Tunnel Infection and Peritonitis Induced by Staphylococcus aureus Due to Decubitus Change of the Anterior Abdominal Wall in a Patient on Peritoneal Dialysis: Case Report
Abstract
1. Introduction
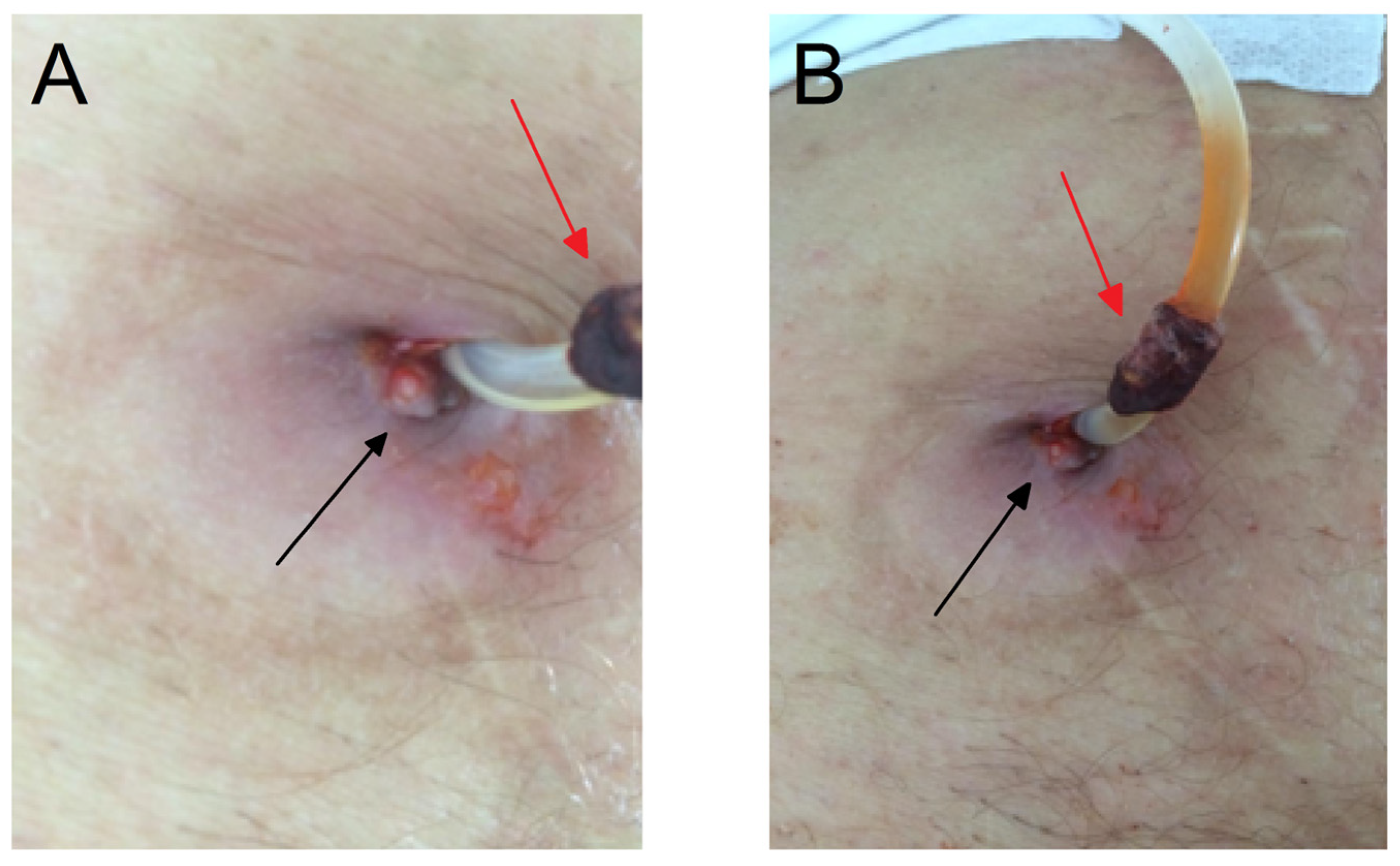
2. Case Presentation Section
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Charles, C.; Ferris, A.-H. Chronic Kidney Disease. Prim. Care 2020, 47, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, I. Peritoneal Dialysis. N. Engl. J. Med. 2021, 385, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-K.; Chow, K.-M.; Van de Luijtgaarden, M.-W.; Johnson, D.-W.; Jager, K.-J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.-Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2017, 13, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Naber, T.; Purohit, S. Chronic Kidney Disease: Role of Diet for a Reduction in the Severity of the Disease. Nutrients 2021, 13, 3277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wattanasatja, V.; Phisutrattanaporn, J.; Doenphai, N.; Sirinual, S.; Kanjanabuch, T. Peritoneal dialysis-associated peritonitis due to infected umbilicus. Med. Mycol. Case Rep. 2024, 45, 100654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baralić, M.; Spasojević, I.; Miljuš, G.; Šunderić, M.; Robajac, D.; Dobrijević, Z.; Gligorijević, N.; Nedić, O.; Penezić, A. Albumin at the intersection between antioxidant and pro-oxidant in patients on peritoneal dialysis. Free. Radic. Biol. Med. 2022, 187, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Enia, G.; Tripepi, G.; Panuccio, V.; Mallamaci, F. Clinical epidemiology of major nontraditional risk factors in peritoneal dialysis patients. Perit. Dial. Int. 2005, 25, S84–S87. [Google Scholar] [CrossRef]
- Zhang, Y.; Corapi, K.-M.; Luongo, M.; Thadhani, R.; Nigwekar, S.-U. Calciphylaxis in peritoneal dialysis patients: A single center cohort study. Int. J. Nephrol. Renov. Dis. 2016, 9, 235–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erkan, D.; Aguiar, C.L.; Andrade, D.; Cohen, H.; Cuadrado, M.J.; Danowski, A.; Levy, R.A.; Ortel, T.L.; Rahman, A.; Salmon, J.E.; et al. 14th International Congress on Antiphospholipid Antibodies: Task force report on antiphospholipid syndrome treatment trends. Autoimmun. Rev. 2014, 13, 685–696. [Google Scholar] [CrossRef]
- Cheung, G.-Y.-C.; Bae, J.-S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markovic, A. Vasculitis and vasculopathy. Acta Medica Croat. 2012, 66 (Suppl. S1), 19–24. [Google Scholar]
- Osaki, Y.; Maeoka, Y.; Sami, M.; Takahashi, A.; Ishiuchi, N.; Sasaki, K.; Masaki, T. Peritoneal dialysis-associated peritonitis, caused by superior mesenteric artery thrombosis with intestinal necrosis: A case report. CEN Case Rep 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Cho, K.-H.; Kim, A.-Y.; Do, J.-Y. Catheter salvage using revision for a peritoneal dialysis catheter with intractable exit site and/or tunnel infections. Semin. Dial. 2023, 36, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Akoh, J.-A. Peritoneal dialysis associated infections: An update on diagnosis and management. World J. Nephrol. 2012, 1, 106–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raphael, E.; Riley, L.W. Infections Caused by Antimicrobial Drug-Resistant Saprophytic Gram-Negative Bacteria in the Environment. Front. Med. 2017, 4, 183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Ma, T.; Hao, J.; Song, D.; Wang, H.; Liu, T.; Zhang, Y.; Abi, N.; Xu, X.; Zhang, M.; et al. Novel equations for estimating intraperitoneal pressure among peritoneal dialysis patients. Clin. Kidney J. 2023, 16, 1447–1456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, R.; Denman, R.; Saltissi, D.; Healy, H.; Muller, M.; Fleming, S. Erosion of a mesenteric vessel by a Tenckhoff catheter. Perit. Dial. Int. 1996, 16, 528–529. [Google Scholar] [CrossRef]
- Tapiawala, S.; Bargman, J.M. An unusual cause of skin ulceration in a very long-term peritoneal dialysis patient. Perit. Dial. Int. 2009, 29, 120–121. [Google Scholar] [CrossRef]
- Taylor, T.A.; Unakal, C.G. Staphylococcus aureus Infection. In StarPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Patel, S.; Preuss, C.V.; Bernice, F. Vancomycin. In StarPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Vila, T.; Kong, E.F.; Montelongo-Jauregui, D.; Van Dijck, P.; Shetty, A.C.; McCracken, C.; Bruno, V.M.; Jabra-Rizk, M.A. Therapeutic implications of C. albicans-S. aureus mixed biofilm in a murine subcutaneous catheter model of polymicrobial infection. Virulence 2021, 12, 835–851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.-W.; Liao, C.-T.; Wu, M.-Y.; Huang, N.-J.; Cherng, Y.-G.; Wu, M.-S.; Hsu, Y.-H.; Chen, C.-H. Pressure induces peritoneal fibrosis and inflammation through CD44 signaling. Ren. Fail. 2024, 46, 2384586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiebalo, T.; Holotka, J.; Habura, I.; Pawlaczyk, K. Nutritional Status in Peritoneal Dialysis: Nutritional Guidelines, Adequacy and the Management of Malnutrition. Nutrients 2020, 12, 1715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Concentration | First Hospitalization | Second Hospitalization | Reference Range |
|---|---|---|---|
| C-reactive protein (mg/L) | 71.8 | 176.2 | 1–8 |
| Leukocytes (×109/L) | 6.4 | 12.7 | 3.6–10.0 |
| Erythrocytes (×1012/L) | 3.38 | 3.35 | 4.34–5.72 |
| Hemoglobin (g/L) | 98 | 101 | 138–175 |
| Hematocrit (L/L) | 0.31 | 0.32 | 0.41–0.53 |
| Total protein (g/L) | 58 | 63 | 62–81 |
| Albumin (g/L) | 42 | 40 | 35–53 |
| Glucose (mmol/L) | 5.5 | 4.8 | 4.4–6.2 |
| Urea (mmol/L) | 22.0 | 24.2 | 1–8 |
| Creatinine (μmol/L) | 486 | 1014 | 40–92 |
| Transferrin (g/L) | 1.7 | / | 1.7–3.8 |
| Ferritin (μg/L) | 227.0 | 264.0 | 30–400 |
| Iron (μmol/L) | 11.5 | 11.8 | 11–30 |
| Transferrin saturation (%) | 28.0 | 26.0 | 20–50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baralić, M.; Bontić, A.; Pavlović, J.; Karadžić-Ristanović, V.; Gajić, S.; Jevtić, J.; Popović, P.; Petrović, K.; Hadži-Tanović, L.; Kezić, A. Tunnel Infection and Peritonitis Induced by Staphylococcus aureus Due to Decubitus Change of the Anterior Abdominal Wall in a Patient on Peritoneal Dialysis: Case Report. Microorganisms 2024, 12, 2608. https://doi.org/10.3390/microorganisms12122608
Baralić M, Bontić A, Pavlović J, Karadžić-Ristanović V, Gajić S, Jevtić J, Popović P, Petrović K, Hadži-Tanović L, Kezić A. Tunnel Infection and Peritonitis Induced by Staphylococcus aureus Due to Decubitus Change of the Anterior Abdominal Wall in a Patient on Peritoneal Dialysis: Case Report. Microorganisms. 2024; 12(12):2608. https://doi.org/10.3390/microorganisms12122608
Chicago/Turabian StyleBaralić, Marko, Ana Bontić, Jelena Pavlović, Vidna Karadžić-Ristanović, Selena Gajić, Jovan Jevtić, Pavle Popović, Kristina Petrović, Lara Hadži-Tanović, and Aleksandra Kezić. 2024. "Tunnel Infection and Peritonitis Induced by Staphylococcus aureus Due to Decubitus Change of the Anterior Abdominal Wall in a Patient on Peritoneal Dialysis: Case Report" Microorganisms 12, no. 12: 2608. https://doi.org/10.3390/microorganisms12122608
APA StyleBaralić, M., Bontić, A., Pavlović, J., Karadžić-Ristanović, V., Gajić, S., Jevtić, J., Popović, P., Petrović, K., Hadži-Tanović, L., & Kezić, A. (2024). Tunnel Infection and Peritonitis Induced by Staphylococcus aureus Due to Decubitus Change of the Anterior Abdominal Wall in a Patient on Peritoneal Dialysis: Case Report. Microorganisms, 12(12), 2608. https://doi.org/10.3390/microorganisms12122608







