Effects of Resistant Starch Infusion, Solely and Mixed with Xylan or Cellulose, on Gut Microbiota Composition in Ileum-Cannulated Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental
2.1.1. DF Material
2.1.2. Animals
2.1.3. Sample Collection
2.2. 16S rRNA Sequence Processing and Bioinformatics Analyses
2.3. Quantification of SCFA
2.4. Blood Biochemistry Analyses
2.5. Statistical Analyses
3. Results
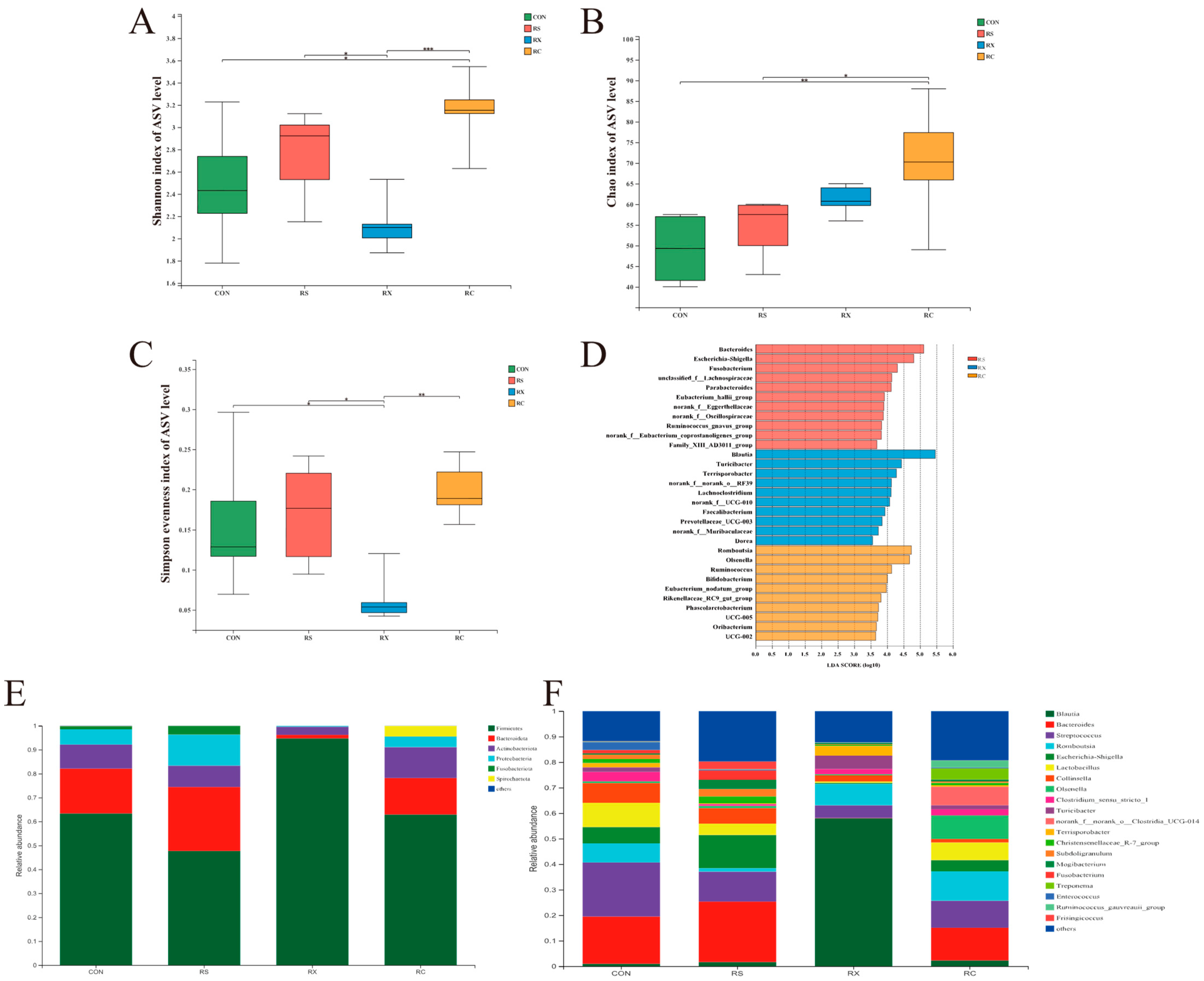
3.1. Fermentation Effects on Gut Microbiota
3.1.1. Microbiome Diversity
3.1.2. Microbiome Composition and Linear Discriminant Analysis Effect Size
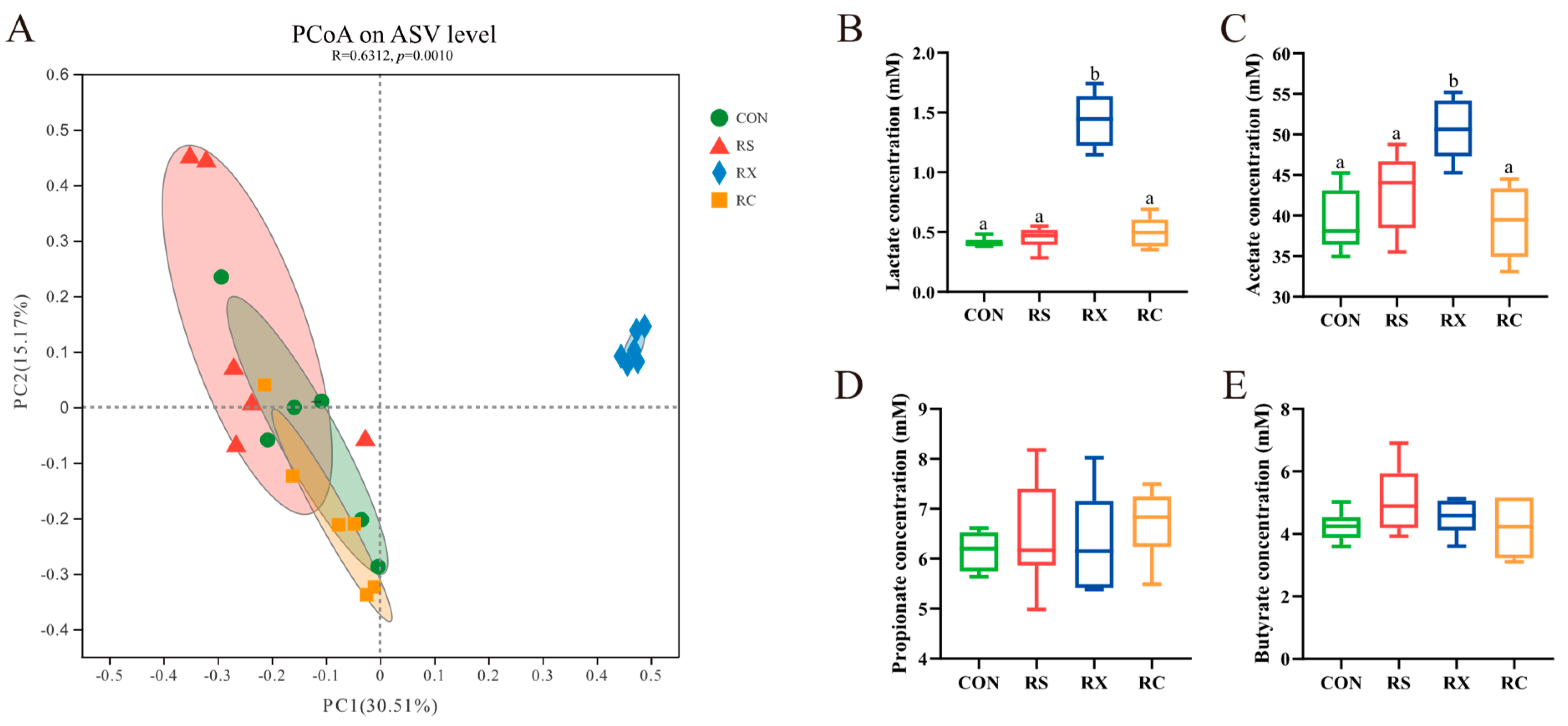
3.1.3. Principle Coordinate Analysis
3.2. Quantification of SCFA
3.3. Blood Biochemistry Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Research advances on structural characterization of resistant starch and its structure-physiological function relationship: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1059–1083. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, X.; Ren, X.; Yang, X.; Raza, H.; Ma, H. Effects of ultrasound-assisted enzymolysis on the physicochemical properties and structure of arrowhead-derived resistant starch. LWT 2021, 147, 111616. [Google Scholar] [CrossRef]
- Pan, S.; Hullar, M.A.J.; Lai, L.A.; Peng, H.; May, D.H.; Noble, W.S.; Raftery, D.; Navarro, S.L.; Neuhouser, M.L.; Lampe, P.D.; et al. Gut Microbial Protein Expression in Response to Dietary Patterns in a Controlled Feeding Study: A Metaproteomic Approach. Microorganisms 2020, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, M.C.; Haenen, D.; Souza da Silva, C.; Bosch, G.; Schols, H.A.; Gruppen, H. Influence of a diet rich in resistant starch on the degradation of non-starch polysaccharides in the large intestine of pigs. Carbohydr. Polym. 2013, 93, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Hermes, G.D.A.; Muller, M.; Bastings, J.; Vaughan, E.E.; van Den Berg, M.A.; Holst, J.J.; Venema, K.; Zoetendal, E.G.; Blaak, E.E. Fiber mixture-specific effect on distal colonic fermentation and metabolic health in lean but not in prediabetic men. Gut Microbes 2022, 14, 2009297. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Sandstrom, B.; Van Amelsvoort, J.M. The effect of ingestion of inulin on blood lipids and gastrointestinal symptoms in healthy females. Br. J. Nutr. 1997, 78, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef]
- Guan, N.; He, X.; Wang, S.; Liu, F.; Huang, Q.; Fu, X.; Chen, T.; Zhang, B. Cell Wall Integrity of Pulse Modulates the in Vitro Fecal Fermentation Rate and Microbiota Composition. J. Agric. Food Chem. 2020, 68, 1091–1100. [Google Scholar] [CrossRef]
- Jonathan, M.; Souza da Silva, C.; Bosch, G.; Schols, H.; Gruppen, H. In vivo degradation of alginate in the presence and in the absence of resistant starch. Food Chem. 2015, 172, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Tuncil, Y.E.; Sikaroodi, M.; Gillevet, P.; Patterson, J.A.; Keshavarzian, A.; Hamaker, B.R. Alterations in the amounts of microbial metabolites in different regions of the mouse large intestine using variably fermentable fibres. Bioact. Carbohydr. Diet. Fibre 2018, 13, 7–13. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, Y.; Pi, Y.; Gerrits, W.J.J.; de Vries, S.; Shang, L.; Tao, S.; Zhang, S.; Han, D.; Zhu, Z.; et al. Xylan alleviates dietary fiber deprivation-induced dysbiosis by selectively promoting Bifidobacterium pseudocatenulatum in pigs. Microbiome 2021, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, X.; Li, N.; Zhao, J.; Ye, H.; Zhang, S.; Yang, H.; Pi, Y.; Tao, S.; Han, D. In vitro fermentation characteristics and fiber-degrading enzyme kinetics of cellulose, arabinoxylan, β-glucan and glucomannan by pig fecal microbiota. Microorganisms 2021, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, Y.; Wang, Z.; Pi, Y.; Zhao, J.; Wang, S.; Han, D.; Wang, J. Amylopectin Partially Substituted by Cellulose in the Hindgut Was Beneficial to Short-Chain Fatty Acid Production and Probiotic Colonization. Microbiol. Spectr. 2023, 11, e0381522. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.; Shipley, C.; Easter, R.A. A technique for inserting a T-cannula into the distal ileum of pregnant sows. J. Anim. Sci. 1998, 76, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Wasielewska, M.; Banel, A.; Zygmunt, B. Applications. Determination of selected organic acids in animal farm water samples by ion chromatography. Int. J. Chem. Eng. Appl. 2012, 3, 178. [Google Scholar]
- Umu, O.C.; Frank, J.A.; Fangel, J.U.; Oostindjer, M.; da Silva, C.S.; Bolhuis, E.J.; Bosch, G.; Willats, W.G.; Pope, P.B.; Diep, D.B. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome 2015, 3, 16. [Google Scholar] [CrossRef]
- Haenen, D.; Souza da Silva, C.; Zhang, J.; Koopmans, S.J.; Bosch, G.; Vervoort, J.; Gerrits, W.J.; Kemp, B.; Smidt, H.; Muller, M.; et al. Resistant starch induces catabolic but suppresses immune and cell division pathways and changes the microbiome in the proximal colon of male pigs. J. Nutr. 2013, 143, 1889–1898. [Google Scholar] [CrossRef]
- Tan, F.P.Y.; Beltranena, E.; Zijlstra, R.T. Resistant starch: Implications of dietary inclusion on gut health and growth in pigs: A review. J. Anim. Sci. Biotechnol. 2021, 12, 124. [Google Scholar] [CrossRef]
- Haenen, D.; Zhang, J.; Souza da Silva, C.; Bosch, G.; van der Meer, I.M.; van Arkel, J.; van den Borne, J.J.; Perez Gutierrez, O.; Smidt, H.; Kemp, B.; et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J. Nutr. 2013, 143, 274–283. [Google Scholar] [CrossRef]
- van Erp, R.J. Nutrient Yield from Starch in Pigs: Consequences for Energy Balance and Meal Patterns. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2019. [Google Scholar]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Sai Prashanthi, G.; Ali, M.H.; Sharma, S.; Tyagi, M.; Shivaji, S. Dysbiosis in the Gut Bacterial Microbiome of Patients with Uveitis, an Inflammatory Disease of the Eye. Indian J. Microbiol. 2018, 58, 457–469. [Google Scholar] [CrossRef]
- Yang, J.; Bindels, L.B.; Segura Munoz, R.R.; Martinez, I.; Walter, J.; Ramer-Tait, A.E.; Rose, D.J. Disparate Metabolic Responses in Mice Fed a High-Fat Diet Supplemented with Maize-Derived Non-Digestible Feruloylated Oligo- and Polysaccharides Are Linked to Changes in the Gut Microbiota. PLoS ONE 2016, 11, e0146144. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Ni, K.; Tsuruta, T.; Nishino, N. Dietary Casein and Soy Protein Isolate Modulate the Effects of Raffinose and Fructooligosaccharides on the Composition and Fermentation of Gut Microbiota in Rats. J. Food Sci. 2016, 81, H2093–H2098. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Gu, J.; Li, D.; Cui, S.; Zhao, J.; Zhang, H.; Chen, W. Effects of different doses of fructooligosaccharides (FOS) on the composition of mice fecal microbiota, especially the Bifidobacterium composition. Nutrients 2018, 10, 1105. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.P.; Singh, A.K.; Jha, R. Fermentation characteristics of resistant starch, arabinoxylan, and β-glucan and their effects on the gut microbial ecology of pigs: A review. Anim. Nutr. 2019, 5, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Varel, V.H.; Yen, J.T. Microbial perspective on fiber utilization by swine. J. Anim. Sci. 1997, 75, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
| Sample | CON | RS | RX | RC | p-Value |
|---|---|---|---|---|---|
| GLU (mmol/L) | 6.56 ± 0.28 | 7.07 ± 0.42 | 6.91 ± 0.25 | 6.90 ± 0.36 | 0.091 |
| TG (mmol/L) | 1.33 ± 0.19 | 1.41 ± 0.25 | 1.53 ± 0.12 | 1.53 ± 0.19 | 0.235 |
| TC (mmol/L) | 2.26 ± 0.22 | 2.36 ± 0.32 | 2.31 ± 0.17 | 2.45 ± 0.16 | 0.517 |
| HDL (mmol/L) | 1.41 ± 0.09 | 1.50 ± 0.11 | 1.40 ± 0.08 | 1.54 ± 0.20 | 0.174 |
| LDL (mmol/L) | 1.43 ± 0.20 | 1.45 ± 0.22 | 1.45 ± 0.13 | 1.57 ± 0.13 | 0.478 |
| TP (g/L) | 58.24 ± 2.69 | 60.39 ± 3.06 | 60.16 ± 1.82 | 61.67 ± 1.48 | 0.123 |
| ALB (g/L) | 22.45 ± 2.06 | 23.17 ± 3.13 | 22.03 ± 1.81 | 22.41 ± 2.26 | 0.868 |
| GLB (g/L) | 36.39 ± 2.46 | 37.93 ± 1.61 | 37.95 ± 1.40 | 36.84 ± 1.47 | 0.350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Bai, Y.; Wang, Z.; Ye, H.; Han, D.; Zhao, J.; Wang, J.; Li, D. Effects of Resistant Starch Infusion, Solely and Mixed with Xylan or Cellulose, on Gut Microbiota Composition in Ileum-Cannulated Pigs. Microorganisms 2024, 12, 356. https://doi.org/10.3390/microorganisms12020356
Zhang Y, Bai Y, Wang Z, Ye H, Han D, Zhao J, Wang J, Li D. Effects of Resistant Starch Infusion, Solely and Mixed with Xylan or Cellulose, on Gut Microbiota Composition in Ileum-Cannulated Pigs. Microorganisms. 2024; 12(2):356. https://doi.org/10.3390/microorganisms12020356
Chicago/Turabian StyleZhang, Yaowen, Yu Bai, Zhenyu Wang, Hao Ye, Dandan Han, Jinbiao Zhao, Junjun Wang, and Defa Li. 2024. "Effects of Resistant Starch Infusion, Solely and Mixed with Xylan or Cellulose, on Gut Microbiota Composition in Ileum-Cannulated Pigs" Microorganisms 12, no. 2: 356. https://doi.org/10.3390/microorganisms12020356
APA StyleZhang, Y., Bai, Y., Wang, Z., Ye, H., Han, D., Zhao, J., Wang, J., & Li, D. (2024). Effects of Resistant Starch Infusion, Solely and Mixed with Xylan or Cellulose, on Gut Microbiota Composition in Ileum-Cannulated Pigs. Microorganisms, 12(2), 356. https://doi.org/10.3390/microorganisms12020356







