Exploring the Mechanistic Interplay between Gut Microbiota and Precocious Puberty: A Narrative Review
Abstract
1. Introduction
2. Methods
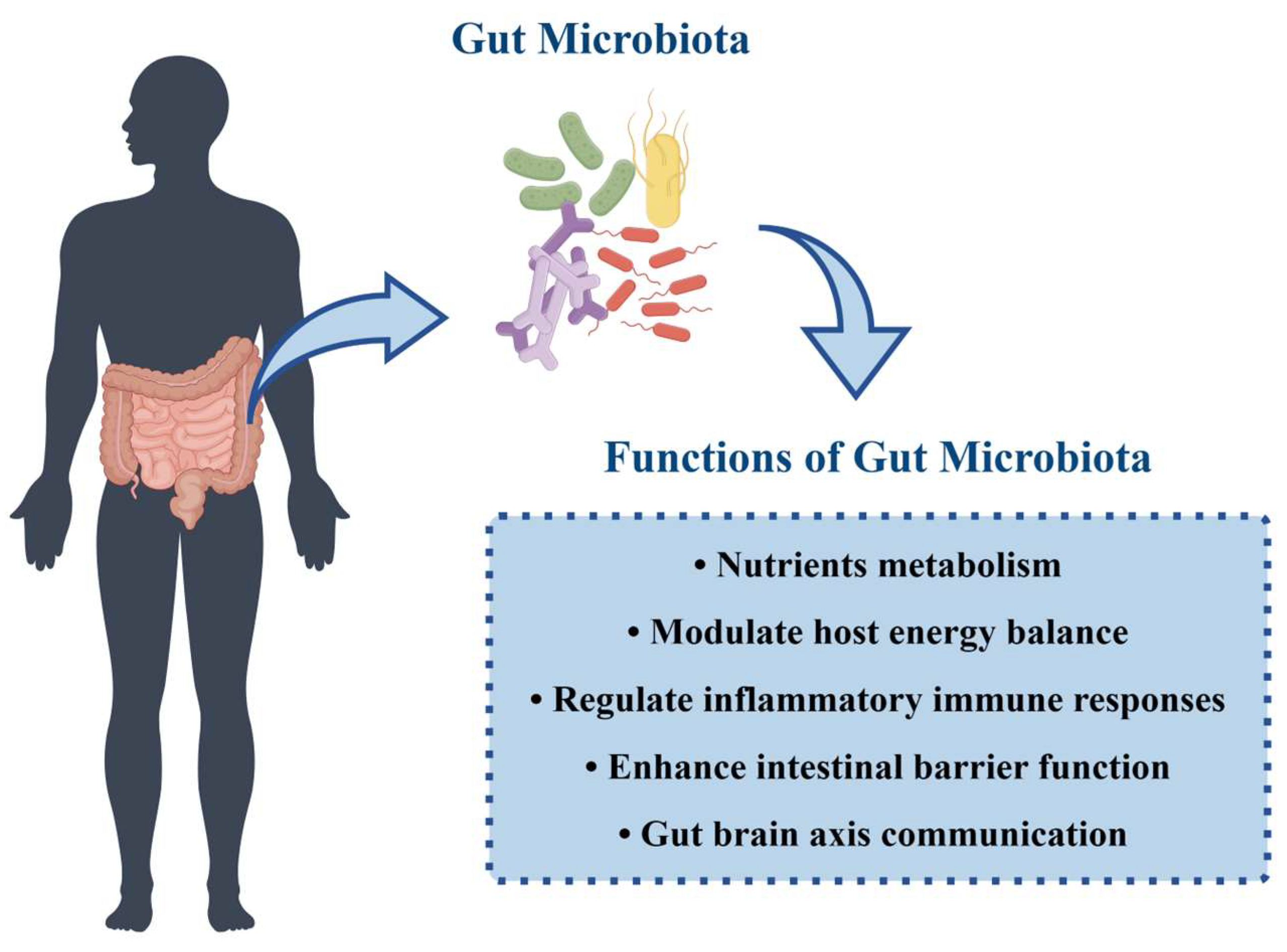
3. Gut Microbiota
3.1. Developmental Trajectory of Gut Microbiota
3.2. Functions of Gut Microbiota
4. Precocious Puberty
4.1. Definition
4.2. Epidemiology of Precocious Puberty
4.3. Etiology and Risks of Precocious Puberty
4.4. Current Diagnosis and Treatment of Precocious Puberty
4.5. Dysbiosis of Gut Microbiota in Precocious Puberty
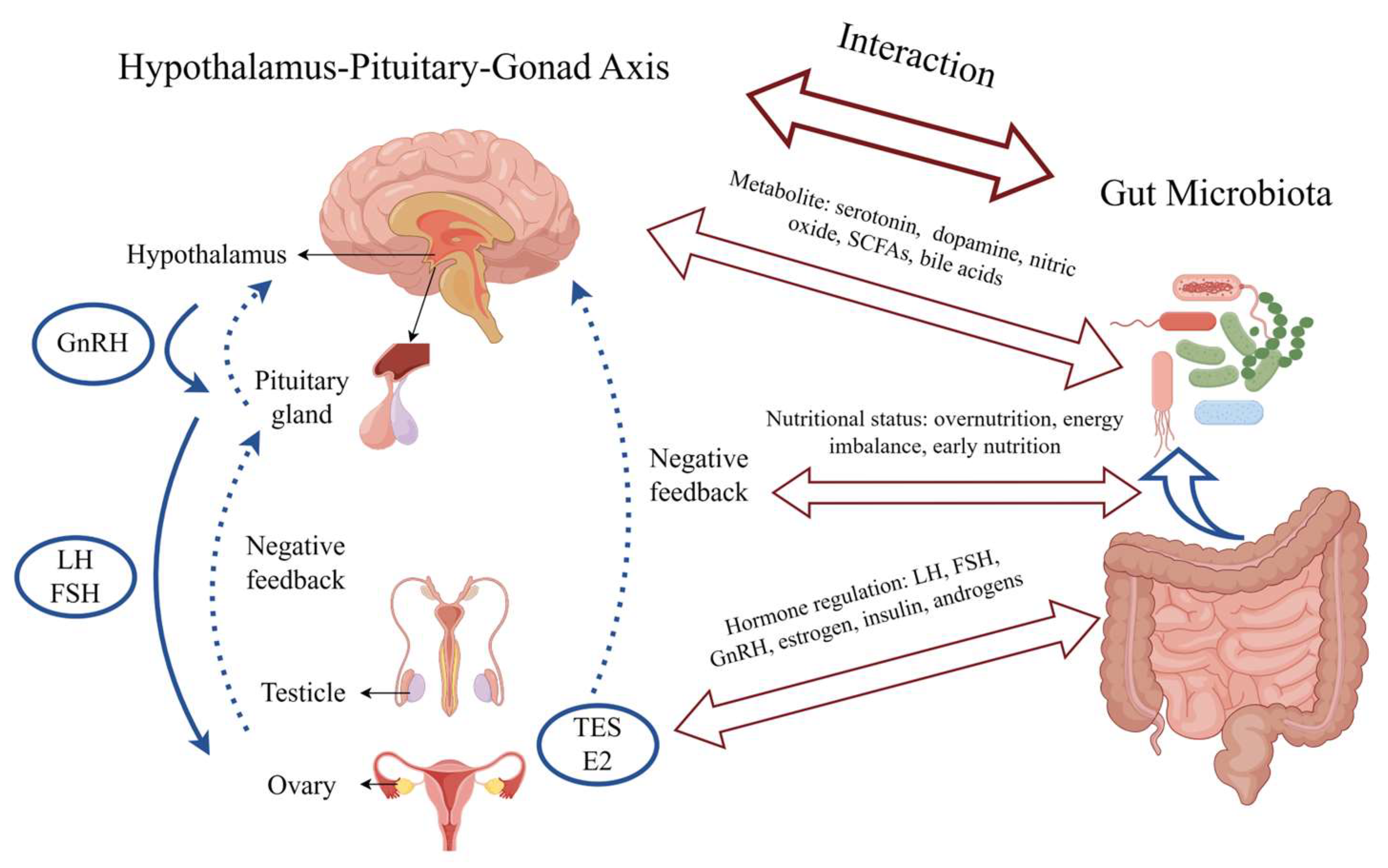
5. Interaction and Potential Mechanisms between Gut Microbiota and Precocious Puberty
5.1. Metabolic Pathways
5.1.1. Neurotransmitter Metabolic Pathways
5.1.2. Amino Acid Metabolic Pathways
5.1.3. Lipid Metabolism
5.1.4. Bile Acid Metabolism
5.2. Hormonal Regulation
5.3. Nutritional Status
5.4. The Potential Role of the Gut Microbiota–Brain Axis in Precocious Puberty
5.5. The Potential Confounding Factors That Could Influence the Observed Associations between Gut Microbiota and Precocious Puberty
6. The Prospects of Microbiota-Associated Therapies
6.1. Probiotics
6.2. Fecal Microbiota Transplantation (FMT)
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef]
- Bergström, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Mølgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of intestinal microbiota during early life: A longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Markle, J.G.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; Von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Agans, R.; Rigsbee, L.; Kenche, H.; Michail, S.; Khamis, H.J.; Paliy, O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol. Ecol. 2011, 77, 404–412. [Google Scholar] [CrossRef]
- Li, Y.; Shen, L.; Huang, C.; Li, X.; Chen, J.; Li, S.C.; Shen, B. Altered nitric oxide induced by gut microbiota reveals the connection between central precocious puberty and obesity. Clin. Transl. Med. 2021, 11, e299. [Google Scholar] [CrossRef] [PubMed]
- Muir, A. Precocious puberty. Pediatr. Rev. 2006, 27, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Latronico, A.C.; Brito, V.N.; Carel, J.-C. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016, 4, 265–274. [Google Scholar] [CrossRef]
- Day, F.R.; Elks, C.E.; Murray, A.; Ong, K.K.; Perry, J.R. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: The UK Biobank study. Sci. Rep. 2015, 5, 11208. [Google Scholar] [CrossRef]
- Cisternino, M.; Arrigo, T.; Pasquino, A.M.; Tinelli, C.; Antoniazzi, F.; Beduschi, L.; Bindi, G.; Borrelli, P.; De Sanctis, V.; Farello, G. Etiology and age incidence of precocious puberty in girls: A multicentric study. J. Pediatr. Endocrinol. Metab. 2000, 13, 695–702. [Google Scholar] [CrossRef]
- Partsch, C.J.; Heger, S.; Sippell, W.G. Management and outcome of central precocious puberty. Clin. Endocrinol. 2002, 56, 129–148. [Google Scholar] [CrossRef]
- Huang, C.; Liu, H.; Yang, W.; Li, Y.; Wu, B.; Chen, J.; Yang, Z.; Liao, C.; Liu, L.; Zhang, X. Distinct gut microbiota structure and function of children with idiopathic central and peripheral precocious puberty. Int. J. Endocrinol. 2022, 2022, 7175250. [Google Scholar] [CrossRef]
- Bo, T.; Liu, M.; Tang, L.; Lv, J.; Wen, J.; Wang, D. Effects of high-fat diet during childhood on precocious puberty and gut microbiota in mice. Front. Microbiol. 2022, 13, 930747. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Tan, B.; Yi, Q.; Liu, H.; Deng, H.; Chen, Y.; Wang, R.; Tian, J.; Zhu, J. Gut microbiota and its derived SCFAs regulate the HPGA to reverse obesity-induced precocious puberty in female rats. Front. Endocrinol. 2022, 13, 1051797. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Y.; Miller, D.; Rehman, N.O.; Cheng, X.; Yeo, J.-Y.; Joe, B.; Hill, J.W. Microbial reconstitution reverses early female puberty induced by maternal high-fat diet during lactation. Endocrinology 2020, 161, bqz041. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, J.; Yang, Z.; Feng, X.; Li, J.; Li, D.; Huang, M.; Li, Y.; Qiu, M.; Lu, X. The association of gut microbiota with idiopathic central precocious puberty in girls. Front. Endocrinol. 2020, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, R.; Gundamaraju, R.; Shastri, M.D.; Shukla, S.D.; Kalpurath, K.; Ball, M.; Tristram, S.; Shankar, E.M.; Ahuja, K.; Eri, R. Gut microbial changes, interactions, and their implications on human lifecycle: An ageing perspective. BioMed Res. Int. 2018, 2018, 4178607. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C. The composition of the gut microbiota throughout life, with an emphasis on early life. Microbes Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Gur, T.L.; Shay, L.; Palkar, A.V.; Fisher, S.; Varaljay, V.A.; Dowd, S.; Bailey, M.T. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav. Immun. 2017, 64, 50–58. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Arrieta, M.-C.; Dimitriu, P.A.; Cheng, J.; Thorson, L.; Lefebvre, D.L.; Azad, M.B.; Subbarao, P.; Mandhane, P.; Becker, A. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin. Sci. 2016, 130, 2199–2207. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Eggesbø, M.; Moen, B.; Peddada, S.; Baird, D.; Rugtveit, J.; Midtvedt, T.; Bushel, P.R.; Sekelja, M.; Rudi, K. Development of gut microbiota in infants not exposed to medical interventions. Apmis 2011, 119, 17–35. [Google Scholar] [CrossRef]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef]
- Korpela, K. Impact of delivery mode on infant gut microbiota. Ann. Nutr. Metab. 2021, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Wampach, L.; Heintz-Buschart, A.; Fritz, J.V.; Ramiro-Garcia, J.; Habier, J.; Herold, M.; Narayanasamy, S.; Kaysen, A.; Hogan, A.H.; Bindl, L. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun. 2018, 9, 5091. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Thorsen, J.; Chawes, B.L.; Schjørring, S.; Krogfelt, K.A.; Bønnelykke, K.; Bisgaard, H. Cesarean section changes neonatal gut colonization. J. Allergy Clin. Immunol. 2016, 138, 881–889.e2. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra382. [Google Scholar] [CrossRef]
- Baumann-Dudenhoeffer, A.M.; D’Souza, A.W.; Tarr, P.I.; Warner, B.B.; Dantas, G. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat. Med. 2018, 24, 1822–1829. [Google Scholar] [CrossRef]
- La Rosa, P.S.; Warner, B.B.; Zhou, Y.; Weinstock, G.M.; Sodergren, E.; Hall-Moore, C.M.; Stevens, H.J.; Bennett, W.E., Jr.; Shaikh, N.; Linneman, L.A. Patterned progression of bacterial populations in the premature infant gut. Proc. Natl. Acad. Sci. USA 2014, 111, 12522–12527. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.; Chou, W.-C.; Conner, M.; Earl, A.; Knight, R.; Bjorkman, P. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S. Environmental factors shaping the gut microbiome in a Dutch population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.-A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The gut microbiota in the first decade of life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Roswall, J.; Olsson, L.M.; Kovatcheva-Datchary, P.; Nilsson, S.; Tremaroli, V.; Simon, M.-C.; Kiilerich, P.; Akrami, R.; Krämer, M.; Uhlén, M. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 2021, 29, 765–776.e3. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X. Sexual dimorphism of gut microbiota at different pubertal status. Microb. Cell Factories 2020, 19, 152. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Wang, F.; Yu, T.; Huang, G.; Cai, D.; Liang, X.; Su, H.; Zhu, Z.; Li, D.; Yang, Y.; Shen, P. Gut microbiota community and its assembly associated with age and diet in Chinese centenarians. J. Microbiol. Biotechnol. 2015, 25, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, P.; Ma, C.; Tang, J.; Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Sangokunle, O.; Singh, P.; Nagpal, R. Gut microbiome-derived metabolites in host health and diseases. In Human-Gut Microbiome; Academic Press: Cambridge, MA, USA, 2022; pp. 81–91. [Google Scholar]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Portune, K.J.; Beaumont, M.; Davila, A.-M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017, 8, 53829. [Google Scholar] [CrossRef]
- Bistoletti, M.; Bosi, A.; Banfi, D.; Giaroni, C.; Baj, A. The microbiota-gut-brain axis: Focus on the fundamental communication pathways. Prog. Mol. Biol. Transl. Sci. 2020, 176, 43–110. [Google Scholar]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Ignatova, V. Influence of gut microbiota on behavior and its disturbances. In Behavioral Neuroscience; Intech Open: London, UK, 2019; pp. 17–43. [Google Scholar]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin. Microbiol. Rev. 2022, 35, e0033820. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D.; Demirkan, A.; Twa, G.; Cohen, G.; Dean, M.N.; Standaert, D.G.; Sampson, T.R.; Payami, H. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat. Commun. 2022, 13, 6958. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Lim, S.Y.; Lang, A.E. The microbiome–gut–brain axis in Parkinson disease—From basic research to the clinic. Nat. Rev. Neurol. 2022, 18, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Ferreiro, A.L.; Choi, J.; Ryou, J.; Newcomer, E.P.; Thompson, R.; Bollinger, R.M.; Hall-Moore, C.; Ndao, I.M.; Sax, L.; Benzinger, T.L. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci. Transl. Med. 2023, 15, eabo2984. [Google Scholar] [CrossRef]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar] [CrossRef]
- Zhu, F.; Ju, Y.; Wang, W.; Wang, Q.; Guo, R.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z. Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun. 2020, 11, 1612. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Pröbstel, A.-K.; Zhou, X.; Baumann, R.; Wischnewski, S.; Kutza, M.; Rojas, O.L.; Sellrie, K.; Bischof, A.; Kim, K.; Ramesh, A. Gut microbiota–specific IgA+ B cells traffic to the CNS in active multiple sclerosis. Sci. Immunol. 2020, 5, eabc7191. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, A. Gut microbiota and depression. Nat. Rev. Microbiol. 2022, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fan, Y.; Xu, L.; Yu, Z.; Wang, S.; Xu, H.; Zhang, J.; Zhang, L.; Liu, W.; Wu, L. Microbiome and tryptophan metabolomics analysis in adolescent depression: Roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 2023, 11, 145. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kotsiliti, E. Gut microbiome and autism spectrum disorder. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.T.; Jin, D.-M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K. Multi-level analysis of the gut–brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat. Neurosci. 2023, 26, 1208–1217. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Konjevod, M.; Perkovic, M.N.; Saiz, J.; Strac, D.S.; Barbas, C.; Rojo, D. Metabolomics analysis of microbiota-gut-brain axis in neurodegenerative and psychiatric diseases. J. Pharm. Biomed. Anal. 2021, 194, 113681. [Google Scholar] [CrossRef]
- Bradley, S.H.; Lawrence, N.; Steele, C.; Mohamed, Z. Precocious puberty. BMJ 2020, 368, I6597. [Google Scholar] [CrossRef] [PubMed]
- Bräuner, E.V.; Busch, A.S.; Eckert-Lind, C.; Koch, T.; Hickey, M.; Juul, A. Trends in the incidence of central precocious puberty and normal variant puberty among children in Denmark, 1998 to 2017. JAMA Netw. Open 2020, 3, e2015665. [Google Scholar] [CrossRef]
- Biro, F.M.; Galvez, M.P.; Greenspan, L.C.; Succop, P.A.; Vangeepuram, N.; Pinney, S.M.; Teitelbaum, S.; Windham, G.C.; Kushi, L.H.; Wolff, M.S. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 2010, 126, e583–e590. [Google Scholar] [CrossRef]
- Sørensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Mogensen, S.S.; Juul, A. Recent secular trends in pubertal timing: Implications for evaluation and diagnosis of precocious puberty. Horm. Res. Paediatr. 2012, 77, 137–145. [Google Scholar] [CrossRef]
- Teilmann, G.; Pedersen, C.B.; Jensen, T.K.; Skakkebæk, N.E.; Juul, A. Prevalence and incidence of precocious pubertal development in Denmark: An epidemiologic study based on national registries. Pediatrics 2005, 116, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Herman-Giddens, M.E.; Slora, E.J.; Wasserman, R.C.; Bourdony, C.J.; Bhapkar, M.V.; Koch, G.G.; Hasemeier, C.M. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings network. Pediatrics 1997, 99, 505–512. [Google Scholar] [CrossRef]
- Kaplowitz, P.; Bloch, C.; Sills, I.N.; Bloch, C.A.; Casella, S.J.; Gonzalez, J.L.; Lynch, J.L.; Wintergerst, K.A. Evaluation and referral of children with signs of early puberty. Pediatrics 2016, 137, e20153732. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Chung, S.J.; Kang, M.J.; Yoon, J.Y.; Lee, J.E.; Lee, Y.A.; Shin, C.H.; Yang, S.W. Boys with precocious or early puberty: Incidence of pathological brain magnetic resonance imaging findings and factors related to newly developed brain lesions. Ann. Pediatr. Endocrinol. Metab. 2013, 18, 183. [Google Scholar] [CrossRef]
- Solorzano, C.M.B.; McCartney, C.R. Obesity and the pubertal transition in girls and boys. Reproduction 2010, 140, 399. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Q.; Deng, X.; Chen, Y.; Liu, S.; Story, M. Association between obesity and puberty timing: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2017, 14, 1266. [Google Scholar] [CrossRef]
- Reinehr, T.; Roth, C.L. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc. Health 2019, 3, 44–54. [Google Scholar] [CrossRef]
- Carel, J.C.; Lahlou, N.; Roger, M.; Chaussain, J.L. Precocious puberty and statural growth. Hum. Reprod. Update 2004, 10, 135–147. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, M.I. Psychosocial aspects in girls with idiopathic precocious puberty. Psychiatry Investig. 2012, 9, 25. [Google Scholar] [CrossRef]
- Elks, C.E.; Ong, K.K.; Scott, R.A.; Van Der Schouw, Y.T.; Brand, J.S.; Wark, P.A.; Amiano, P.; Balkau, B.; Barricarte, A.; Boeing, H. Age at menarche and type 2 diabetes risk: The EPIC-InterAct study. Diabetes Care 2013, 36, 3526–3534. [Google Scholar] [CrossRef]
- Prentice, P.; Viner, R.M. Pubertal timing and adult obesity and cardiometabolic risk in women and men: A systematic review and meta-analysis. Int. J. Obes. 2013, 37, 1036–1043. [Google Scholar] [CrossRef]
- Bodicoat, D.H.; Schoemaker, M.J.; Jones, M.E.; McFadden, E.; Griffin, J.; Ashworth, A.; Swerdlow, A.J. Timing of pubertal stages and breast cancer risk: The Breakthrough Generations Study. Breast Cancer Res. 2014, 16, R18. [Google Scholar] [CrossRef] [PubMed]
- Carel, J.-C.; Léger, J. Precocious puberty. N. Engl. J. Med. 2008, 358, 2366–2377. [Google Scholar] [CrossRef]
- Brito, V.N.; Latronico, A.C.; Arnhold, I.J.; Mendonça, B.B. Update on the etiology, diagnosis and therapeutic management of sexual precocity. Arq. Bras. Endocrinol. Metabol. 2008, 52, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Neely, E.K.; Hintz, R.L.; Wilson, D.M.; Lee, P.A.; Gautier, T.; Argente, J.; Stene, M. Normal ranges for immunochemiluminometric gonadotropin assays. J. Pediatr. 1995, 127, 40–46. [Google Scholar] [CrossRef]
- Carretto, F.; Salinas-Vert, I.; Granada-Yvern, M.; Murillo-Vallés, M.; Gómez-Gómez, C.; Puig-Domingo, M.; Bel, J. The usefulness of the leuprolide stimulation test as a diagnostic method of idiopathic central precocious puberty in girls. Horm. Metab. Res. 2014, 46, 959–963. [Google Scholar] [CrossRef]
- Carel, J.-C.; Eugster, E.A.; Rogol, A.; Ghizzoni, L.; Palmert, M.R.; on behalf of the members of the ESPE-LWPES GnRH Analogs Consensus Conference Group. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009, 123, e752–e762. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, N.; Carel, J.-C.; Chaussain, J.-L.; Roger, M. Pharmacokinetics and pharmacodynamics of GnRH agonists: Clinical implications in pediatrics. J. Pediatr. Endocrinol. Metab. 2000, 13, 723–738. [Google Scholar] [CrossRef]
- Carel, J.-C.; Lahlou, N.; Jaramillo, O.; Montauban, V.; Teinturier, C.c.; Colle, M.; Lucas, C.; French Leuprorelin Trial Group; Chaussain, J.L. Treatment of central precocious puberty by subcutaneous injections of leuprorelin 3-month depot (11.25 mg). J. Clin. Endocrinol. Metab. 2002, 87, 4111–4116. [Google Scholar] [CrossRef]
- Johnson, S.R.; Nolan, R.C.; Grant, M.T.; Price, G.J.; Siafarikas, A.; Bint, L.; Choong, C.S. Sterile abscess formation associated with depot leuprorelin acetate therapy for central precocious puberty. J. Paediatr. Child Health 2012, 48, E136–E139. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Zou, H.; Huang, P.; Luo, H.; Li, H.; Cai, Y.; Liu, L.; Li, Y.; He, X. Gut microbiome combined with metabolomics reveals biomarkers and pathways in central precocious puberty. J. Transl. Med. 2023, 21, 316. [Google Scholar] [CrossRef]
- Ng, Q.X.; Yau, C.E.; Yaow, C.Y.L.; Chong, R.I.H.; Chong, N.Z.-Y.; Teoh, S.E.; Lim, Y.L.; Soh, A.Y.S.; Ng, W.K.; Thumboo, J. What Has Longitudinal ‘Omics’ Studies Taught Us about Irritable Bowel Syndrome? A Systematic Review. Metabolites 2023, 13, 484. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, V.; Liberati, M.; D’Antonio, F.; Masuccio, F.; Capanna, R.; Verrotti, A.; Chiarelli, F.; Mohn, A. GNRH analog therapy in girls with early puberty is associated with the achievement of predicted final height but also with increased risk of polycystic ovary syndrome. Eur. J. Endocrinol. 2010, 163, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, H.; Yu, Z.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lü, M. The gut microbiome and sex hormone-related diseases. Front. Microbiol. 2021, 12, 711137. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Nestler, J.E.; Jakubowicz, D.J.; Falcon de Vargas, A.; Brik, C.; Quintero, N.; Medina, F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J. Clin. Endocrinol. Metab. 1998, 83, 2001–2005. [Google Scholar] [PubMed]
- Franasiak, J.M.; Scott, R.T., Jr. Introduction: Microbiome in human reproduction. Fertil. Steril. 2015, 104, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Yurtdaş, G.; Akdevelioğlu, Y. A new approach to polycystic ovary syndrome: The gut microbiota. J. Am. Coll. Nutr. 2020, 39, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; De Sanctis, V.; Elalaily, R. Nutrition and pubertal development. Indian J. Endocrinol. Metab. 2014, 18, S39. [Google Scholar] [CrossRef]
- Hong, Y.H.; Woo, Y.J.; Lee, J.H.; Shin, Y.-L.; Lim, H.-S. Association between dietary habits and parental health with obesity among children with precocious puberty. Children 2020, 7, 220. [Google Scholar] [CrossRef]
- Huang, A.; Roth, C.L. The link between obesity and puberty: What is new? Curr. Opin. Pediatr. 2021, 33, 449–457. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; Sottotetti, F.; Rossi, V.; Loperfido, F.; Zuccotti, G. Breast and Formula Milk and Early Puberty Onset. Children 2023, 10, 1686. [Google Scholar] [CrossRef]
- Hvidt, J.J.; Brix, N.; Ernst, A.; Lunddorf, L.L.; Ramlau-Hansen, C.H. Breast feeding and timing of puberty in boys and girls: A nationwide cohort study. Paediatr. Perinat. Epidemiol. 2021, 35, 578–589. [Google Scholar] [CrossRef]
- Al-Sahab, B.; Adair, L.; Hamadeh, M.J.; Ardern, C.I.; Tamim, H. Impact of breastfeeding duration on age at menarche. Am. J. Epidemiol. 2011, 173, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.K.; Emmett, P.; Northstone, K.; Golding, J.; Rogers, I.; Ness, A.R.; Wells, J.C.; Dunger, D.B. Infancy weight gain predicts childhood body fat and age at menarche in girls. J. Clin. Endocrinol. Metab. 2009, 94, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Pi, Y.; Mu, C.L.; Peng, Y.; Huang, Z.; Zhu, W.Y. Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J. Neurochem. 2018, 146, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Mannerås-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Gladh, A.H.; Seeley, R.J.; Drucker, D.J.; Bäckhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021, 35, 109163. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, Y.; Li, L.; Ge, W.; Yu, S.; Hao, Y.; Shen, W.; Han, X.; Ma, D.; Yin, S. Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut 2020, 70, 222–225. [Google Scholar] [CrossRef]
- Toppari, J.; Juul, A. Trends in puberty timing in humans and environmental modifiers. Mol. Cell. Endocrinol. 2010, 324, 39–44. [Google Scholar] [CrossRef]
- Bleil, M.E.; Booth-LaForce, C.; Benner, A.D. Race disparities in pubertal timing: Implications for cardiovascular disease risk among African American women. Popul. Res. Policy Rev. 2017, 36, 717–738. [Google Scholar] [CrossRef]
- Bonder, M.J.; Kurilshikov, A.; Tigchelaar, E.F.; Mujagic, Z.; Imhann, F.; Vila, A.V.; Deelen, P.; Vatanen, T.; Schirmer, M.; Smeekens, S.P. The effect of host genetics on the gut microbiome. Nat. Genet. 2016, 48, 1407–1412. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Wijmenga, C.; Fu, J.; Zhernakova, A. Host genetics and gut microbiome: Challenges and perspectives. Trends Immunol. 2017, 38, 633–647. [Google Scholar] [CrossRef]
- Imhann, F.; Vila, A.V.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; Van Dullemen, H.M. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018, 67, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Dzierozynski, L.; Queen, J.; Sears, C.L. Subtle, persistent shaping of the gut microbiome by host genes: A critical determinant of host biology. Cell Host Microbe 2023, 31, 1569–1573. [Google Scholar] [CrossRef]
- Leijs, M.M.; Koppe, J.G.; Olie, K.; van Aalderen, W.M.; de Voogt, P.; Vulsma, T.; Westra, M.; Ten Tusscher, G.W. Delayed initiation of breast development in girls with higher prenatal dioxin exposure; a longitudinal cohort study. Chemosphere 2008, 73, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Eskenazi, B.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Holland, N.; Calafat, A.M.; Ye, X.; Harley, K.G. Association of prenatal urinary concentrations of phthalates and bisphenol A and pubertal timing in boys and girls. Environ. Health Perspect. 2018, 126, 097004. [Google Scholar] [CrossRef]
- Andrade, J.C.; Kumar, S.; Kumar, A.; Černáková, L.; Rodrigues, C.F. Application of probiotics in candidiasis management. Crit. Rev. Food Sci. Nutr. 2022, 62, 8249–8264. [Google Scholar] [CrossRef] [PubMed]
- Newlove-Delgado, T.; Abbott, R.A.; Martin, A.E. Probiotics for children with recurrent abdominal pain. JAMA Pediatr. 2019, 173, 183–184. [Google Scholar] [CrossRef]
- Ettinger, G.; MacDonald, K.; Reid, G.; Burton, J.P. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes 2014, 5, 719–728. [Google Scholar] [CrossRef]
- Han, M.; Lei, W.; Liang, J.; Li, H.; Hou, M.; Gao, Z. The single-cell modification strategies for probiotics delivery in inflammatory bowel disease: A review. Carbohydr. Polym. 2023, 324, 121472. [Google Scholar] [CrossRef]
- Yuan, X.; Shangguan, H.; Zhang, Y.; Lin, X.; Chen, R. Intervention Effect of Probiotics on the Early Onset of Puberty Induced by Daidzein in Female Mice. Mol. Nutr. Food Res. 2023, 67, e2200501. [Google Scholar] [CrossRef]
- Cowan, C.S.; Richardson, R. Early-life stress leads to sex-dependent changes in pubertal timing in rats that are reversed by a probiotic formulation. Dev. Psychobiol. 2019, 61, 679–687. [Google Scholar] [CrossRef]
- Ng, Q.X.; Lim, Y.L.; Yaow, C.Y.L.; Ng, W.K.; Thumboo, J.; Liew, T.M. Effect of probiotic supplementation on gut microbiota in patients with major depressive disorders: A systematic review. Nutrients 2023, 15, 1351. [Google Scholar] [CrossRef]
- Staley, C.; Khoruts, A.; Sadowsky, M.J. Contemporary applications of fecal microbiota transplantation to treat intestinal diseases in humans. Arch. Med. Res. 2017, 48, 766–773. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Gulati, M.; Singh, S.K.; Corrie, L.; Kaur, I.P.; Chandwani, L. Delivery routes for faecal microbiota transplants: Available, anticipated and aspired. Pharmacol. Res. 2020, 159, 104954. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Kuntz, T.; Shaik, S.M.; Baufeld, C.; Leibowitz, J.; Zhang, X.; Gottel, N.; Zhang, X.; Butovsky, O.; Gilbert, J.A. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 2019, 216, 1542–1560. [Google Scholar] [CrossRef]
- Fujii, Y.; Nguyen, T.T.T.; Fujimura, Y.; Kameya, N.; Nakamura, S.; Arakawa, K.; Morita, H. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci. Biotechnol. Biochem. 2019, 83, 2144–2152. [Google Scholar] [CrossRef]
- Parker, A.; Romano, S.; Ansorge, R.; Aboelnour, A.; Le Gall, G.; Savva, G.M.; Pontifex, M.G.; Telatin, A.; Baker, D.; Jones, E. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome 2022, 10, 1–25. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Chen, C.; Bobr, A.; Yao, D.; Lu, Y.; Nelson, V.M.; Sadowsky, M.J.; Khoruts, A. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 306, G310–G319. [Google Scholar] [CrossRef]
- Wang, H.; Lee, I.-S.; Braun, C.; Enck, P. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J. Neurogastroenterol. Motil. 2016, 22, 589. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Villar-García, J.; Güerri-Fernández, R.; Moya, A.; Gonzalez, A.; Hernandez, J.J.; Lerma, E.; Guelar, A.; Sorli, L.; Horcajada, J.P.; Artacho, A. Impact of probiotic Saccharomyces boulardii on the gut microbiome composition in HIV-treated patients: A double-blind, randomised, placebo-controlled trial. PLoS ONE 2017, 12, e0173802. [Google Scholar] [CrossRef]
- Lundelin, K.; Poussa, T.; Salminen, S.; Isolauri, E. Long-term safety and efficacy of perinatal probiotic intervention: Evidence from a follow-up study of four randomized, double-blind, placebo-controlled trials. Pediatr. Allergy Immunol. 2017, 28, 170–175. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Location | Study Type | Sample Details | Methods | Key Findings |
|---|---|---|---|---|---|
| Li et al., 2021 [7] | China | Observational study | n = 73, 27 CPP girls, 24 over-weighted girls, 22 healthy controls | 16S rRNA sequencing | 1. The CPP group exhibited increased α-diversity in GM, significant elevations in Alistipes, Klebsiella, and Sutterella bacteria, enhanced inter-bacterial correlations 2. In the CPP group, increased nitric oxide synthesis positively correlated with FSH and insulin. |
| Dong et al., 2020 [17] | China | Observational study | n = 58, 25 ICPP girls, 23 healthy controls | 16S rDNA sequencing | 1. The ICPP group had higher GM diversity. 2. 36 candidate GM biomarkers for patients with ICPP screening were identified. 3. The GM of the ICPP group was enriched for the microbial functions of cell motility, signal transduction, and environmental adaptation. 4. Positive correlations were also detected between Fusobacterium and FSH, and Gemmiger and LH. |
| Wang et al., 2020 [16] | The United States | Animal experiment | C57BL/6 mice | 16S rRNA sequencing | 1. Co-housing reversed early puberty induced by MHFD during lactation through the fecal–oral route via increasing GM richness. 2. Co-housing reversed insulin insensitivity in offspring induced by MHFD during lactation. |
| Wang et al., 2022 [15] | China | Animal experiment | female Sprague Dawley rats | 16S rDNA sequencing | SCFAs can act on Kiss1 neurons and their receptor GPR54 and then reduce the release of hypothalamic GnRH and pituitary LH and FSH through the PKC-ERK1/2 pathway and delay the development of the ovary and uterus. |
| Bo et al., 2022 [14] | China | Animal experiment | C57 mice | 16S rDNA sequencing, untargeted metabolomics sequencing | 1. HFD after weaning caused precocious puberty in mice. 2 “HFD-microbiota” transplantation promoted the precocious puberty of mice. 3. Estrogen changes the composition and proportion of gut microbiota and promotes precocious puberty in mice. |
| Cowan et al., 2018 [142] | Australia | Animal experiment | Sprague Dawley-derived rats | Probiotic treatment | 1. Stressed females exhibited earlier pubertal onset compared to standard-reared females, whereas stressed males matured later than their standard-reared counterparts. 2. A probiotic treatment restores the normative timing of puberty onset in rodents of both sexes. |
| Yuan et al., 2022 [141] | China | Animal experiment | female c57/bl mice | 16S rRNA sequencing, Probiotic treatment | 95% daidzein has the potential to advance the timing of puberty onset in female mice, and gut microbiome can be a therapeutic target to regulate the timing of puberty onset. |
| Huang et al., 2023 [103] | China | Observational study | n = 150, 91 CPP patients, 59 healthy controls | 16S rRNA sequencing, untargeted metabolomics sequencing | 1. Identified the altered microorganisms and metabolites during the development of CPP and constructed a classifier for distinguishing CPP. 2. Revealed the nitric oxide synthesis was closely associated with the progression of CPP and the genus Streptococcus could be a candidate molecular marker for CPP treatment. |
| Author, Year | Study Type | Sample Details | Methods | Key Findings | Methodological Limitations |
|---|---|---|---|---|---|
| Li et al., 2021 [7] | Observational study | n = 73, 27 CPP girls, 24 over-weighted girls, 22 healthy controls | 16S rRNA sequencing | 1. The CPP group exhibited increased α-diversity in GM, significant elevations in Alistipes, Klebsiella, and Sutterella bacteria, enhanced inter-bacterial correlations 2. In the CPP group, increased nitric oxide synthesis positively correlated with FSH and insulin. | 16S rRNA sequencing provides taxa resolution up to the genus level and is unable to yield information on the functional characteristics compared to newer techniques such as shotgun-metagenome sequencing. |
| Dong et al., 2020 [17] | Observational study | n = 58, 25 ICPP girls, 23 healthy controls | 16S rDNA sequencing | 1. The ICPP group had higher GM diversity. 2. 36 candidate GM biomarkers for patients with ICPP screening were identified. 3. The GM of the ICPP group was enriched for the microbial functions of cell motility, signal transduction, and environmental adaptation. 4. Positive correlations were also detected between Fusobacterium and FSH, and Gemmiger and LH. | 1. 16S rDNA cannot provide taxa information on the functional characteristics. 2. Fecal metabolomics were not investigated. 3. Researchers did not administer and analyze dietary questionnaires. |
| Huang et al., 2023 [103] | Observational study | n = 150, 91 CPP patients, 59 healthy controls | 16S rRNA sequencing, untargeted metabolomics sequencing | 1. Identified the altered microorganisms and metabolites during the development of CPP and constructed a classifier for distinguishing CPP. 2. Revealed the nitric oxide synthesis was closely associated with the progression of CPP and the genus Streptococcus could be a candidate molecular marker for CPP treatment. | 1. Although 16 s rRNA sequencing was widely used to characterize microbial communities, it existed limitations in explaining complete genetic information compared to metagenomic sequencing. 2. Candidate microorganisms need to be further cultured to judge the origin of metabolites more accurately. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, M.; Zhang, L. Exploring the Mechanistic Interplay between Gut Microbiota and Precocious Puberty: A Narrative Review. Microorganisms 2024, 12, 323. https://doi.org/10.3390/microorganisms12020323
Yue M, Zhang L. Exploring the Mechanistic Interplay between Gut Microbiota and Precocious Puberty: A Narrative Review. Microorganisms. 2024; 12(2):323. https://doi.org/10.3390/microorganisms12020323
Chicago/Turabian StyleYue, Min, and Lei Zhang. 2024. "Exploring the Mechanistic Interplay between Gut Microbiota and Precocious Puberty: A Narrative Review" Microorganisms 12, no. 2: 323. https://doi.org/10.3390/microorganisms12020323
APA StyleYue, M., & Zhang, L. (2024). Exploring the Mechanistic Interplay between Gut Microbiota and Precocious Puberty: A Narrative Review. Microorganisms, 12(2), 323. https://doi.org/10.3390/microorganisms12020323




