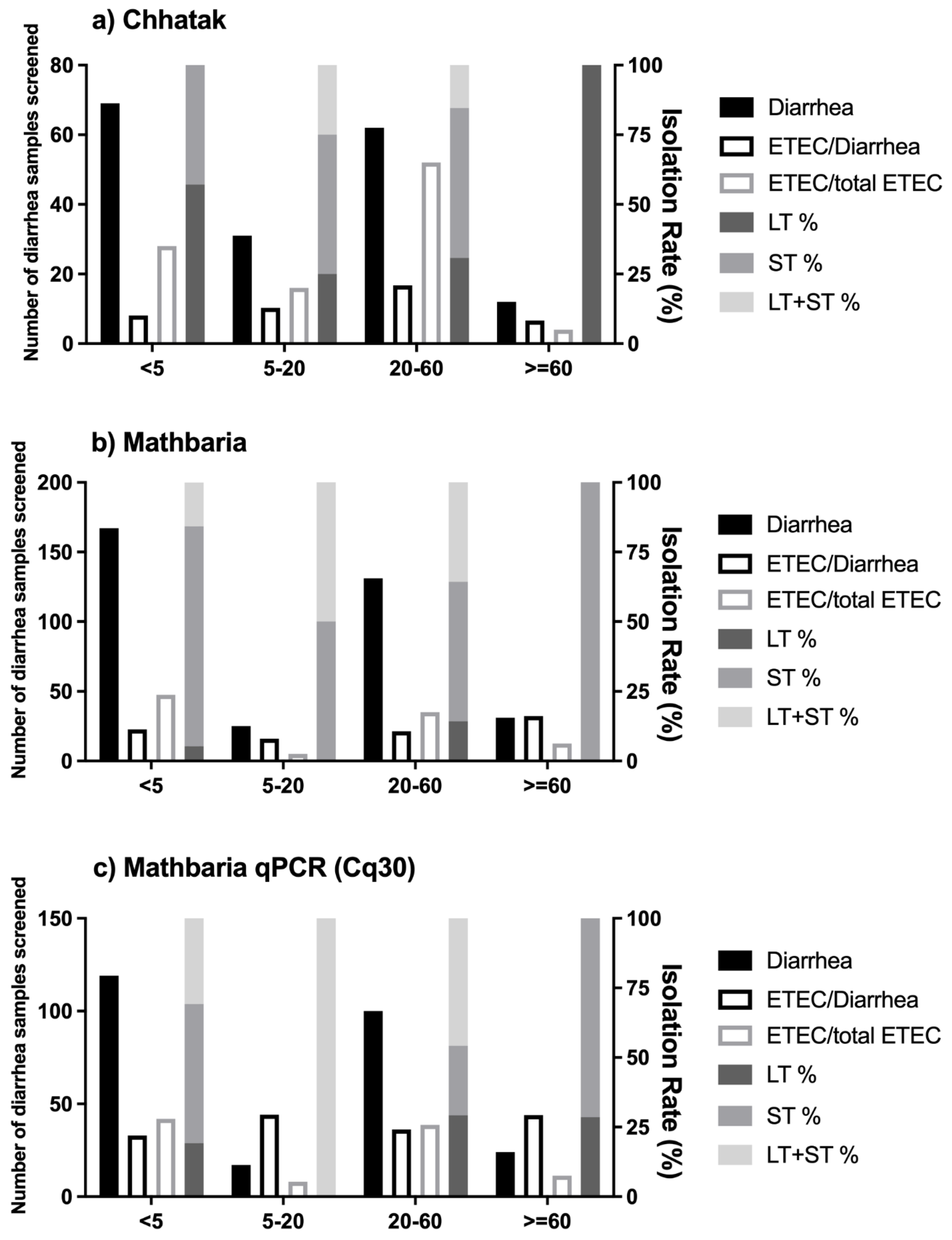
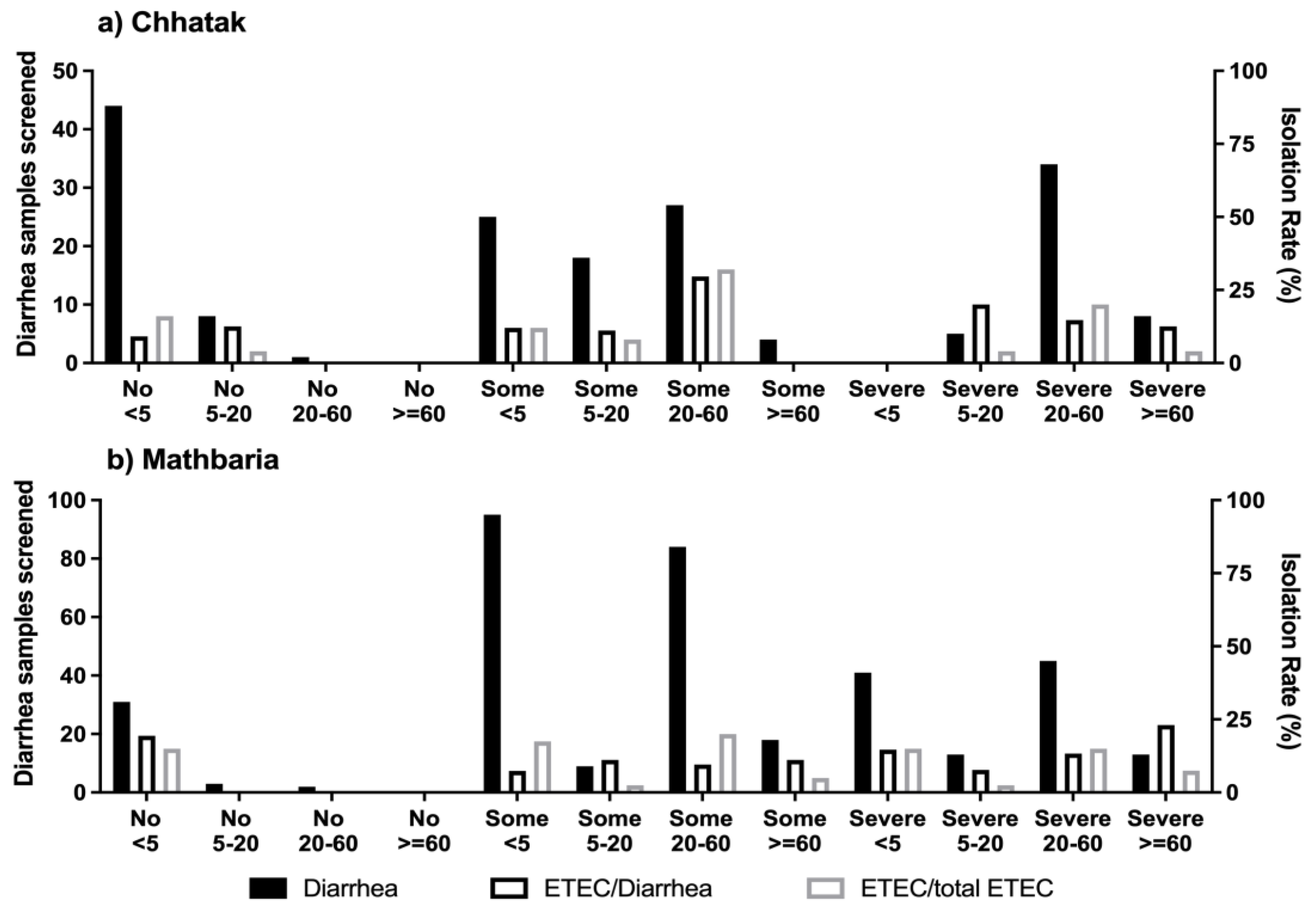
3.1. The Risk of ETEC Diarrhea among Age Groups
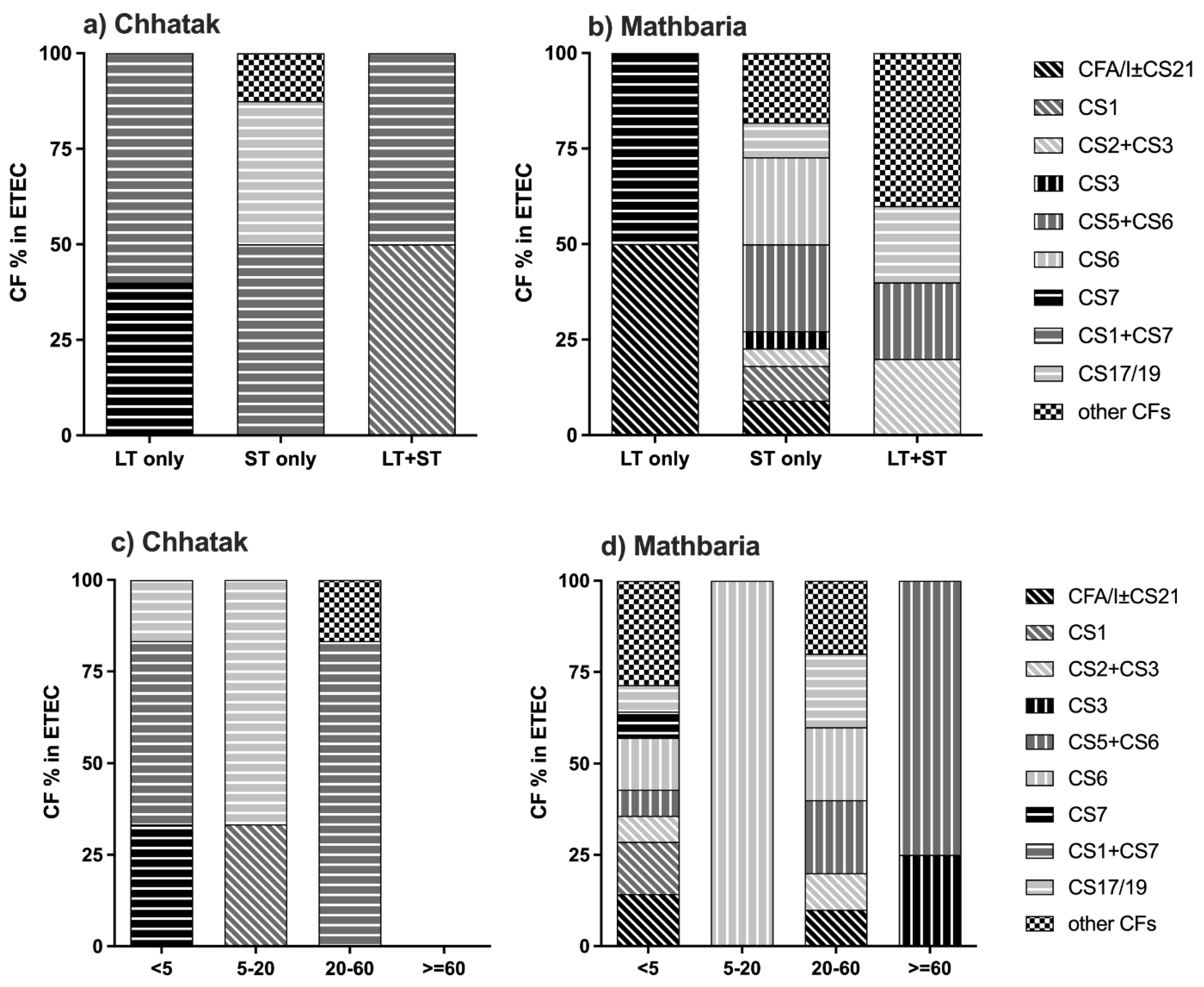
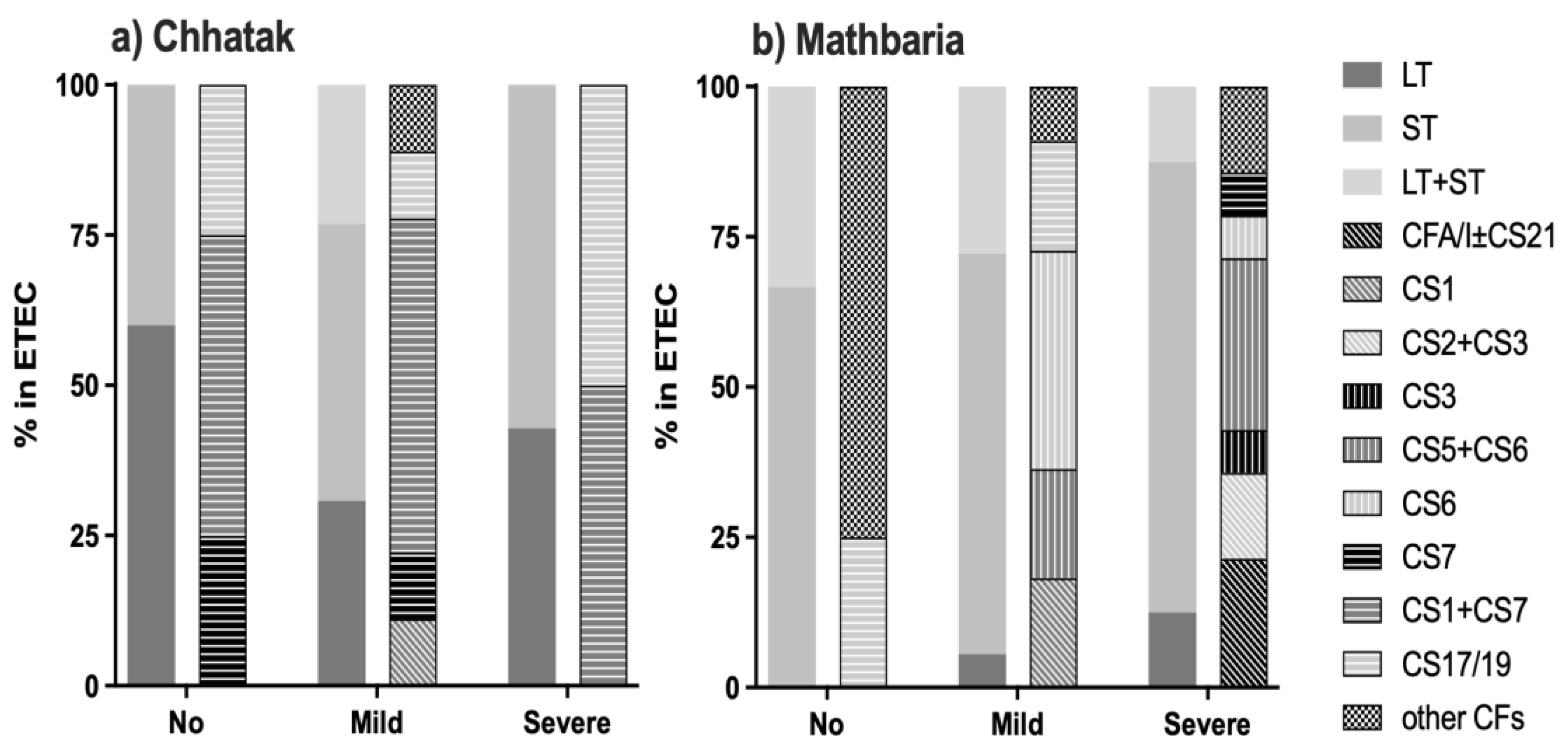
3.2. CFs and O ETEC Serogroups in Chhatak and Mathbaria
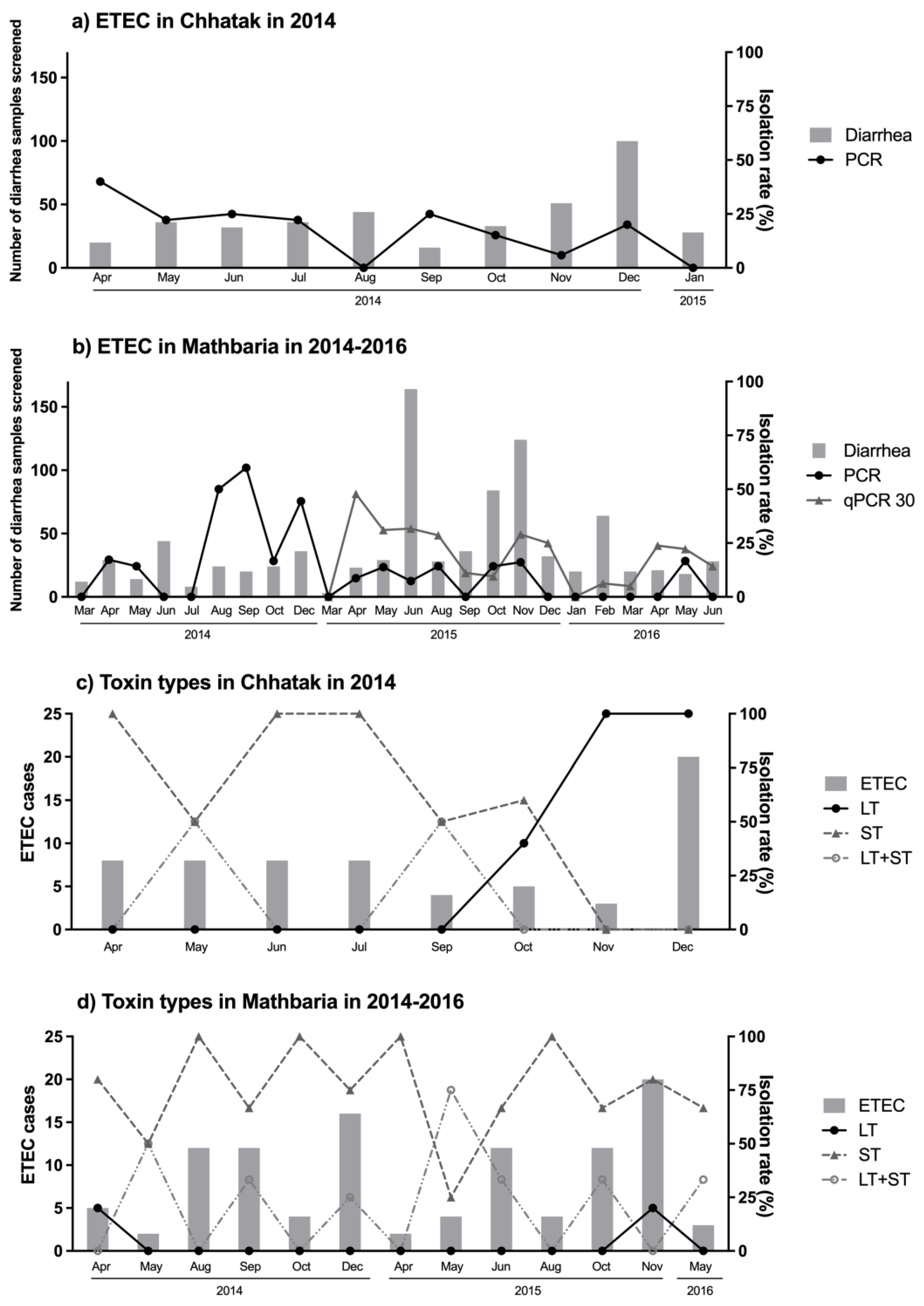
3.3. ETEC Toxin and CF Patterns over Two and Half Years in Mathbaria
3.4. Seasonal Occurrence of ETEC
3.5. Clinical Severity of ETEC Diarrhea
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qadri, F.; Svennerholm, A.M.; Faruque, A.S.G.; Sack, R.B. Enterotoxigenic Escherichia coli in Developing Countries: Epidemiology, Microbiology, Clinical Features, Treatment, and Prevention. Clin. Microbiol. Rev. 2005, 18, 465–483. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and Aetiology of Diarrhoeal Disease in Infants and Young Children in Developing Countries (the Global Enteric Multicenter Study, GEMS): A Prospective, Case-Control Study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Khalil, I.; Walker, R.; Porter, C.K.; Muhib, F.; Chilengi, R.; Cravioto, A.; Guerrant, R.; Svennerholm, A.M.; Qadri, F.; Baqar, S.; et al. Enterotoxigenic Escherichia coli (ETEC) Vaccines: Priority Activities to Enable Product Development, Licensure, and Global Access. Vaccine 2021, 39, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Hasso-Agopsowicz, M.; Lopman, B.A.; Lanata, C.F.; Rogawski McQuade, E.T.; Kang, G.; Prudden, H.J.; Khalil, I.; Platts-Mills, J.A.; Kotloff, K.; Jit, M.; et al. World Health Organization Expert Working Group: Recommendations for Assessing Morbidity Associated with Enteric Pathogens. Vaccine 2021, 39, 7521–7525. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.R.; Beeching, N.J. Travelers’ Diarrhea. Curr. Opin. Infect. Dis. 2010, 23, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-D.; Lowe, B.; Verenkar, M.P.; Ashley, D.; Steffen, R.; Tornieporth, N.; Von Sonnenburg, F.; Waiyaki, P.; Dupont, H.L. Prevalence of Enteric Pathogens among International Travelers with Diarrhea Acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J. Infect. Dis. 2002, 185, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.D.; Bagamian, K.H.; Muhib, F.; Baral, R.; Laytner, L.A.; Amaya, M.; Wierzba, T.; Rheingans, R. Potential Impact and Cost-Effectiveness of Future ETEC and Shigella Vaccines in 79 Low- and Lower Middle-Income Countries. Vaccine X 2019, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Kantele, A.; Riekkinen, M.; Jokiranta, T.S.; Pakkanen, S.H.; Pietilä, J.P.; Patjas, A.; Eriksson, M.; Khawaja, T.; Klemets, P.; Marttinen, K.; et al. Safety and immunogenicity of ETVAX®, an oral inactivated vaccine against enterotoxigenic Escherichia coli diarrhoea: A double-blinded, randomized, placebo-controlled trial amongst Finnish travellers to Benin, West Africa. J. Travel Med. 2023, 30, taad045. [Google Scholar] [CrossRef] [PubMed]
- Begum, Y.A.; Baby, N.I.; Faruque, A.S.G.; Jahan, N.; Cravioto, A.; Svennerholm, A.M.; Qadri, F. Shift in Phenotypic Characteristics of Enterotoxigenic Escherichia coli (ETEC) Isolated from Diarrheal Patients in Bangladesh. PLoS Neglected Trop. Dis. 2014, 8, e3031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khalil, I.; Troeger, C.E.; Blacker, B.F.; Reiner, R.C. Capturing the True Burden of Shigella and ETEC: The Way Forward. Vaccine 2019, 37, 4784–4786. [Google Scholar] [CrossRef]
- Connor, S.; Velagic, M.; Zhang, X.; Johura, F.T.; Chowdhury, G.; Mukhopadhyay, A.K.; Dutta, S.; Alam, M.; Sack, D.A.; Wierzba, T.F.; et al. Evaluation of a Simple, Rapid and Field-Adapted Diagnostic Assay for Enterotoxigenic E. Coli and Shigella. PLoS Neglected Trop. Dis. 2022, 16, e0010192. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.L.; Wierzba, T.F.; Walker, R.I. Status of Vaccine Research and Development for Enterotoxigenic Escherichia coli. Vaccine 2016, 34, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Hosangadi, D.; Smith, P.G.; Kaslow, D.C.; Giersing, B.K. WHO Consultation on ETEC and Shigella Burden of Disease, Geneva, 6–7th April 2017: Meeting Report. Vaccine 2019, 37, 7381–7390. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, L.M.; Bourgeois, A.L.; Fischer Walker, C.L.; Black, R.E.; Sack, D. Estimating Diarrheal Illness and Deaths Attributable to Shigellae and Enterotoxigenic Escherichia coli among Older Children, Adolescents, and Adults in South Asia and Africa. PLoS Neglected Trop. Dis. 2014, 8, e2705. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Das, S.K.; Faruque, A.S.G.; Fuchs, G.J.; Albert, M.J.; Bradley Sack, R.; Svennerholm, A.-M. Prevalence of Toxin Types and Colonization Factors in Enterotoxigenic Escherichia coli Isolated during a 2-Year Period from Diarrheal Patients in Bangladesh. J. Clin. Microbiol. 2000, 38, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Begum, Y.A.; Talukder, K.A.; Azmi, I.J.; Shahnaij, M.; Sheikh, A.; Sharmin, S.; Svennerholm, A.M.; Qadri, F. Resistance Pattern and Molecular Characterization of Enterotoxigenic Escherichia coli (ETEC) Strains Isolated in Bangladesh. PLoS ONE 2016, 11, e0157415. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, P.; Kar, A.; Sakib, N.; Shibly, A.Z.; Zohora, F.T.; Hasan, M.N. Prevalence, Antimicrobial Resistance, and Pathogenic Potential of Enterotoxigenic and Enteropathogenic Escherichia coli Associated with Acute Diarrheal Patients in Tangail, Bangladesh. Foodborne Pathog. Dis. 2020, 17, 434–439. [Google Scholar] [CrossRef]
- Reiner, R.C.; Graetz, N.; Casey, D.C.; Troeger, C.; Garcia, G.M.; Mosser, J.F.; Deshpande, A.; Swartz, S.J.; Ray, S.E.; Blacker, B.F.; et al. Variation in Childhood Diarrheal Morbidity and Mortality in Africa, 2000–2015. N. Engl. J. Med. 2018, 379, 1128–1138. [Google Scholar] [CrossRef]
- Alam, M.; Kasan, N.A.; Sadique, A.; Bhuiyan, N.A.; Ahmed, K.U.; Nusrin, S.; Nair, G.B.; Siddique, A.K.; Sack, R.B.; Sack, D.A.; et al. Seasonal Cholera Caused by Vibrio cholerae Serogroups O1 and O139 in the Coastal Aquatic Environment of Bangladesh. Appl. Environ. Microbiol. 2006, 72, 4096–4104. [Google Scholar] [CrossRef] [PubMed]
- Johura, F.-T.; Sultana, M.; Sadique, A.; Monira, S.; Sack, D.A.; Sack, R.B.; Alam, M.; Chakraborty, S. Antimicrobial resistance of enterotoxigenic Escherichia coli from diarrheal patients and environment over the years in two geographically distinct rural areas in Bangladesh. Microorganisms 2024, 12, 301. [Google Scholar] [CrossRef]
- Rodas, C.; Iniguez, V.; Qadri, F.; Wiklund, G.; Svennerholm, A.M.; Sjoling, Å. Development of Multiplex PCR Assays for Detection of Enterotoxigenic Escherichia coli Colonization Factors and Toxins. J. Clin. Microbiol. 2009, 47, 1218–1220. [Google Scholar] [CrossRef]
- Ørskov, F.; Ørskov, I. Serotyping of Escherichia coli. In Methods in Microbiology; Bergan, T., Ed.; Academic Press: London, UK, 1984; Volume 14, pp. 43–112. [Google Scholar]
- Leach, S.; Lundgren, A.; Carlin, N.; Löfstrand, M.; Svennerholm, A.M. Cross-Reactivity and Avidity of Antibody Responses Induced in Humans by the Oral Inactivated Multivalent Enterotoxigenic Escherichia coli (ETEC) Vaccine ETVAX. Vaccine 2017, 35, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, A.M.; Lundgren, A.; Leach, S.; Akhtar, M.; Qadri, F. Mucosal Immune Responses Against an Oral Enterotoxigenic Escherichia Coli Vaccine Evaluated in Clinical Trials. J. Infect. Dis. 2021, 224, S821–S828. [Google Scholar] [CrossRef] [PubMed]
- Mubanga, C.; Simuyandi, M.; Mwape, K.; Chibesa, K.; Chisenga, C.; Chilyabanyama, O.N.; Randall, A.; Liang, X.; Glashoff, R.H.; Chilengi, R. Use of an ETEC Proteome Microarray to Evaluate Cross-Reactivity of ETVAX® Vaccine-Induced IgG Antibodies in Zambian Children. Vaccines 2023, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Ahmed, S.; Ferdous, F.; Farzana, F.D.; Chisti, M.J.; Latham, J.R.; Talukder, K.A.; Rahman, M.; Begum, Y.A.; Qadri, F.; et al. Etiological Diversity of Diarrhoeal Disease in Bangladesh. J. Infect. Dev. Ctries. 2013, 7, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, F.; Ahmed, S.; Farzana, F.D.; Das, J.; Malek, M.A.; Das, S.K.; Salam, M.A.; Faruque, A.S.G. Aetiologies of Diarrhoea in Adults from Urban and Rural Treatment Facilities in Bangladesh. Epidemiol. Infect. 2015, 143, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Harro, C.; Louis Bourgeois, A.; Sack, D.; Walker, R.; DeNearing, B.; Brubaker, J.; Maier, N.; Fix, A.; Dally, L.; Chakraborty, S.; et al. Live Attenuated Enterotoxigenic Escherichia coli (ETEC) Vaccine with DmLT Adjuvant Protects Human Volunteers against Virulent Experimental ETEC Challenge. Vaccine 2019, 37, 1978–1986. [Google Scholar] [CrossRef]
- Qadri, F.; Akhtar, M.; Bhuiyan, T.R.; Chowdhury, M.I.; Ahmed, T.; Rafique, T.A.; Khan, A.; Rahman, S.I.A.; Khanam, F.; Lundgren, A.; et al. Safety and Immunogenicity of the Oral, Inactivated, Enterotoxigenic Escherichia coli Vaccine ETVAX in Bangladeshi Children and Infants: A Double-Blind, Randomised, Placebo-Controlled Phase 1/2 Trial. Lancet Infect. Dis. 2020, 20, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Lothigius, Å.; Janzon, A.; Begum, Y.; Sjöling, Å.; Qadri, F.; Svennerholm, A.-M.; Bölin, I. Enterotoxigenic Escherichia coli is detectable in water samples from an endemic area by real-time PCR. J. Appl. Microbiol. 2008, 104, 1128–1136. [Google Scholar] [CrossRef]

| Chhatak | Mathbaria | ||||
|---|---|---|---|---|---|
| April 2014–January 2015 | March 2014–June 2016 | March–December 2014 | March–December 2015 | January–June 2016 | |
| Total diarrhea patients screened | 174 | 354 | 94 | 173 | 87 |
| Male–Female Ratio | 0.71 | 1.3 | 1.09 | 1.75 | 0.89 |
| Patients with ETEC | 25 (14.4%) | 40 (11.3%) | 18 (19.1%) | 19 (11.0%) | 3 (3.4%) |
| Male–Female Ratio for ETEC | 1.08 | 0.82 | 0.8 | 0.9 | 0.5 |
| PCR from E. coli Isolates n (%) | qPCR from Stool n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total ETEC | LT-ETEC | ST-ETEC | LT+ST-ETEC | Total ETEC | LT-ETEC | ST-ETEC | LT+ST-ETEC | |
| Chhatak 2014–2015 | 25 | 10 (40.0%) | 12 (48.0%) | 3 (12.0%) | ND | |||
| Mathbaria 2014–2016 | 40 | 3 (7.5%) | 28 (70.0%) | 9 (22.5%) | ||||
| Mathbaria 2014 | 18 | 1 (5.6%) | 14 (77.8%) | 3 (16.7%) | ND | |||
| Mathbaria 2015 | 19 | 2 (10.5%) | 12 (63.2%) | 5 (26.3%) | 50 | 10 (20.0%) | 22 (44.0%) | 18 (36%) |
| Mathbaria 2016 | 3 | 0 | 2 (66.7%) | 1 (33.3%) | 12 | 4 (33.3%) | 2 (16.7%) | 6 (50.0%) |
| ETEC Strain | Proportion of ETEC Strains that Will Be Covered | |
|---|---|---|
| Chhatak | Mathbaria | |
| LT-ETEC | 40% | 7.5% |
| LT-ETEC + LT+ST-ETEC | 52% | 30% |
| LT-ETEC + LT+ST-ETEC + CFs (CFA/I, CS3, CS5 and CS6) | 59% | 76% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, S.; Johura, F.-T.; Sultana, M.; Zhang, X.; Sadique, A.; George, C.M.; Monira, S.; Sack, D.A.; Sack, R.B.; Alam, M. Epidemiology of Enterotoxigenic Escherichia coli among Children and Adults Seeking Care at Hospitals in Two Geographically Distinct Rural Areas in Bangladesh. Microorganisms 2024, 12, 359. https://doi.org/10.3390/microorganisms12020359
Chakraborty S, Johura F-T, Sultana M, Zhang X, Sadique A, George CM, Monira S, Sack DA, Sack RB, Alam M. Epidemiology of Enterotoxigenic Escherichia coli among Children and Adults Seeking Care at Hospitals in Two Geographically Distinct Rural Areas in Bangladesh. Microorganisms. 2024; 12(2):359. https://doi.org/10.3390/microorganisms12020359
Chicago/Turabian StyleChakraborty, Subhra, Fatema-Tuz Johura, Marzia Sultana, Xueyan Zhang, Abdus Sadique, Christine M. George, Shirajum Monira, David A. Sack, Richard Bradley Sack, and Munirul Alam. 2024. "Epidemiology of Enterotoxigenic Escherichia coli among Children and Adults Seeking Care at Hospitals in Two Geographically Distinct Rural Areas in Bangladesh" Microorganisms 12, no. 2: 359. https://doi.org/10.3390/microorganisms12020359
APA StyleChakraborty, S., Johura, F.-T., Sultana, M., Zhang, X., Sadique, A., George, C. M., Monira, S., Sack, D. A., Sack, R. B., & Alam, M. (2024). Epidemiology of Enterotoxigenic Escherichia coli among Children and Adults Seeking Care at Hospitals in Two Geographically Distinct Rural Areas in Bangladesh. Microorganisms, 12(2), 359. https://doi.org/10.3390/microorganisms12020359






