Effects of the Application of Pseudomonas cedrina DY1-3 on the Growth of Maize Plants and the Structure of Soil Bacterial Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Strains and Experimental Materials
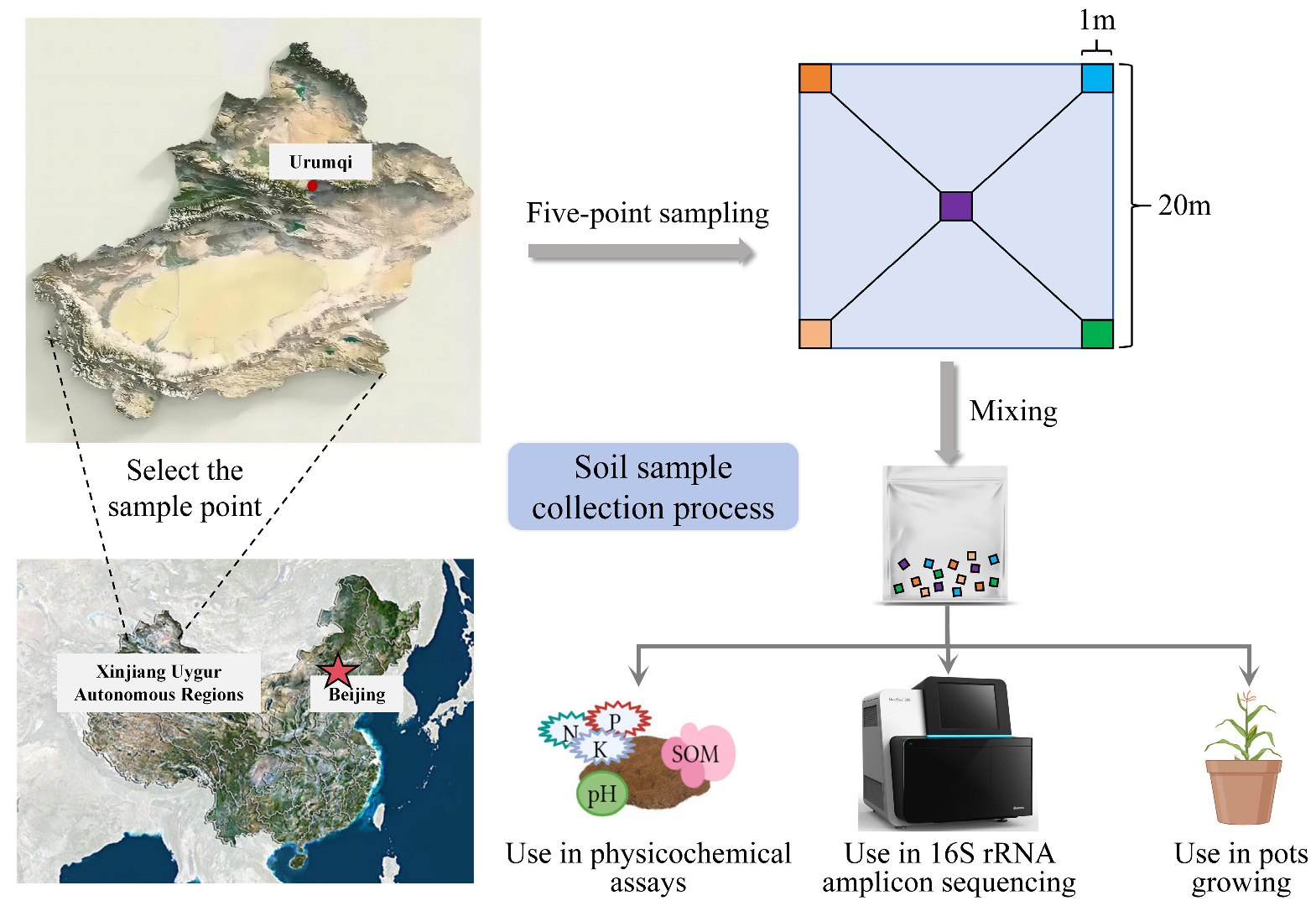
2.2. Soil Sample Collection
2.3. Determination of Soil Physical and Chemical Properties
2.4. Sequencing of Amplicons of Soil Bacterial 16S rRNA V3-V4 Region
2.5. Potting Experiment
2.6. Data Analysis
3. Results
3.1. Physico-Chemical Properties of Virgin Soil and Sequencing Analysis of 16S rRNA V3-V4 Region Amplicon of Soil Bacteria
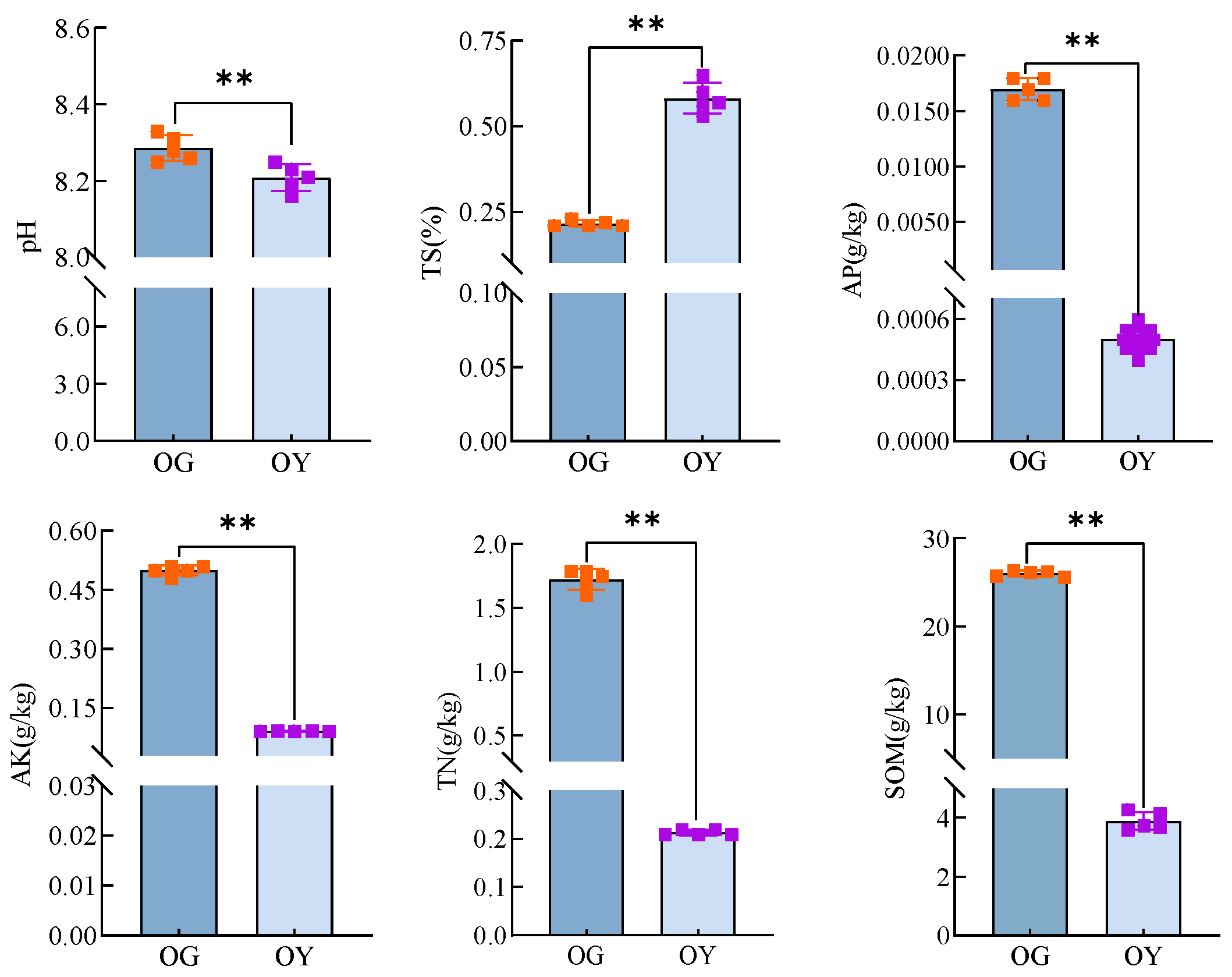
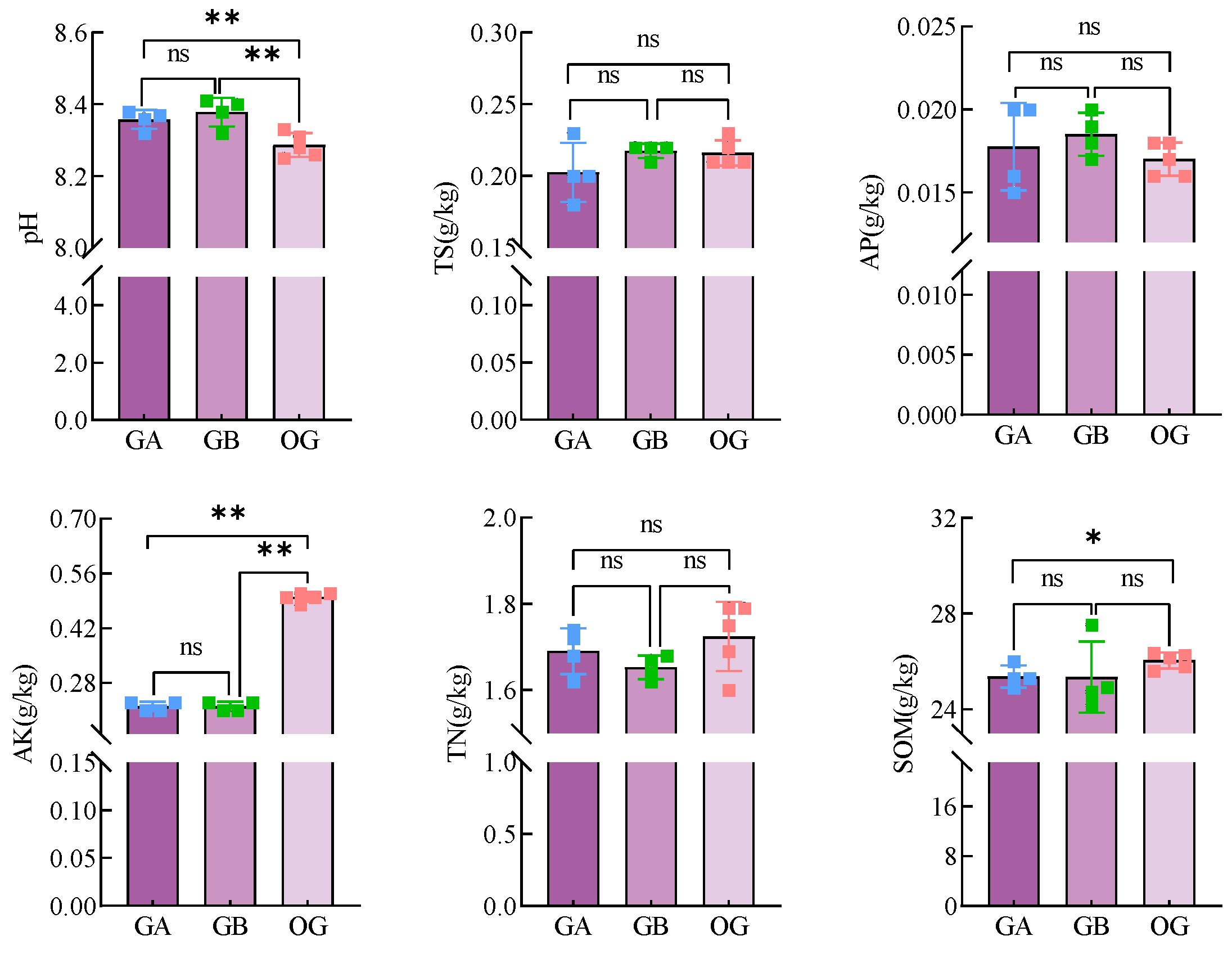
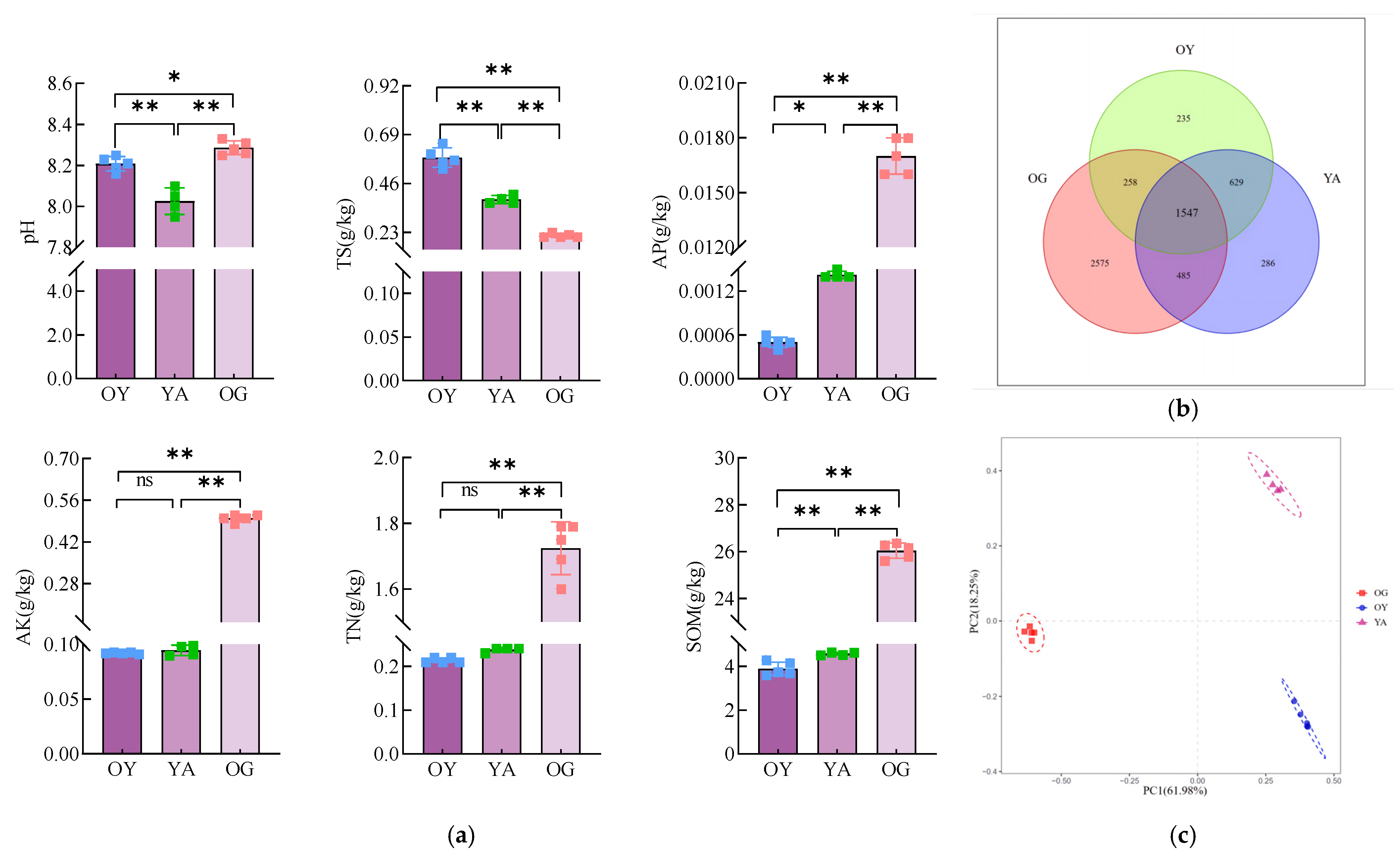
3.1.1. Physico-Chemical Properties of the Original Soil
3.1.2. Raw Soil Data Pre-Processing Statistics, Quality Control, and OUT Distribution Analysis
3.1.3. Analysis of Alpha Diversity Indices of Pristine Soil Bacterial Communities
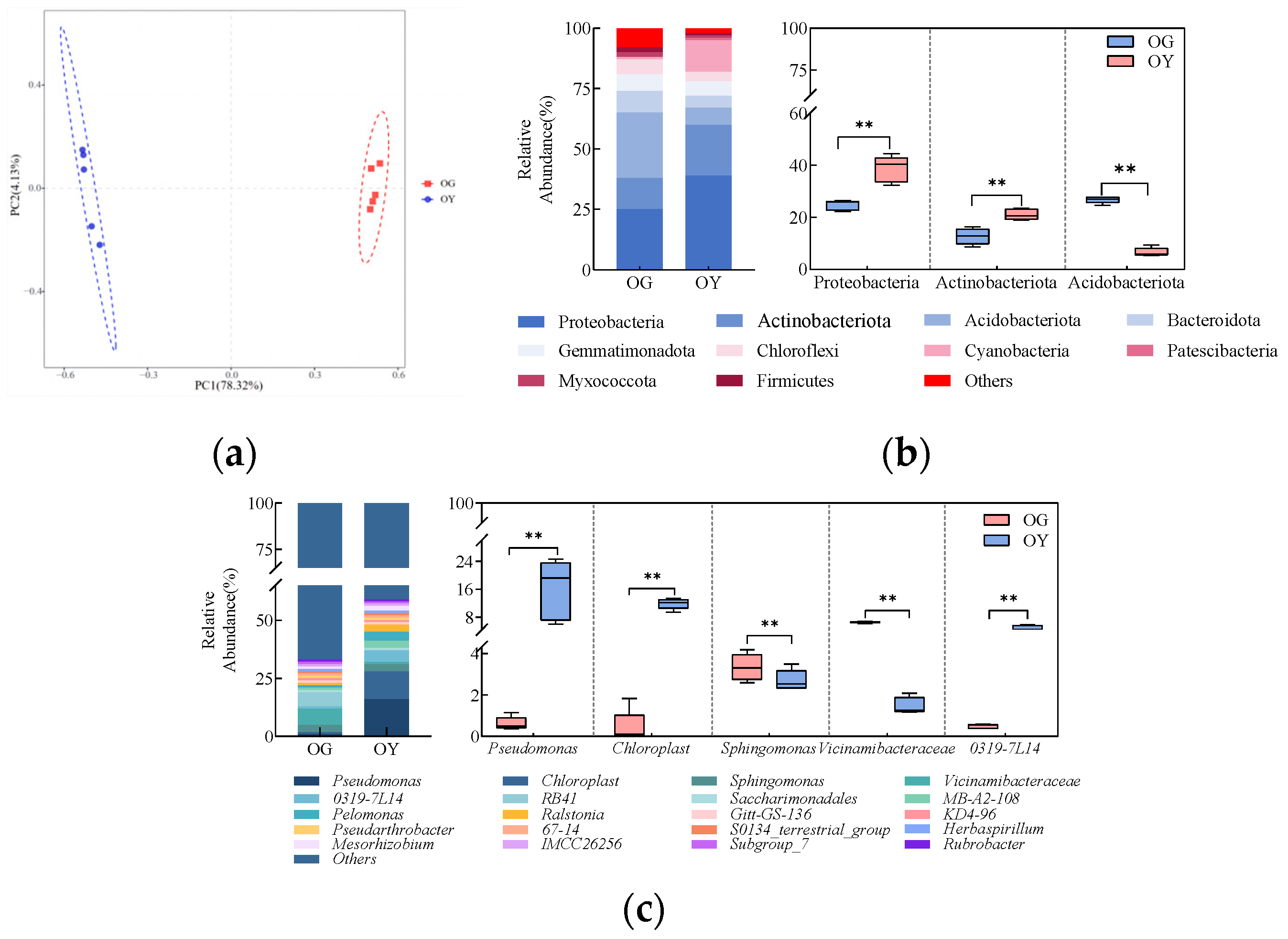
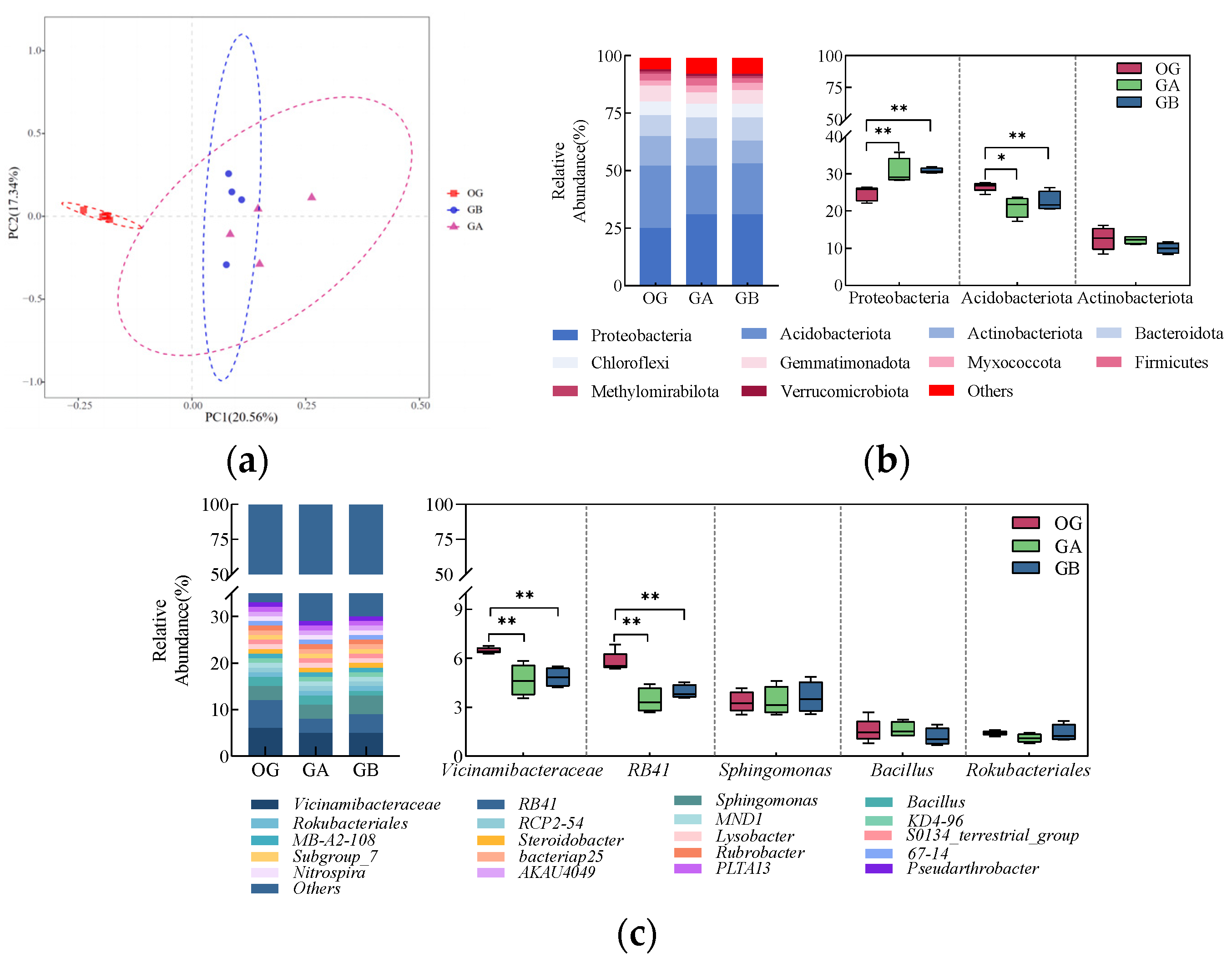
3.1.4. Analysis of the Composition of the Original Soil Bacterial Community
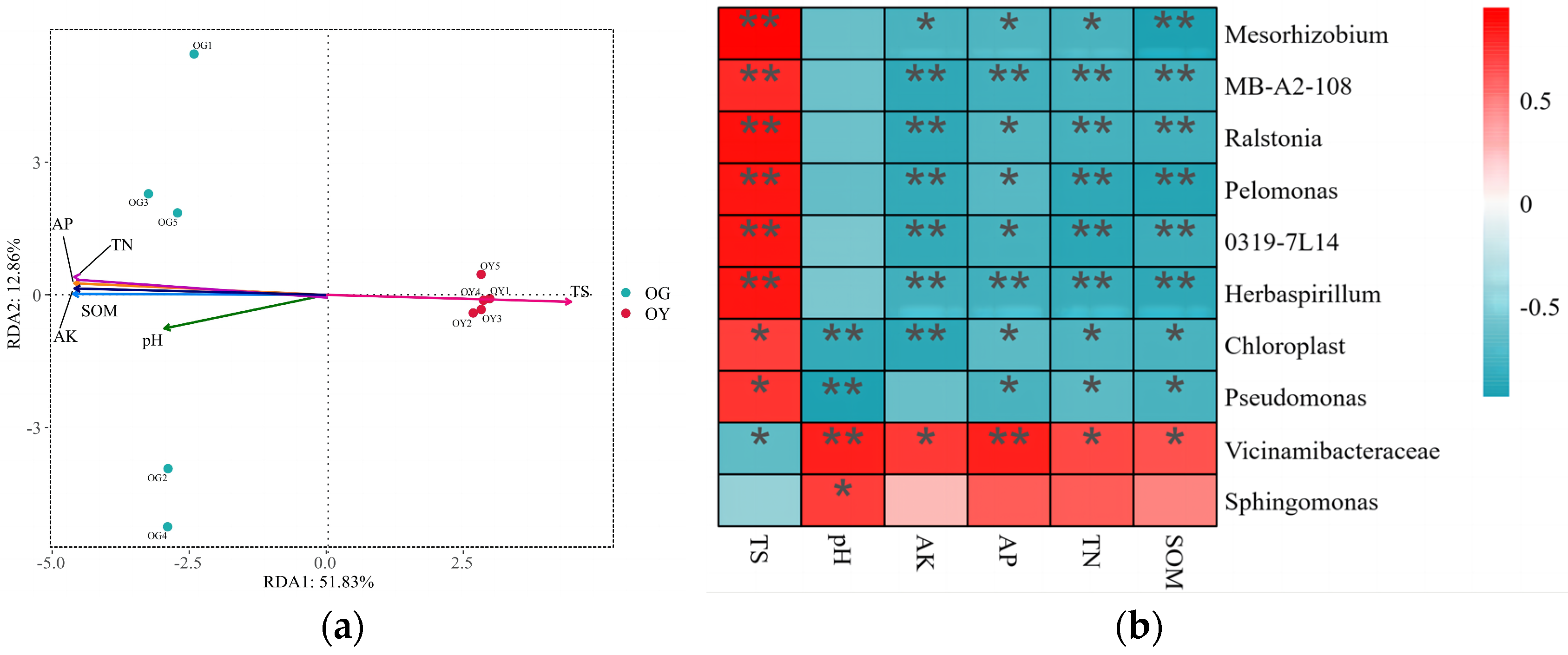
3.1.5. Analysis of Primary Soil Bacterial Community Structure and Environmental Factors
3.2. Investigating the Effects of the Application of DY1-3 Bacterial Suspension in Arable Soil on the Growth of Maize Seedlings and the Structure of Soil Bacterial Communities
3.2.1. Analysis of the Results of the Determination of Growth and Physiological Indexes of Potted Plants in Arable Soil
3.2.2. Analysis of the Results of the Determination of Physico-Chemical Properties of Soil in Pots of Arable Soil
3.2.3. Statistics on Pre-Processing of Arable Soil Data After Potting, Quality Control and Analysis of OUT Distribution
3.2.4. Analysis of Alpha Diversity Indices of Soil Bacterial Communities in Arable Land After Potting Plants
3.2.5. Analysis of Bacterial Community Composition in Arable Soil After Potting
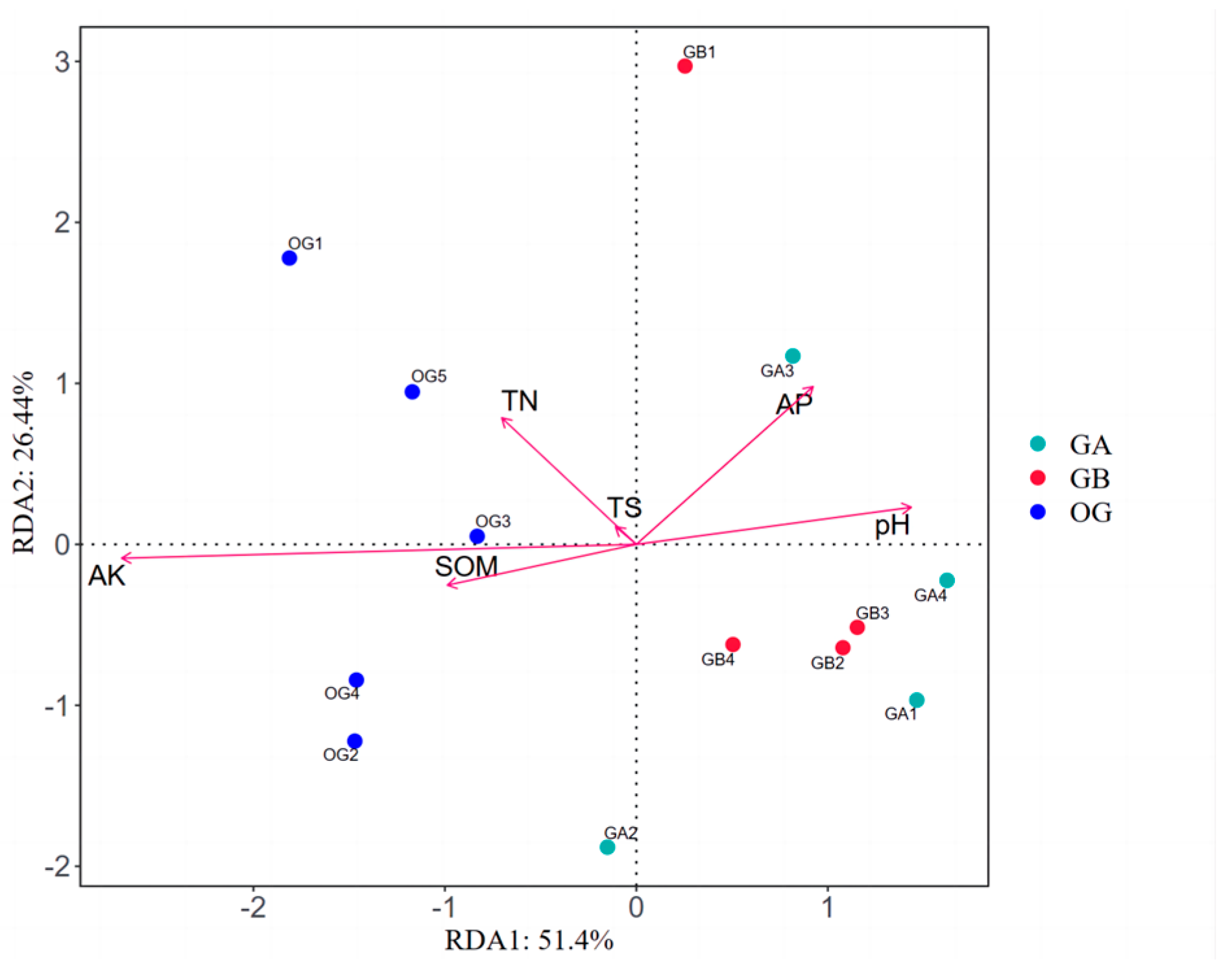
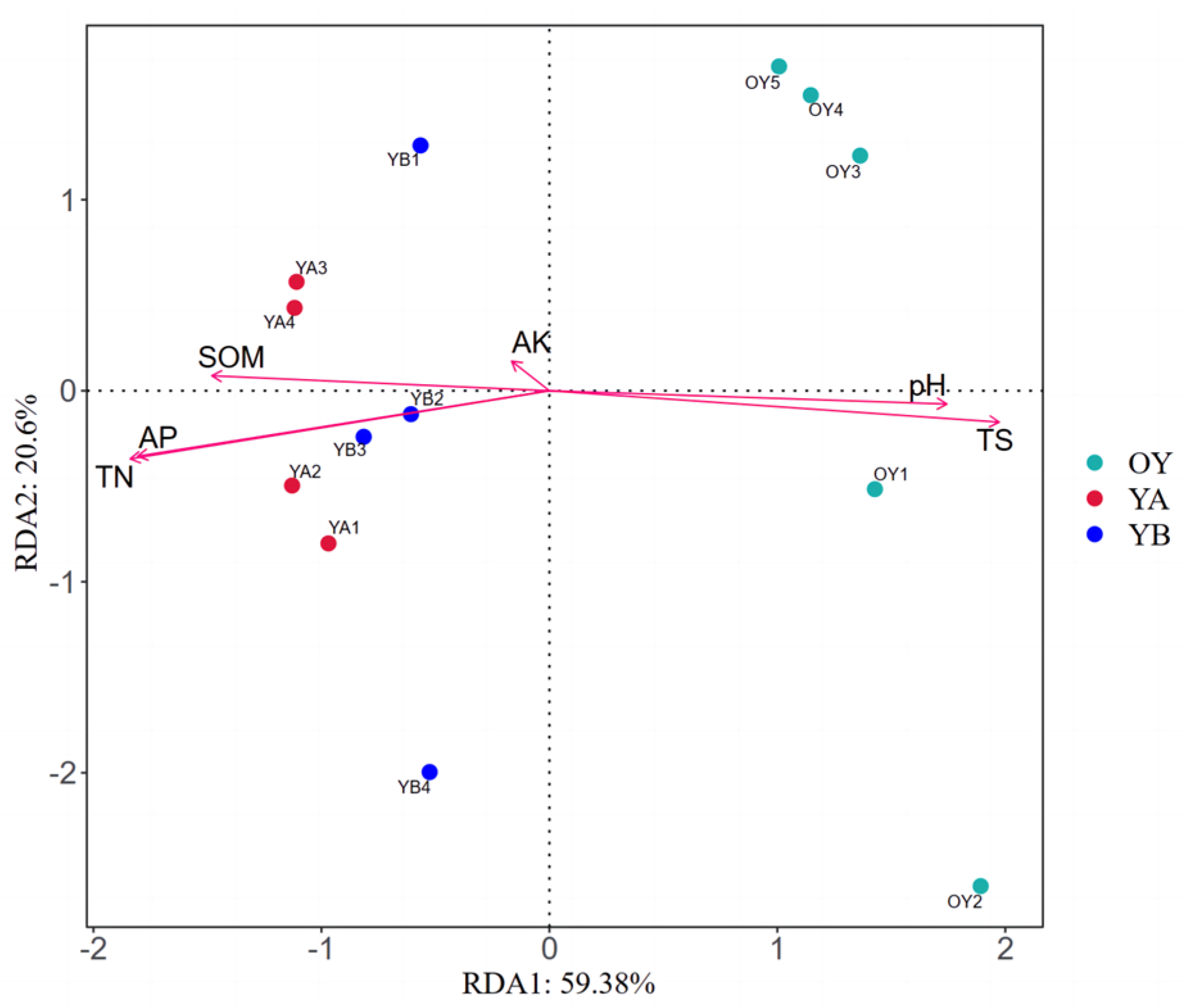
3.2.6. RDA Analysis Between Soil Bacterial Community Structure Composition and Soil Physicochemical Factors in Arable Soil
3.3. Investigating the Effect of the Application of DY1-3 Bacterial Suspension in Saline Soil on the Growth of Maize Seedlings and the Structure of Soil Bacterial Community
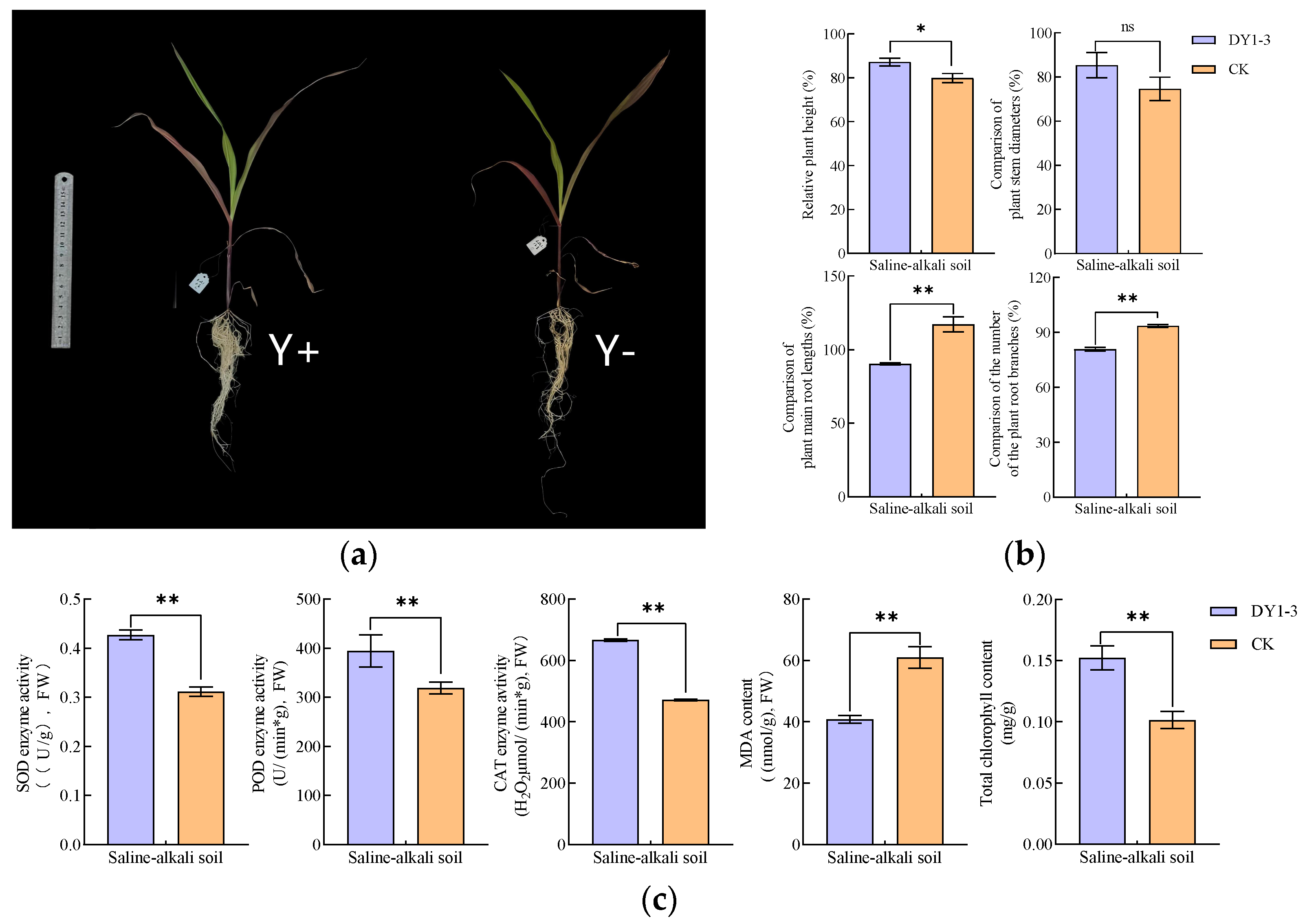
3.3.1. Measurement Results of Growth and Physiological Indexes of Potted Plants in Saline Soil
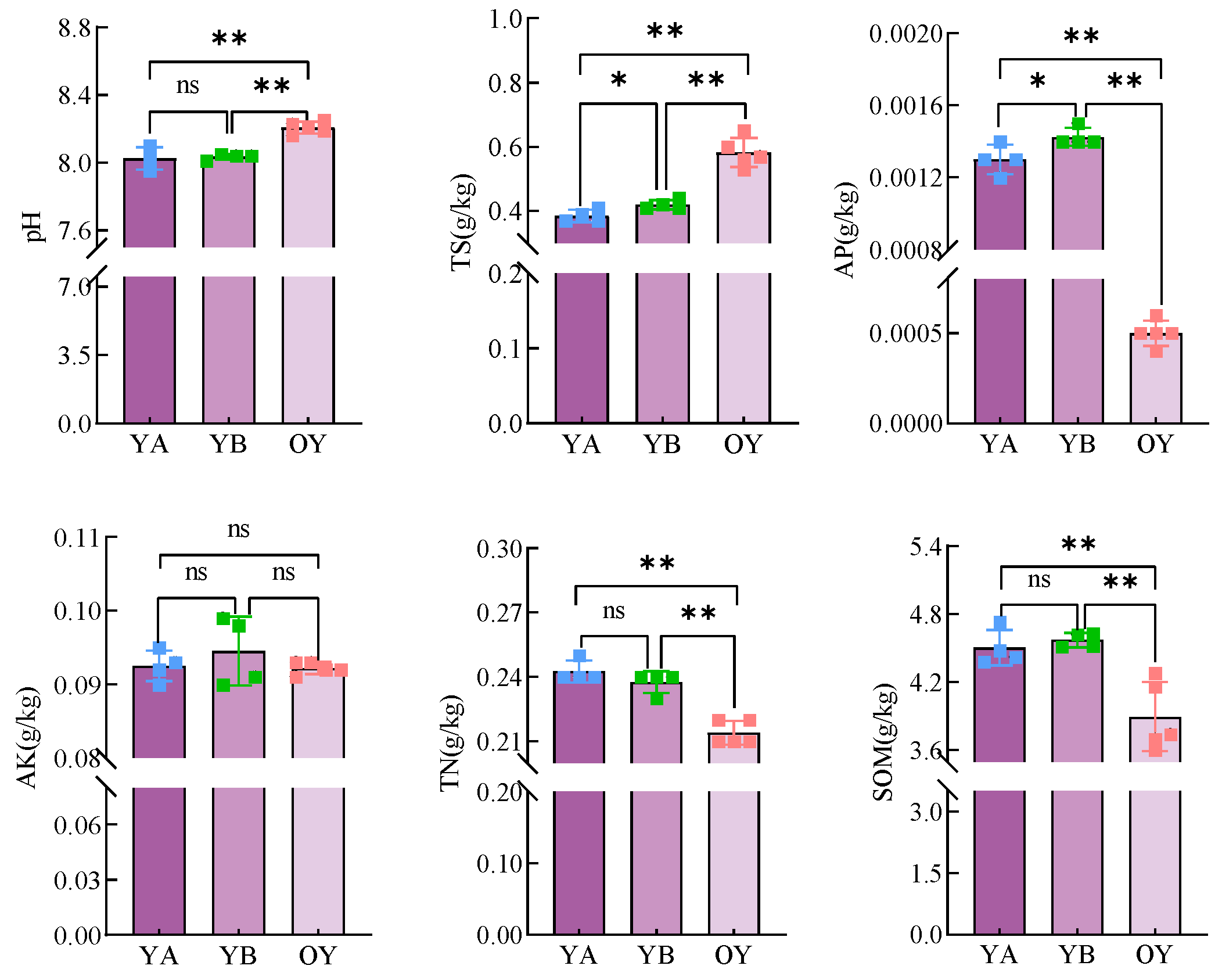
3.3.2. Results of Physicochemical Property Determination of Soil in Potting Saline Soil
3.3.3. Pre-Processing Statistics, Quality Control and OUT Distribution Analysis of Saline Soil Data After Potting Treatment
3.3.4. Analysis of Alpha Diversity Indices of Bacterial Communities in Saline Soils After Potting
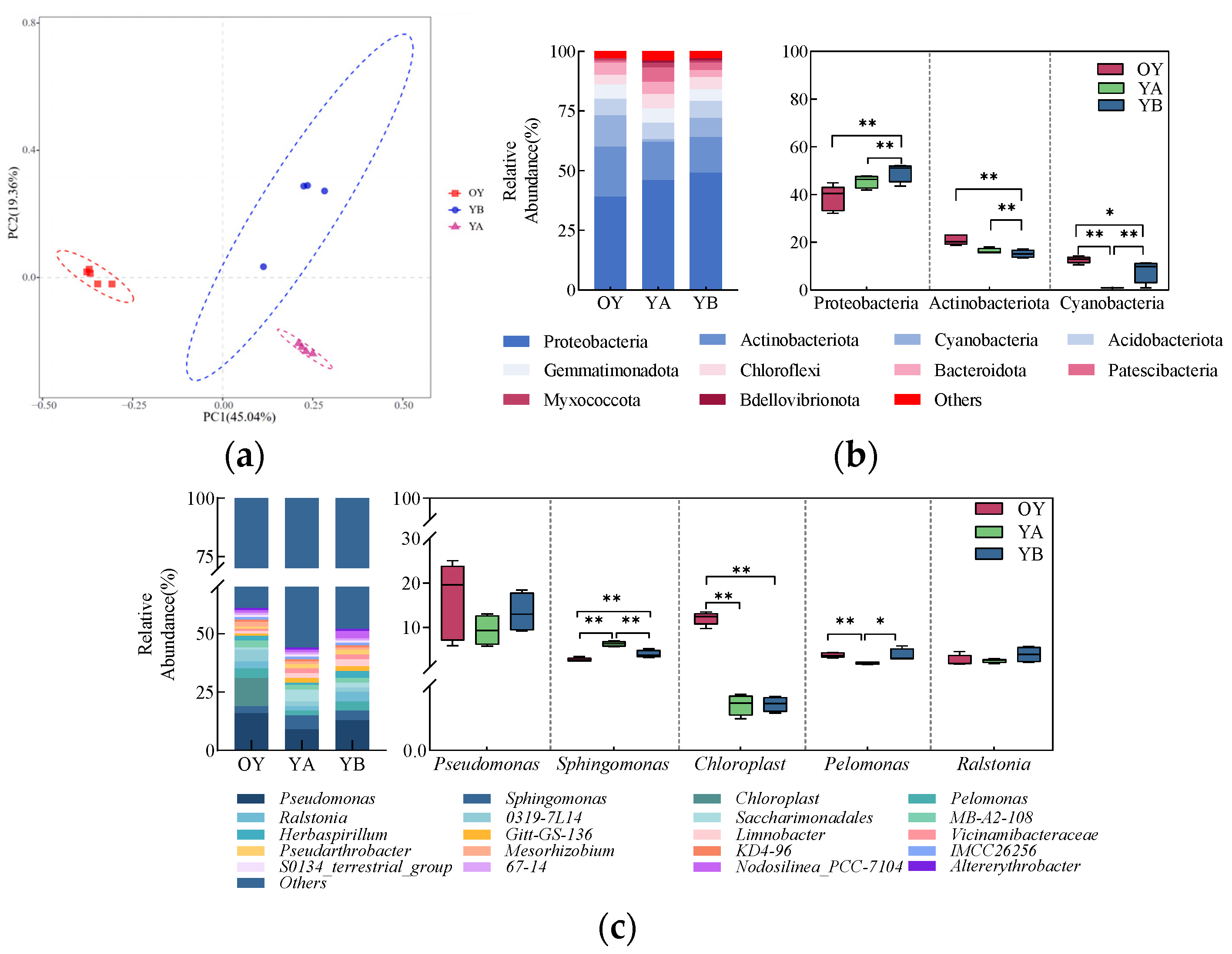
3.3.5. Analysis of Bacterial Community Composition of Saline Soil After Potting
3.3.6. RDA Analysis of the Structural Composition of Saline–Alkaline Soil Bacterial Community and Soil Physicochemical Factors
3.4. Investigate the Effects of DY1-3 Strain Application and Planting of Maize Seedlings on Soil Improvement in Saline and Alkaline Soils
4. Discussion
4.1. Differences in Physico-Chemical Properties and Microbial Community Structure of Different Soil Types
4.2. Effects of DY1-3 Solution on the Growth of Maize Seedlings, Soil Physico-Chemical Properties and Bacterial Community Structure in Arable Soil
4.3. Effects of the Application of DY1-3 Bacterial Suspension on the Growth of Maize Seedlings, Soil Physico-Chemical Properties, and Bacterial Community Structure in Saline Soil
4.4. Amelioration Effect of DY1-3 Bacterial Suspension Application and Planting of Maize Seedlings on Saline Soil
5. Conclusions
- There were significant differences between arable and saline soils in terms of soil physico-chemical properties and bacterial community structure composition, while TS was the main environmental factor influencing the composition and diversity of microbial communities. Differences in microbial metabolism between the two soils due to differences in various soil physico-chemical properties and differences in microbial community composition and diversity, mainly due to differences in TS content, may be a key determinant of differences in agricultural use of the soils.
- In arable soils, the application of DY1-3 bacterial suspension did not have much effect on the physicochemical factors and soil bacterial communities we measured, but more on the pro-biotic aspect of the plants, which could increase the plant height, SOD, and chlorophyll content of maize seedlings, but the elemental variations in the soil could still have an effect on the abundance of microbial communities. This may be related to the fact that our bacteria come from stressful environments, in which they are better able to carry out soil amelioration effects, whereas they may produce and metabolise more pro-biotic biomass to help plant growth in more suitable soil environments. Moreover, maize utilised more AK in soil during growth, while in the RDA analysis of soil physicochemical factors and bacterial community composition, it was found that AK content was also a key factor influencing the structural composition of the bacterial community.
- In saline soil, the application of DY1-3 bacterial suspension also had a significant positive effect on the growth of maize plants and the richness of bacterial community, as well as an obvious alleviation effect on the environmental stresses suffered by maize plants during the growth process. It increased the plant height, SOD, POD, CAT, and chlorophyll content and reduced the root length, root branch number, and MDA content of maize seedlings, which could lead to an increase in the diversity and abundance of the bacterial community in saline soil. And the application of DY1-3 bacterial suspension and the planting of potted plants can make the TS in saline soil decrease significantly, playing a role saline soil improvement.
- The application of DY1-3 bacterial suspension and the planting of maize seedlings had a certain positive effect on the improvement of saline soil and the improvement of fertility, which made the content of TS, AP, TN, and SOM, as well as the composition of the bacterial community in saline soil, appear close to that of arable soil.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paulo, P.; Damià, B.; Panos, P. Soil and water threats in a changing environment. Environ. Res. 2020, 186, 109501. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Xu, X.; Yang, J.; Zheng, G.; Xiao, H. Research Advances on Alkaline Soil Improvement by Flue Gas Desulphurization Byproducts. Soils 2010, 42, 352–357. [Google Scholar]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Yanmin, Y.; Licheng, W.; Hongtao, W.; Zhenhua, X.; Haiying, L.; Chunxu, L.; Zhongyi, S.; Ping, Y. Effects of Saline-Alkali Stress on Growth Characteristics and Yield of Rice. Heilongjiang Agric. Sci. 2024, 3, 1–5. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar] [CrossRef]
- Chendi, S. Long-Term Positioning Study on the Effects of Different Treatments of Reed Straw on Improving Saline-Alkali Land. Int. J. Ecol. 2020, 09, 13–17. [Google Scholar] [CrossRef]
- Li, S.; Chen, P.; Liu, H.; Hao, J.; Zhou, Y.; Shi, L. Mechanism and ecological effects of arbuscular mycorrhizal fungi on improving salt tolerance of plants in coastal saline–alkaline land. Ecol. Environ. Sci. 2019, 28, 411–418. [Google Scholar] [CrossRef]
- Wang, X.F.; Yang, S. An Overview of Saline-alkali Land Improvement Measures. Constr. Technol. 2020, 49, 220–222. [Google Scholar]
- Yin, X.L.; Cheng, C.; Ding, G.D.; Zhang, X.M.; Gao, G.L.; Bao, S.Z. Improvement and effects of calcium-containing wastes on saline-alkali soil and growth of Lespedeza bicolor Turcz. Sci. Soil Water Conserv. 2020, 18, 115–122. [Google Scholar] [CrossRef]
- Liu, C.; Wan, C.C.; Song, X.; Xia, G.F.; Ao, N.; Wang, K.M.; Wang, J. Effects of effective microorganisms on growth promotion and the rhizosphere eukaryotic community structure of pepper in Xinjiang, China. Chin. J. Appl. Ecol. 2024, 35, 1599–1607. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, D.; Zhao, F.; Hou, X.; Li, Q.; Zeng, L.; Lu, H.; Wang, B. Effects of Different Microbial Agents on the Growth of Foxtail Millet and Enzyme Activities of Soil. Heilongjiang Agric. Sci. 2024, 5, 27–31. [Google Scholar] [CrossRef]
- László, K.; Rita, B.; Dóra, B.; Henrietta, A.; Orsolya, K.; Gordana, R.; András, V.; Viktor, D.; Csaba, V. Recent advances in the use of Trichoderma-containing multicomponent microbial inoculants for pathogen control and plant growth promotion. World J. Microbiol. Biotechnol. 2024, 40, 162. [Google Scholar] [CrossRef]
- Li, J.; Wen, Y.; Fang, Z.; Yang, W.; Song, X. Application of cold-adapted microbial agents in soil contaminate remediation: Biodegradation mechanisms, case studies, and safety assessments. RSC Adv. 2024, 14, 12720–12734. [Google Scholar] [CrossRef]
- Yao, D.; Niu, S.Q.; Zhao, Q.; Cao, J.; Han, Q.; Li, H.; Gou, J.; Zhang, J. Induced salt tolerance of ryegrass by Bacillus subtilis strain WM13-24 from the rhizosphere of Haloxylon ammodendron. Acta Ecol. Sin. 2020, 40, 7419–7429. [Google Scholar]
- Jordan, E.; Guilhem, E.; Marie-Lara, E.; Bruno, T.; Yvan, M.; Laurent, L.; Florence, W.; Claire, P. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2020, 21, 49–68. [Google Scholar] [CrossRef]
- Jiawei, W.; Aoping, T.; Jianli, L.; Wanpeng, T.; Jiamin, Y.; Miao, G.; Biwen, S.; Zhirou, S. Screening of rhizosphere growth\|promoting bacteria of desert plants and its promoting effects on silage maize seedlings. Agric. Res. Arid Areas 2024, 42, 210–221. [Google Scholar] [CrossRef]
- Abdelkefi, N.; Louati, I.; Mechichi, H.Z.; Sayahi, N.; El-Sayed, W.; Nayal, A.; Ismail, W.; Hanin, M.; Mechichi, T. Enhanced salt stress tolerance in tomato plants following inoculation with newly isolated plant growth-promoting rhizobacteria. Sci. Hortic. 2024, 328, 112921. [Google Scholar] [CrossRef]
- Chamkhi, I.; El Omari, N.; Balahbib, A.; Menyiy, N.; Benali, T.; Ghoulam, C. Is—the rhizosphere a source of applicable multi-beneficial microorganisms for plant enhancement? Saudi J. Biol. Sci. 2022, 29, 1246–1259. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Wang, L.; Yue, Y.; Wang, L.; Yang, X. The effects of plant growth-promoting rhizobacteria on plants under temperature stress:A meta-analysis. Rhizosphere 2023, 28, 100788. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, S.; Zhang, X.; Guan, F.; Wu, H.; Zai, D.; Li, C.; Xu, L.; Li, X.; Zou, Y. Studies on the application of inter-root biotrophic bacteria to salt stress in plants. Agric. Technol. 2023, 43, 13–16. [Google Scholar] [CrossRef]
- Wu, Z.; Song, J.; Zhu, S.; Zhao, X.; Yang, X.; Ren, J.; Chen, F. Effects of Plant Growth-Promoting Microorganisms on Rhizosphere Microbial Community and the Leaf Pigment Composition of Liquidambar formosana. Sci. Silvae Sin. 2023, 59, 125–136. [Google Scholar] [CrossRef]
- Yuan, B.; Tian, C.; Hu, Y. Effects of Biochar and Plant Growth Promting Rhizobacteria on soil physico-chemical properties and Microbial Comunity Composition of Salinized Soil. Shandong Agric. Sci. 2023, 55, 101–109. [Google Scholar] [CrossRef]
- Guo, H.; Wang, F.; Tian, L.; Wang, C.; Li, W.; Fang, B.; Li, X.; Wang, Z. Bacterial diversity and influencing factors in soil sediments of Yuncheng Salt Lake, Shanxi. Acta Microbiol. Sin. 2024, 64, 1891–1905. [Google Scholar] [CrossRef]
- Li, M.; Bi, J.; Wang, J. Bacterial community structure and key influence factors in saline soil of different sites in Ningxia. Acta Ecol. Sin. 2020, 40, 1316–1330. [Google Scholar] [CrossRef]
- He, H.; Chen, J.; Wang, T.; Tang, M.; Liang, J.; Cao, Z.; Xiao, M.; He, M.; Li, Y.; Li, X. Influence of soil physicochemical properties on microbial diversity in different production areas of Chinese Cordyceps in Qinghai province. Southwest China J. Agric. Sci. 2024, 37, 824–834. [Google Scholar] [CrossRef]
- Ma, L.; Gao, W.; Mi, H.; Tang, J.; Li, M.; Huang, S. Soil microbial community characteristics in greenhouse vegetable production under different fertilization patterns based on metagenomic analysis. Plant Nutr. Fertil. Sci. 2021, 27, 403–416. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, E.; Zhang, Y.; Liu, J.; Xu, L.; Chen, Y. Isolation and characterization of Bacillus subtilis S1 capable of inducing resistance against powdery mildew and promoting cucumber growth from cucumber rhizosphere. Microbiol. China 2024, 51, 2141–2157. [Google Scholar] [CrossRef]
- Hou, Y.; Wei, C.; Zeng, W.; Hou, M.; Wang, Z.; Xu, G.; Huang, J. Application of rhizobacteria to improve microbial community structure and maize (Zea mays L.) growth in saline soil. Environ. Sci. Pollut. Res. Int. 2024, 31, 2481–2494. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, Y.; Feng, Y.; Zhang, Y.; Fan, Y. Bacterial Diversity Analysis and Screening for ACC Deaminase-Producing Strains in Moss-Covered Soil at Different Altitudes in Tianshan Mountains—A Case Study of Glacier No. 1. Microorganisms 2023, 11, 1521. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, Y.; Liu, Z.; Fan, Y.; Liu, M.; Ningjing, M.; Li, Y. Promotional Properties of ACC Deaminase-Producing Bacterial Strain DY1-3 and Its Enhancement of Maize Resistance to Salt and Drought Stresses. Microorganisms 2023, 11, 2654. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Liu, X.; Yang, J.; Yu, J.; Wang, Z.; Zhou, D.; Yu, Y.; Ning, K. Effects of returning years from farmland to wetland on the content and distribution of soil iron oxides in the Yellow River Delta. Chin. J. Ecol. 2023, 42, 2359–2367. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000; ISBN 978-7-109-06644-1. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, Y.; Chang, J.; Li, M.; Zhang, Y.; Dang, Y.; Wang, M.; Cheng, Y.; Zhang, B. Screening, resistance and growth-promoting effect of endophytic bacteria with ACC deaminase activity isolated from soybean nodules. Acta Microbiol. Sin. 2016, 56, 1009–1021. [Google Scholar] [CrossRef]
- Tianyun, S.; Jianjing, Z.; Anhong, L. Effects of soil physicochemical properties on microbial communities in different ecological niches in coastal area. Appl. Soil Ecol. 2020, 150, 103486. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi, B.M.; Chu, H. Salinity Is a Key Determinant for Soil Microbial Communities in a Desert Ecosystem. mSystems 2019, 4, e00225-18. [Google Scholar] [CrossRef]
- Hu, F.; Wang, F.; Han, X.; Xu, M.; Fu, Y.; Yan, J.; Jia, Z.; James, M.; Jiang, X. Succession of Microbial Community in Typical Black Soil under Different Land Use Pattern. Acta Pedol. Sin. 2022, 59, 1238–1247. [Google Scholar] [CrossRef]
- Zvyagintsev, D.G.; Zenova, G.M.; Oborotov, G.V. Moderately haloalkaliphilic actinomycetes in salt-affected soils. Eurasian Soil Sci. 2009, 42, 1515–1520. [Google Scholar] [CrossRef]
- Li, W.; Zhang, T.; Li, Y.; Li, X.; Huang, H.; Huang, M. Effect of Rhizobacteria Containing ACC Deaminase on Growth of Rose Bush. Fujian J. Agric. Sci. 2023, 38, 423–430. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Zhang, Y.; Zhang, T. Genetic 16S rRNA Diversity of Soil Microbes in Rhizosphere of Chinese Yam and Its Influencing Factors. Acta Pedol. Sin. 2019, 56, 1235–1246. [Google Scholar] [CrossRef]
- Li, C.; Wei, X.; Zhao, Z. Effects of different fertiliser ratios on the growth and nitrogen, phosphorus and potassium content of maize stover. J. Zhejiang Agric. Sci. 2017, 58, 747–749. [Google Scholar] [CrossRef]
- Pei, Y.; Ren, S.; Li, C.; Chen, X.; Xia, T.; Jiang, M.; Yin, M. Study on arbuscular mycorrhizal fungi difference in roots and rhizosphere soil in healthy and black shank infected tobacco Yunyan 121. J. South. Agric. 2022, 53, 1502–1512. [Google Scholar] [CrossRef]
- Yang, S.; Deng, L.; Huang, B.; Wang, Y.; Huang, Y.; Xiao, W.; Zheng, X.; Su, Y.; Ren, Z.; Yin, M. Correlation analysis between diversity of arbuscular mycorrhi-zal fungi in roots and soil physicochemical properties of Panax notoginseng (Burk.)F.H.Chen at different ages. J. South. Agric. 2023, 54, 3217–3227. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhao, X.; Zhao, L. Effect of Straw Addition on Acidity and Buffering Performance of Soil with Different Organic Contents. J. Soil Water Conserv. 2020, 34, 361–368. [Google Scholar] [CrossRef]
- Wu, L.; Lin, X.; Lin, W. Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chin. J. Plant Ecol. 2014, 38, 298–310. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, D.; Wang, Y.; Wu, F.; Zhou, X. Effects of Applying Different Rhizosphere Growth-promoting Bacteria on Pepper Growth and Soil Microbial Community. China Veg. 2022, 47–55. [Google Scholar] [CrossRef]
- Elizabeth, T.; Dwipendra, T.; Mohan Chandra, K.; Chandradev, K.S.; Narayan, C.T. Root colonization by host-specific rhizobacteria alters indigenous root endophyte and rhizosphere soil bacterial communities and promotes the growth of mandarin orange. Eur. J. Soil Biol. 2017, 79, 48–56. [Google Scholar] [CrossRef]
- He, M.; Xi, W.; Wang, D.; Pan, H.; Zhu, X.; Liu, Z. Effect of Microbial Fertilizer on Heavy Metal Content, Microbial Community Structure and Function in Aquacultural Sediment. Saf. Environ. Eng. 2023, 30, 234–243. [Google Scholar] [CrossRef]
- Lv, G. Effect of microbial fertiliser on soybean yield and soil microbial diversity. China Agric. Technol. Ext. 2023, 39, 79–81. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Tang, G.; Li, A.; Zhou, W.; Wu, T.; Hou, S.; Hou, Y.; Zeng, W. Effects of Rhizosphere Plant Growth Promoting Bacteria on Maize Growth Under Salt Stress. Water Sav. Irrig. 2023, 2, 121–127. [Google Scholar] [CrossRef]
- Zhao, W. Research Progress of Soil Salinization Stress on Maize. Heilongjiang Agric. Sci. 2019, 1, 140–143. [Google Scholar] [CrossRef]
- Suman, S.; Arpita, T.; Chandan Singh, C.; Deepti, B.; Pooja, S.; Vikas, K.P.; Poornima, V. Cold stress alleviation using individual and combined inoculation of ACC deaminase producing microbes in Ocimum sanctum. Environ. Sustain. 2020, 3, 289–301. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, L. Stoichiometric characteristics and influencing factors of soil nutrients under different land use types in an alpine mountain region. Acta Ecol. Sin. 2022, 42, 4415–4427. [Google Scholar] [CrossRef]
- Liu, G.; Gao, Y.; Shao, Z.; Dei, C. Research Progress of Soil Salinization-Alkalization Remediation Technology. Heilongjiang Agric. Sci. 2024, 1, 99–107. [Google Scholar] [CrossRef]
- Xing, J.; Yu, H. Effects of planting alfalfa on improving saline-alkali soil in Huanghua City, Hebei Province. Anim. Breed. Feed 2024, 23, 43–45. [Google Scholar] [CrossRef]
- He, G.; Song, J.; Wen, Y.; Liu, C.; Qi, J. Effects of different rhizobium fertilizers on alfalfa productivity and soil fertility. Acta Pratacult. Sin. 2020, 29, 109–120. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Q.; Li, S.; Lu, X.; Dong, L.; Wang, P.; Zhang, X.; Su, Z.; Ma, P. Application of Bacillus subtilis NCD-2 can suppress cotton verticillium wilt and its effect on abundant and rare microbial communities in rhizosphere. Biol. Control 2022, 165, 104812. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Li, X.; Wang, S.; Liu, Q.; Yin, K.; Zhang, X. Effects of three saline-alkali tolerant growth-promoting bacteria on the rhizosphere microecology of mung bean. Agric. Res. Arid Areas 2022, 40, 139–145. [Google Scholar] [CrossRef]
- Zhao, B.; Pan, F.; Wang, W.; Meng, C.; Zhang, X.; Liu, Z.; Liu, H.; Han, X. Effects of Biological Agent on Promoting Mahaleb Growth and Rhizospheric Bacterial Community. J. Shenyang Agric. Univ. 2018, 49, 286–292. [Google Scholar]
- Chou, D.; Liao, Z.; Xing, Q.; Wang, H.; Liu, J.; Zhao, B. The correlation analyses between bacterial community and the crucial environmental factors in saline-alkali soil of Songnen Plain. Microbiol. China 2022, 49, 2037–2049. [Google Scholar] [CrossRef]
- Wu, X.; Wang, R.; Gao, C.; Gao, S.; Du, L.; Khan, A.; Barmon, M.; Guo, S. Variations of soil properties effect on microbial community structure and functional structure under land uses. Acta Ecol. Sin. 2021, 41, 7989–8002. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, J.; Wei, Y.; Wang, Y.; Li, Q.; Teri, G.L.; Xia, J.; Lu, Y.; Xini, N.G. Spatial differentiation of Cyanobacterial communities and their relationship with environmental factors in Xilin River basin. Acta Sci. Circumstantiae 2020, 40, 4338–4348. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, Y.; Du, W. Effects of planting techniques for two-season forage grass per year on soil enzyme activities in the alpine pasturing area of Northwesten Sichuan in Qinghai-Tibet Plateau. Grassl. Turf 2024, 44, 57–66. [Google Scholar] [CrossRef]
- Navidi, M.; Sheidai-Karkaj, E.; Plaza-Alvarez, P.A.; Xu, X.; Sasanifar, S.; Nazarnejad, H.; Ortega, R.; Lucas-Borja, M.E.; Zema, D.A. Effects of banqueting on water infiltration and physico-chemical properties of soil in semi-arid lands. J. Arid Environ. 2024, 222, 105173. [Google Scholar] [CrossRef]
- Wang, D.; Niu, S.; Xu, H.; Zhao, W.; Yang, X.; Li, W.; Ma, W.; Sun, Z. Status of soil fertility, nutrient balance, and environmental risk assessment in yam produc-tion of North China Plain. Chin. J. Appl. Ecol. 2021, 32, 2818–2828. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Wang, Y.; Fan, B. Effects of Alfalfa Cultivation on Physiochemical Properties of Farmland Soil. Anim. Husb. Feed Sci. 2023, 44, 98–102. [Google Scholar] [CrossRef]
- Li, H. Microbial Fertilizers and Soil Conditioners Improvement Effect of Combined Application on Secondary Salinization Soil. North. Hortic. 2024, 85–92. [Google Scholar]
- Yang, X.; Zhang, K.; Chang, T.; Shaghaleh, H.; Qi, Z.; Zhang, J.; Ye, H.; Hamoud, A. Interactive Effects of Microbial Fertilizer and Soil Salinity on the Hydraulic Properties of Salt-Affected Soil. Plants 2024, 13, 473. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Edgar, R.C. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 2016. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, L.; Du, H.; Liu, Y.; Jin, X.; Chen, J.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; et al. Treatment with microbial agents increases the relative abundance of beneficial microorganisms in the rhizosphere soil of ginger. Plant Prot. 2023, 49, 55–66. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, Y.; Jiang, C.; Li, X.; Liu, T.; Wang, J.; Chen, C.; Gao, J. Identification and Characterization of Arthrobacter nicotinovorans JI39, a Novel Plant Growth-Promoting Rhizobacteria Strain from Panax ginseng. Front. Plant Sci. 2022, 13, 873621. [Google Scholar] [CrossRef] [PubMed]
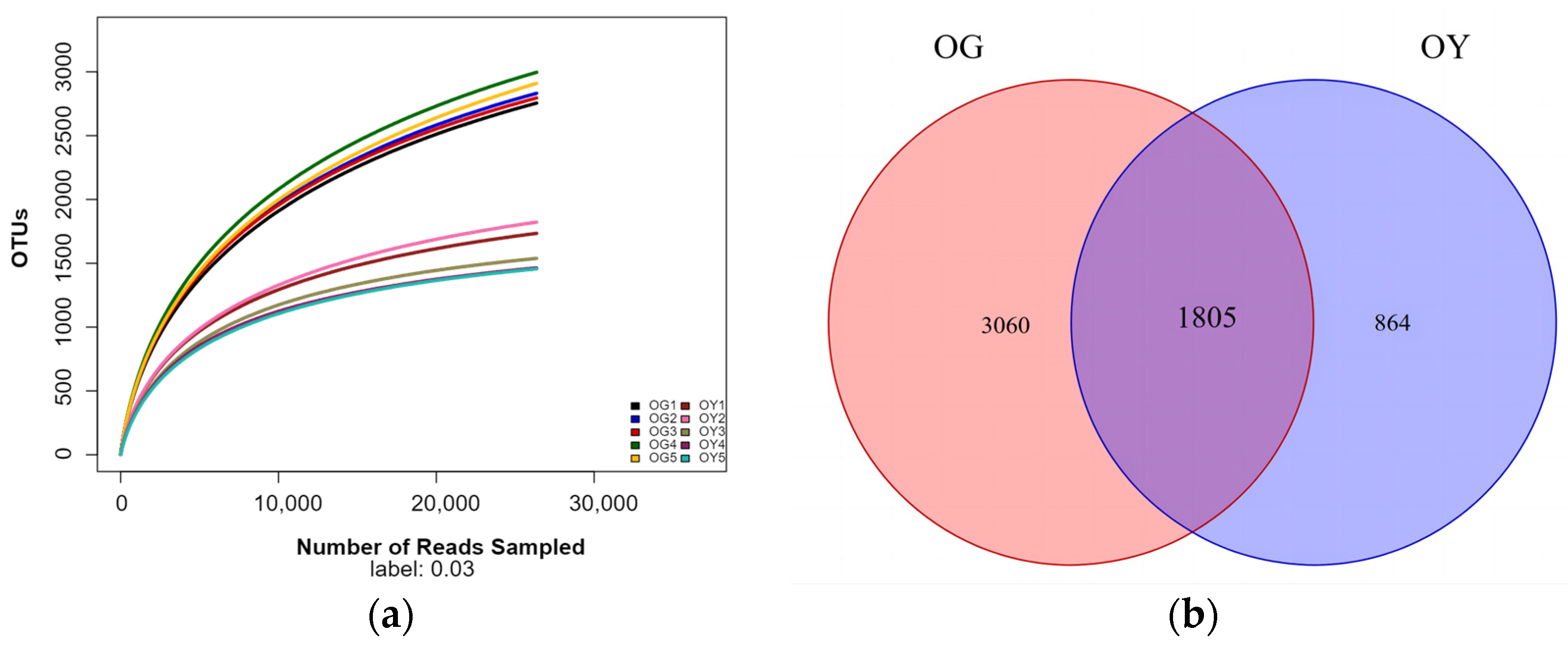

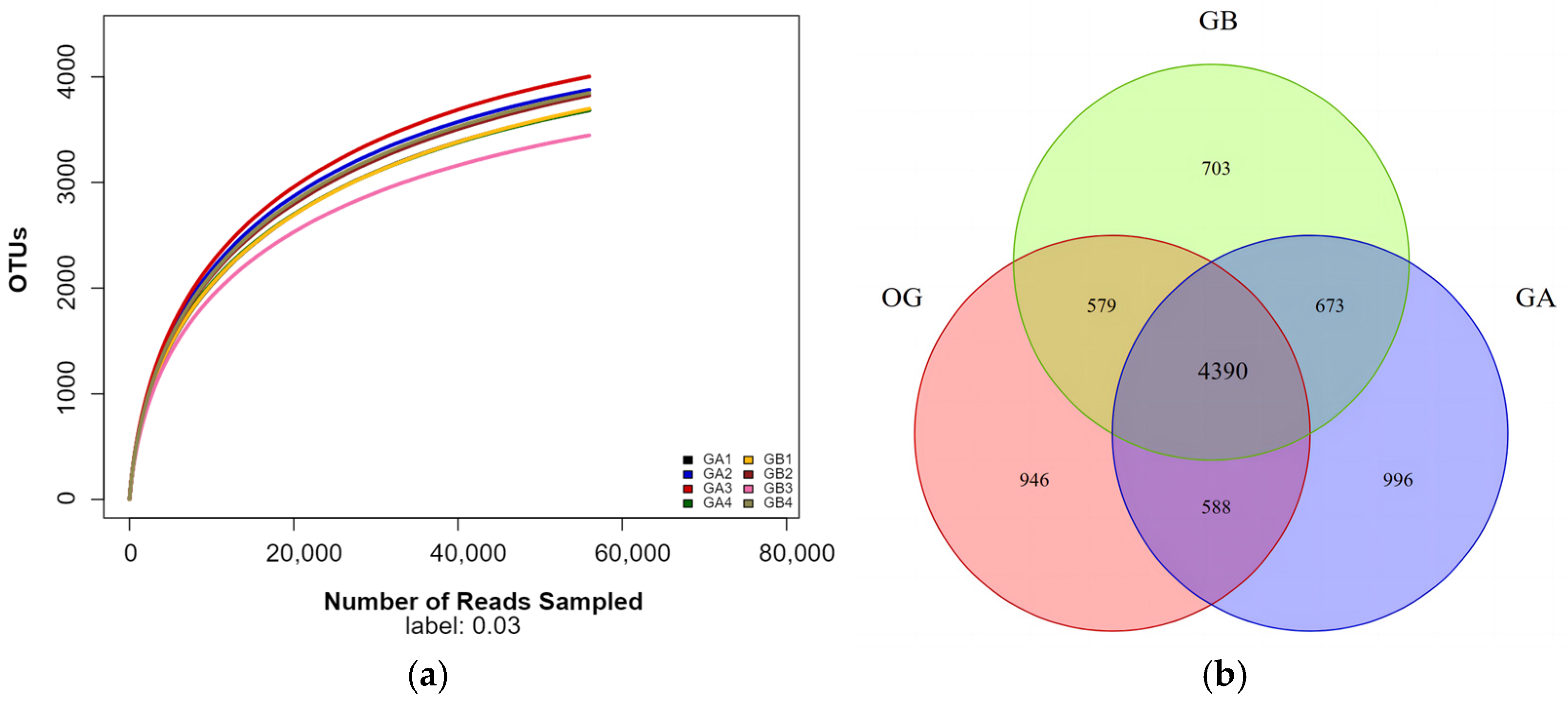

| Sample Name | Raw Reads | Clean Reads | Effective% | OTUs |
|---|---|---|---|---|
| OG1 | 74,173 | 72,497 | 97.74 | 2755 |
| OG2 | 55,163 | 53,284 | 96.59 | 2831 |
| OG3 | 65,880 | 64,168 | 97.40 | 2795 |
| OG4 | 70,811 | 68,672 | 96.98 | 2996 |
| OG5 | 72,199 | 69,562 | 96.35 | 2909 |
| OY1 | 35,280 | 34,935 | 99.02 | 1734 |
| OY2 | 56,820 | 56,307 | 99.10 | 1822 |
| OY3 | 34,110 | 33,903 | 99.39 | 1538 |
| OY4 | 27,619 | 27,431 | 99.32 | 1464 |
| OY5 | 38,195 | 37,911 | 99.26 | 1456 |
| Sample | OTUs | Shannon | Simpson | Chao1 | Goods Coverage |
|---|---|---|---|---|---|
| OG | 2857 ± 96 a | 9.7569 ± 0.0960 a | 0.9967 ± 0.0002 a | 3691.515 ± 155.813 a | 0.9652 ± 0.0018 a |
| OY | 1602 ± 166 b | 7.4808 ± 0.5097 b | 0.9563 ± 0.0197 b | 1866.832 ± 216.090 b | 0.9861 ± 0.0027 b |
| Sample Name | Raw Reads | Clean Reads | Effective% | OTUs |
|---|---|---|---|---|
| GA1 | 63,783 | 60,552 | 94.93 | 3871 |
| GA2 | 70,853 | 68,170 | 96.21 | 4107 |
| GA3 | 74,169 | 70,836 | 95.51 | 4274 |
| GA4 | 77,721 | 72,862 | 93.75 | 3867 |
| GB1 | 77,223 | 74,558 | 96.55 | 3956 |
| GB2 | 74,538 | 71,714 | 96.21 | 4022 |
| GB3 | 71,954 | 69,852 | 97.08 | 3662 |
| GB4 | 76,699 | 73,792 | 96.21 | 4020 |
| Sample | OTUs | Shannon | Simpson | Chao1 | Goods Coverage |
|---|---|---|---|---|---|
| GA | 4029 ± 198 a | 10.1499 ± 0.1251 a | 0.9977 ± 0.0002 a | 4870.657 ± 284.790 a | 0.9783 ± 0.0016 a |
| GB | 3915 ± 171 a | 10.0085 ± 0.1465 a | 0.9973 ± 0.0003 b | 4864.216 ± 230.542 a | 0.9777 ± 0.0011 a |
| OG | 3880 ± 125 a | 9.9436 ± 0.1012 b | 0.9970 ± 0.0002 c | 4681.391 ± 131.868 a | 0.9789 ± 0.0009 a |
| Sample Name | Raw Reads | Clean Reads | Effective% | OTUs |
|---|---|---|---|---|
| YA1 | 32,519 | 31,988 | 98.37 | 1894 |
| YA2 | 45,575 | 44,645 | 97.96 | 1927 |
| YA3 | 39,510 | 38,830 | 98.28 | 1841 |
| YA4 | 40,389 | 39,622 | 98.10 | 1772 |
| YB1 | 33,552 | 32,136 | 95.78 | 1543 |
| YB2 | 47,322 | 45,222 | 95.56 | 1588 |
| YB3 | 64,320 | 60,962 | 94.78 | 1709 |
| YB4 | 72,840 | 70,647 | 96.99 | 1615 |
| Sample | OTUs | Shannon | Simpson | Chao1 | Goods Coverage |
|---|---|---|---|---|---|
| YA | 1858 ± 68 a | 8.7032 ± 0.1808 a | 0.9895 ± 0.0039 a | 2120.417 ± 66.787 a | 0.9851 ± 0.0007 b |
| YB | 1613 ± 70 b | 7.9511 ± 0.2400 b | 0.9812 ± 0.0052 a | 1894.056 ± 103.166 b | 0.9858 ± 0.0010 b |
| OY | 1578 ± 156 b | 7.5832 ± 0.5175 b | 0.9613 ± 0.0200 b | 1821.878 ± 190.620 c | 0.9870 ± 0.0020 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Shi, Y.; Yuan, Y.; Fan, Y.; Chen, P.; Feng, Y.; Ningjing, M.; Li, H.; Li, D.; Wu, L. Effects of the Application of Pseudomonas cedrina DY1-3 on the Growth of Maize Plants and the Structure of Soil Bacterial Community. Microorganisms 2024, 12, 2556. https://doi.org/10.3390/microorganisms12122556
Liu Z, Shi Y, Yuan Y, Fan Y, Chen P, Feng Y, Ningjing M, Li H, Li D, Wu L. Effects of the Application of Pseudomonas cedrina DY1-3 on the Growth of Maize Plants and the Structure of Soil Bacterial Community. Microorganisms. 2024; 12(12):2556. https://doi.org/10.3390/microorganisms12122556
Chicago/Turabian StyleLiu, Zhenzhen, Yanlei Shi, Ye Yuan, Yonghong Fan, Peng Chen, Yingying Feng, Mengkedala Ningjing, Haocheng Li, Daiping Li, and Lewei Wu. 2024. "Effects of the Application of Pseudomonas cedrina DY1-3 on the Growth of Maize Plants and the Structure of Soil Bacterial Community" Microorganisms 12, no. 12: 2556. https://doi.org/10.3390/microorganisms12122556
APA StyleLiu, Z., Shi, Y., Yuan, Y., Fan, Y., Chen, P., Feng, Y., Ningjing, M., Li, H., Li, D., & Wu, L. (2024). Effects of the Application of Pseudomonas cedrina DY1-3 on the Growth of Maize Plants and the Structure of Soil Bacterial Community. Microorganisms, 12(12), 2556. https://doi.org/10.3390/microorganisms12122556






