Straw Soil Conditioner Modulates Key Soil Microbes and Nutrient Dynamics across Different Maize Developmental Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Soil Sampling
2.3. Soil Physicochemical Analysis
2.4. DNA Extraction and High-Throughput Sequencing
2.5. Statistical Analyses
3. Results
3.1. Soil Properties
3.2. Microbial Beta Diversity
3.3. Microbial α-Diversity
3.4. Relationships between Regression Coefficients and Bacterial and Fungal Abundance
3.5. Correlation between Soil Microbial Community and Soil Physiochemical Properties
3.6. Crop Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Xia, H.; Jiang, C.; Riaz, M.; Yang, L.; Chen, Y.; Fan, X.; Xia, X. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef]
- Wu, Y.; He, J.; Liu, W.; Cheng, W.; Shaaban, M.; Jiang, Y. The effects of continuous straw returning strategies on SOC balance upon fresh straw incorporation. Environ. Res. 2023, 232, 116225. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Laird, D.A. The Charcoal Vision: A Win–Win–Win Scenario for Simultaneously Producing Bioenergy, Permanently Sequestering Carbon, while Improving Soil and Water Quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Lehmann, J.; Da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Lenka, N.K.; Lal, R. Soil aggregation and greenhouse gas flux after 15 years of wheat straw and fertilizer management in a no-till system. Soil Tillage Res. 2013, 126, 78–89. [Google Scholar] [CrossRef]
- Qian, S.; Zhou, X.; Fu, Y.; Song, B.; Yan, H.; Chen, Z.; Sun, Q.; Ye, H.; Qin, L.; Lai, C. Biochar-compost as a new option for soil improvement: Application in various problem soils. Sci. Total Environ. 2023, 870, 162024. [Google Scholar] [CrossRef]
- Jiang, C.; Yan, H.; Shen, X.; Zhang, Y.; Wang, Y.; Sun, S.; Jiang, H.; Zang, H.; Zhao, X.; Hou, N.; et al. Genome Functional Analysis of the Psychrotrophic Lignin-Degrading Bacterium Arthrobacter sp. C2 and the Role of DyP in Catalyzing Lignin Degradation. Front. Microbiol. 2022, 13, 921549. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Sun, X.; McLaughlin, N.B.; Jia, S.; Liang, A.; Zhang, S. The driving mechanism of soil organic carbon biodegradability in the black soil region of Northeast China. Sci. Total Environ. 2023, 884, 163835. [Google Scholar] [CrossRef]
- Schostag, M.D.; Albers, C.N.; Jacobsen, C.S.; Prieme, A. Low Turnover of Soil Bacterial rRNA at Low Temperatures. Front. Microbiol. 2020, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Brookes, P.C.; He, Y.; Wu, J.; Xu, J. An evaluation of a microbial inoculum in promoting organic C decomposition in a paddy soil following straw incorporation. J. Soils Sediments 2016, 16, 1776–1786. [Google Scholar] [CrossRef]
- Cabrera, W.G.; Avila, C.J.; Cabrera, N.; Nava, D.E.; de Sene, P.A.; Weber, D.C. Biology and Management of Pest Diabrotica Species in South America. Insects 2020, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.S.; Yin, Y.; Chen, H.; Ma, Z. The escalating threat of Rhizoctonia cerealis, the causal agent of sharp eyespot in wheat. Pest Manag. Sci. 2011, 67, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Wang, S.; Zhang, C.; Ma, Z. Influence of maize straw amendment on soil-borne diseases of winter wheat. Front. Agric. China 2009, 3, 7–12. [Google Scholar] [CrossRef]
- Bai, N.; Zhang, H.; Zhou, S.; Sun, H.; Zhao, Y.; Zheng, X.; Li, S.; Zhang, J.; Lv, W. Long-term effects of straw return and straw-derived biochar amendment on bacterial communities in soil aggregates. Sci. Rep. 2020, 10, 7891. [Google Scholar] [CrossRef]
- Li, Y.; Gupta, R.; Zhang, Q.; You, S. Review of biochar production via crop residue pyrolysis: Development and perspectives. Bioresour. Technol. 2023, 369, 128423. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar-manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef]
- Yang, H.; Feng, J.; Zhai, S.; Dai, Y.; Xu, M.; Wu, J.; Shen, M.; Bian, X.; Koide, R.T.; Liu, J. Long-term ditch-buried straw return alters soil water potential, temperature, and microbial communities in a rice-wheat rotation system. Soil Tillage Res. 2016, 163, 21–31. [Google Scholar] [CrossRef]
- Du, M.; Zhang, J.; Wang, G.; Liu, C.; Wang, Z. Response of bacterial community composition and co-occurrence network to straw and straw biochar incorporation. Front. Microbiol. 2022, 13, 999399. [Google Scholar] [CrossRef]
- Luo, S.; Tian, L.; Chang, C.; Wang, S.; Zhang, J.; Zhou, X.; Li, X.; Tran, L.S.P.; Tian, C. Grass and maize vegetation systems restore saline-sodic soils in the Songnen Plain of northeast China. Land Degrad. Dev. 2018, 29, 1107–1119. [Google Scholar] [CrossRef]
- Yadav, R.L.; Dwivedi, B.S.; Prasad, K.; Tomar, O.K.; Shurpali, N.J.; Pandey, P.S. Yield trends, and changes in soil organic-C and available NPK in a long-term rice–wheat system under integrated use of manures and fertilisers. Field Crops Res. 2000, 68, 219–246. [Google Scholar] [CrossRef]
- Bergmann, G.T.; Bates, S.T.; Eilers, K.G.; Lauber, C.L.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol. Biochem. 2011, 43, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. 38—Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Grunsky, E.C. R: A data analysis and statistical programming environment—An emerging tool for the geosciences. Comput. Geosci. 2002, 28, 1219–1222. [Google Scholar] [CrossRef]
- Clark, J.S.; Nemergut, D.; Seyednasrollah, B.; Turner, P.J.; Zhang, S. Generalized joint attribute modeling for biodiversity analysis: Median-zero, multivariate, multifarious data. Ecol. Monogr. 2017, 87, 34–56. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Leite, M.F.A.; Kuramae, E.E. You must choose, but choose wisely: Model-based approaches for microbial community analysis. Soil Biol. Biochem. 2020, 151, 108042. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macêdo, J.L.V.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef]
- Mekuria, W.; Noble, A.; Sengtaheuanghoung, O.; Hoanh, C.T.; Bossio, D.; Sipaseuth, N.; McCartney, M.; Langan, S. Organic and Clay-Based Soil Amendments Increase Maize Yield, Total Nutrient Uptake, and Soil Properties in Lao PDR. Agroecol. Sustain. Food 2015, 8, 936–961. [Google Scholar] [CrossRef]
- Bello, A.; Wang, B.; Zhao, Y.; Yang, W.; Ogundeji, A.; Deng, L.; Egbeagu, U.U.; Yu, S.; Zhao, L.; Li, D.; et al. Composted biochar affects structural dynamics, function and co-occurrence network patterns of fungi community. Sci. Total Environ. 2021, 775, 145672. [Google Scholar] [CrossRef]
- Hellequin, E.; Binet, F.; Klarzynski, O.; Hallin, S.; Juhanson, J.; Daburon, V.; Monard, C. Shaping of soil microbial communities by plants does not translate into specific legacy effects on organic carbon mineralization. Soil Biol. Biochem. 2021, 163, 108449. [Google Scholar] [CrossRef]
- Yang, Y.; Bao, X.; Xie, H.; He, H.; Zhang, X.; Shao, P.; Zhu, X.; Jiang, Y.; Liang, C. Frequent stover mulching builds healthy soil and sustainable agriculture in Mollisols. Agric. Ecosyst. Environ. 2022, 326, 107815. [Google Scholar] [CrossRef]
- Ge, Z.; Li, S.; Bol, R.; Zhu, P.; Peng, C.; An, T.; Cheng, N.; Liu, X.; Li, T.; Xu, Z.; et al. Differential long-term fertilization alters residue-derived labile organic carbon fractions and microbial community during straw residue decomposition. Soil Tillage Res. 2021, 213, 105120. [Google Scholar] [CrossRef]
- Partovi, Z.; Ramezani Etedali, H.; Kaviani, A. Effects of applying biochar and straw on nitrate leaching and maize yield production. Water Environ. J. 2021, 35, 943–950. [Google Scholar] [CrossRef]
- Gleason, C.; Chaudhuri, S.; Yang, T.; Munoz, A.; Poovaiah, B.W.; Oldroyd, G.E. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 2006, 441, 1149–1152. [Google Scholar] [CrossRef]
- Primieri, S.; Magnoli, S.M.; Koffel, T.; Stürmer, S.L.; Bever, J.D. Perennial, but not annual legumes synergistically benefit from infection with arbuscular mycorrhizal fungi and rhizobia: A meta-analysis. New Phytol. 2022, 233, 505–514. [Google Scholar] [CrossRef]
- Hrbackova, M.; Luptovciak, I.; Hlavackova, K.; Dvorak, P.; Ticha, M.; Samajova, O.; Novak, D.; Bednarz, H.; Niehaus, K.; Ovecka, M.; et al. Overexpression of alfalfa SIMK promotes root hair growth, nodule clustering and shoot biomass production. Plant Biotechnol. J. 2021, 19, 767–784. [Google Scholar] [CrossRef]
- Geddes, B.A.; Kearsley, J.; Huang, J.; Zamani, M.; Muhammed, Z.; Sather, L.; Panchal, A.K.; DiCenzo, G.C.; Finan, T.M. Minimal gene set from Sinorhizobium (Ensifer) meliloti pSymA required for efficient symbiosis with Medicago. Proc. Natl. Acad. Sci. USA 2021, 118, e2018015118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, Y.; Liu, Q.; Yang, H.; Wang, Z.; Zhang, M. Effects of Drought-Tolerant Ea-DREB2B Transgenic Sugarcane on Bacterial Communities in Soil. Front. Microbiol. 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hao, Z.; Li, K.; Sha, Y.; Wang, E.; Sui, X.; Mi, G.; Tian, C.; Chen, W. Effects of growth-promoting rhizobacteria on maize growth and rhizosphere microbial community under conservation tillage in Northeast China. Microb. Biotechnol. 2021, 14, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Azman, S.; Khadem, A.F.; van Lier, J.B.; Zeeman, G.; Plugge, C.M. Presence and Role of Anaerobic Hydrolytic Microbes in Conversion of Lignocellulosic Biomass for Biogas Production. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2523–2564. [Google Scholar] [CrossRef]
- Suksong, W.; Kongjan, P.; Prasertsan, P.; O-Thong, S. Thermotolerant cellulolytic Clostridiaceae and Lachnospiraceae rich consortium enhanced biogas production from oil palm empty fruit bunches by solid-state anaerobic digestion. Bioresour. Technol. 2019, 291, 121851. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Jiang, B.; Huang, F.; Hu, X. Nitrogen removal mechanism and microbial community changes of bioaugmentation subsurface wastewater infiltration system. Bioresour. Technol. 2019, 294, 122140. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, P.; He, Y.; Zeng, F.; Xu, J.; He, L. Enantioselective effects of cyflumetofen on microbial community and related nitrogen cycle gene function in acid-soil. Sci. Total Environ. 2021, 771, 144831. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Feng, Y.; Zhao, D.Q.; Jiang, J.X. Evaluation of cellulases produced from four fungi cultured on furfural residues and microcrystalline cellulose. Biodegradation 2012, 23, 465–472. [Google Scholar] [CrossRef]
- Simões, M.F.; Antunes, A.; Ottoni, C.A.; Amini, M.S.; Alam, I.; Alzubaidy, H.; Mokhtar, N.; Archer, J.A.C.; Bajic, V.B. Soil and Rhizosphere Associated Fungi in Gray Mangroves (Avicennia marina) from the Red Sea—A Metagenomic Approach. Genom. Proteom. Bioinform. 2015, 13, 310–320. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Q.; Zhang, H.; Zhang, J.; Chen, Y.; Yao, F.; Chen, Y. Effects of exogenous organic matter addition on agricultural soil microbial communities and relevant enzyme activities in southern China. Sci. Rep. 2023, 13, 8045. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Rengel, Z.; Bowden, J.W. Carbon, nitrogen and sulphur cycling following incorporation of canola residue of different sizes into a nutrient-poor sandy soil. Soil Biol. Biochem. 2006, 38, 32–42. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; Wang, X.; George, T.S.; Feng, G.; Zhang, L. Simulated root exudates stimulate the abundance of Saccharimonadales to improve the alkaline phosphatase activity in maize rhizosphere. Appl. Soil Ecol. 2022, 170, 104274. [Google Scholar] [CrossRef]


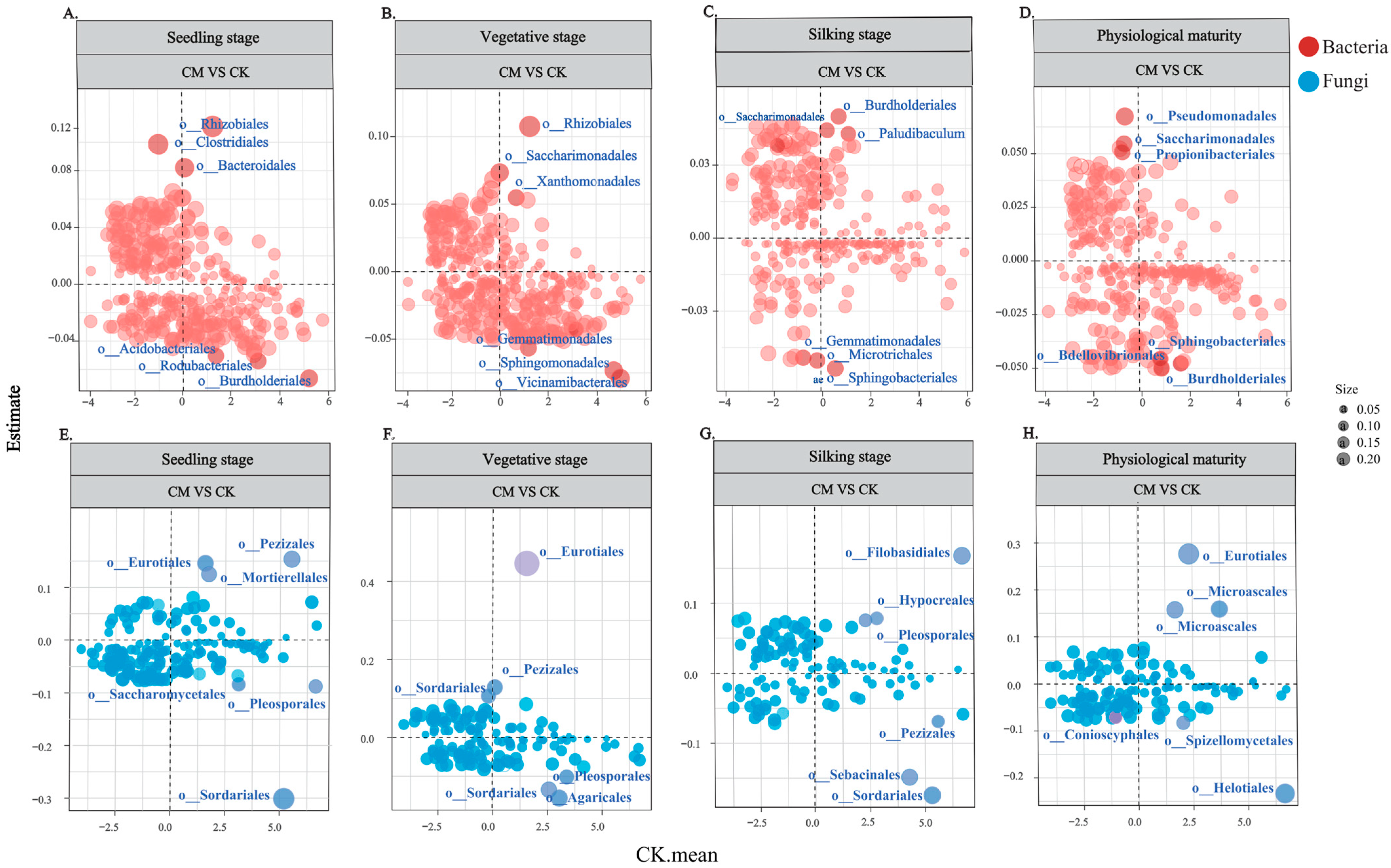
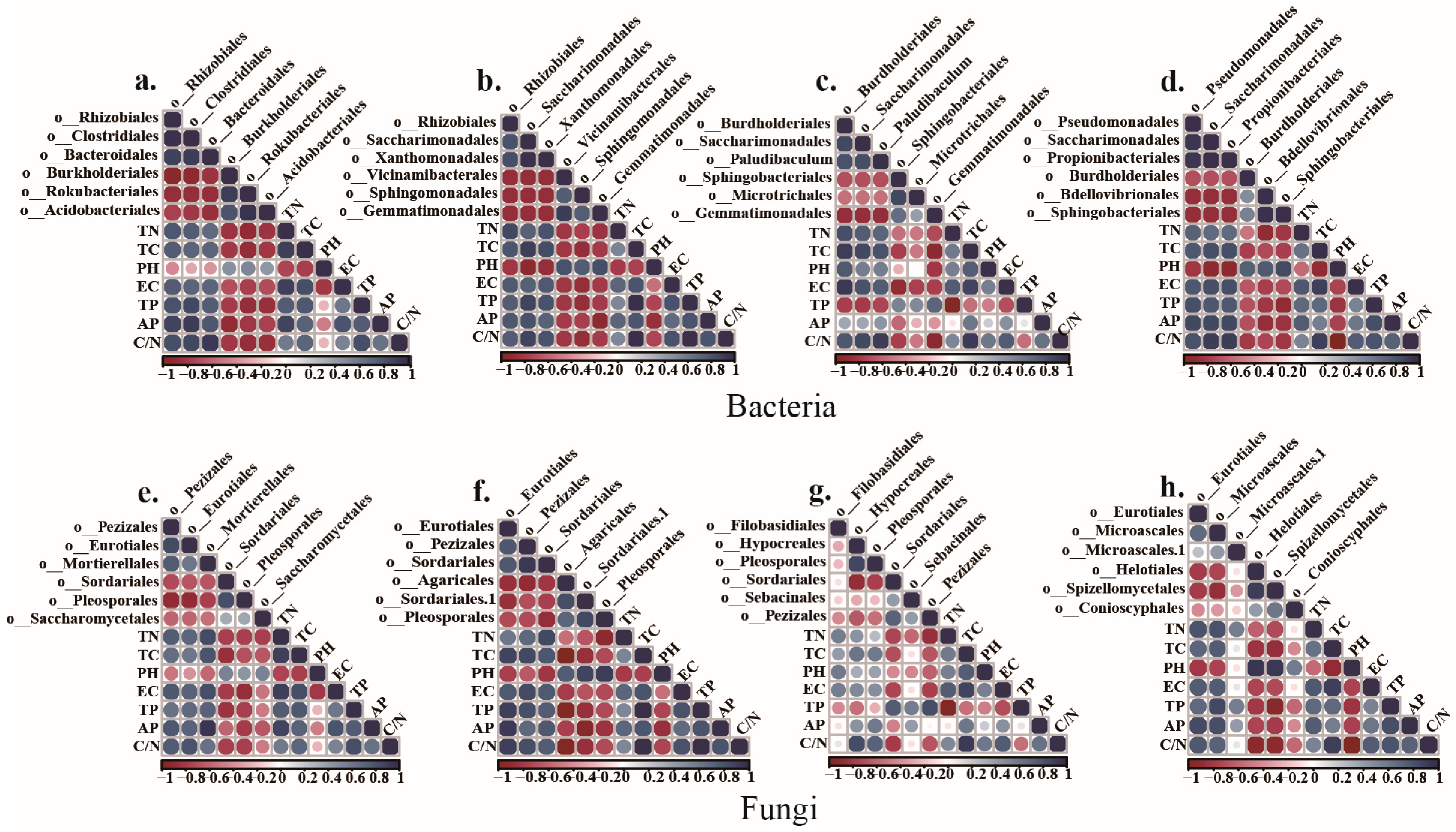
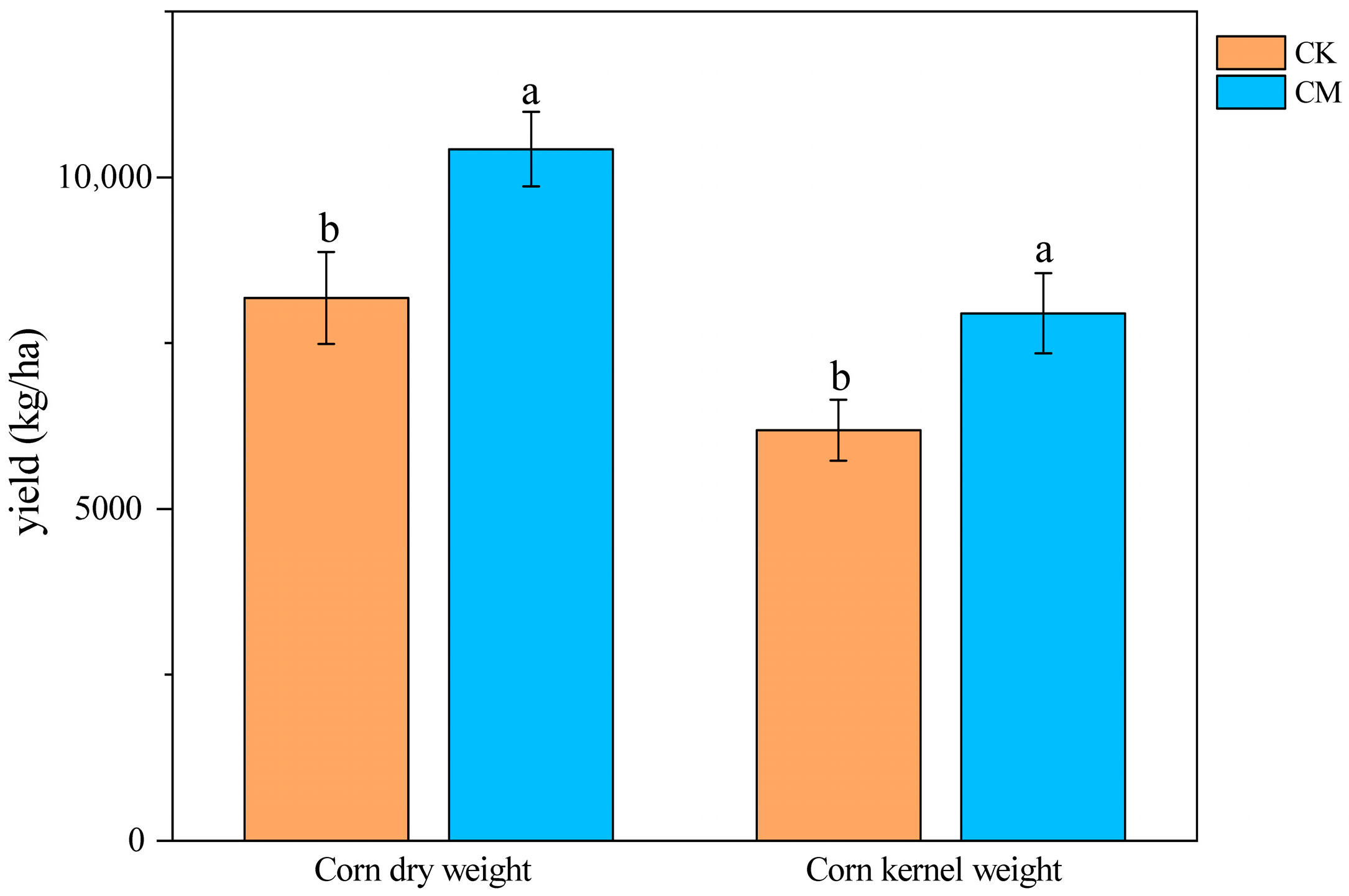
| Treatments | Growth Stage | pH | EC | TC (%) | TN (%) | C/N | TP (mg/kg) | AP (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| CK | Seedling stage | 6.45 ± 0.04 a | 55.83 ± 1.21 b | 2.02 ± 0.04 b | 0.21 ± 0.01 b | 9.5 ± 0.13 b | 4.85 ± 0.18 b | 2.14 ± 0.32 b |
| Vegetative stage | 6.49 ± 0.05 a | 66.87 ± 4.41 b | 3.22 ± 0.02 b | 0.25 ± 0.02 b | 12.78 ± 0.91 b | 7.18 ± 0.06 b | 3.16 ± 0.08 b | |
| Silking stage | 5.96 ± 0.13 a | 73.77 ± 0.42 b | 2.5 ± 0.02 b | 0.23 ± 0.02 b | 10.94 ± 0.91 b | 8.32 ± 0.45 a | 3.48 ± 0.18 a | |
| Physiological maturity | 6.33 ± 0.01 a | 55.2 ± 2.01 b | 1.71 ± 0.02 b | 0.18 ± 0.02 b | 9.35 ± 0.96 b | 5.92 ± 0.32 b | 2.75 ± 0.04 b | |
| CM | Seedling stage | 6.39 ± 0.08 a | 169.83 ± 19.8 a | 18.02 ± 0.02 a | 0.79 ± 0.03 a | 22.95 ± 0.83 a | 10.34 ± 0.32 a | 3.49 ± 0.17 a |
| Vegetative stage | 6.4 ± 0.02 b | 549 ± 17 a | 20.26 ± 0.02 a | 0.93 ± 0.02 a | 21.79 ± 0.53 a | 16.7 ± 0.15 a | 5.3 ± 0.42 a | |
| Silking stage | 6.1 ± 0.02 a | 85.37 ± 0.45 a | 12.94 ± 0.01 a | 0.59 ± 0.01 a | 21.84 ± 0.19 a | 6.78 ± 0.45 b | 3.54 ± 0.18 a | |
| Physiological maturity | 5.95 ± 0.13 b | 124.6 ± 1.35 a | 12.04 ± 0.04 a | 0.57 ± 0.02 a | 20.97 ± 0.6 a | 10.55 ± 0.97 a | 4.07 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ye, L.; Chang, J.; Wang, E.; Wang, C.; Zhang, H.; Pang, Y.; Tian, C. Straw Soil Conditioner Modulates Key Soil Microbes and Nutrient Dynamics across Different Maize Developmental Stages. Microorganisms 2024, 12, 295. https://doi.org/10.3390/microorganisms12020295
Zhang J, Ye L, Chang J, Wang E, Wang C, Zhang H, Pang Y, Tian C. Straw Soil Conditioner Modulates Key Soil Microbes and Nutrient Dynamics across Different Maize Developmental Stages. Microorganisms. 2024; 12(2):295. https://doi.org/10.3390/microorganisms12020295
Chicago/Turabian StyleZhang, Jianfeng, Libo Ye, Jingjing Chang, Enze Wang, Changji Wang, Hengfei Zhang, Yingnan Pang, and Chunjie Tian. 2024. "Straw Soil Conditioner Modulates Key Soil Microbes and Nutrient Dynamics across Different Maize Developmental Stages" Microorganisms 12, no. 2: 295. https://doi.org/10.3390/microorganisms12020295
APA StyleZhang, J., Ye, L., Chang, J., Wang, E., Wang, C., Zhang, H., Pang, Y., & Tian, C. (2024). Straw Soil Conditioner Modulates Key Soil Microbes and Nutrient Dynamics across Different Maize Developmental Stages. Microorganisms, 12(2), 295. https://doi.org/10.3390/microorganisms12020295






