A Seed Endophytic Bacterium Cronobacter dublinensis BC-14 Enhances the Growth and Drought Tolerance of Echinochloa crus-galli
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection and Endophytic Bacterial Isolation
2.2. Plant Growth Promotion
2.3. Identification of C. dublinensis BC-14
2.4. Indole Acetic Acid (IAA) Production of C. dublinensis BC-14
2.5. Phosphate-Solubilizing and Siderophore Production of C. dublinensis BC-14
2.6. GFP-Labeled C. Dublinensis BC-14 and Endophytic Establishment Studies
2.7. Pot Assay for Barnyard Grass Applied with C. dublinensis BC-14 Under Drought Stress
2.8. Statistical Analysis
3. Results
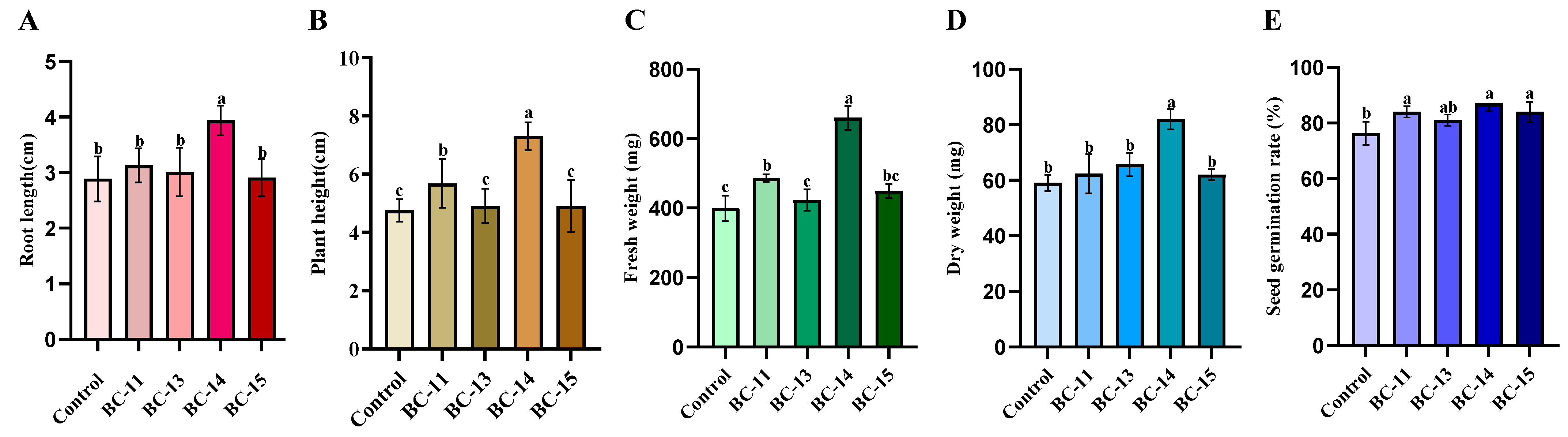
3.1. Selection of Endophytic C. dublinensis BC-14 from Barnyard Grass Seeds
3.2. Identification of C. dublinensis BC-14
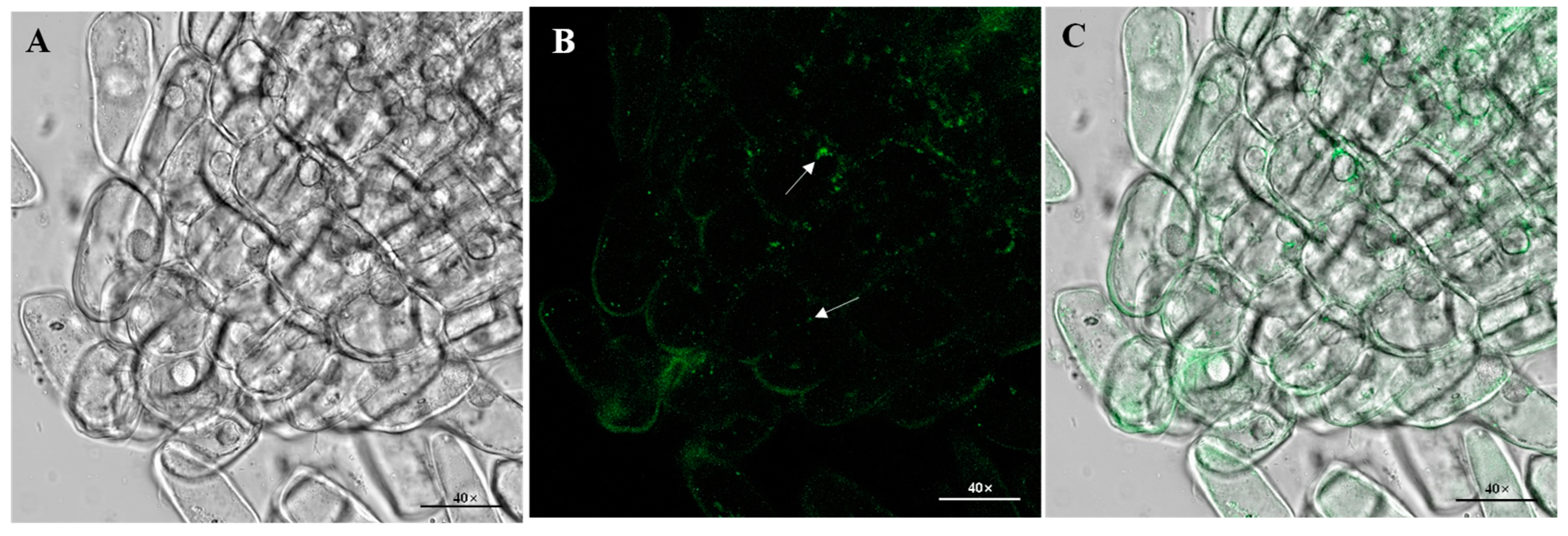
3.3. Functional Detection of C. dublinensis BC-14 and Its Colonization
3.4. Pot Study on Drought Stress
3.5. Genome Sequencing and Annotation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Trenberth, K.E.; Dai, A.; Van Der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Trivedi, G.; Shah, R.; Patel, P.; Saraf, M. Role of endophytes in agricultural crops under drought stress: Current and future prospects. J. Adv. Microbiol. 2017, 3, 174–188. [Google Scholar] [CrossRef]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Yang, X. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.; Mahawar, H.; Agarrwal, R.; Gautam, V.; Deshmukh, R. Advancement in the molecular perspective of plant-endophytic interaction to mitigate drought stress in plants. Front. Microbiol. 2022, 13, 981355. [Google Scholar] [CrossRef]
- Akbari, D.L.; Golakiya, B.A.; Bhadania, R.A. Screening and plant growth promoting activity of drought tolerant endophytic bacteria isolated from wild Poaceae. J. Pure Appl. Microbiol. 2015, 9, 2619–2627. [Google Scholar]
- Chauhan, B.S.; Johnson, D.E. Ecological studies on Echinochloa crus-galli and the implications for weed management in direct-seeded rice. Crop Prot. 2011, 30, 1385–1391. [Google Scholar] [CrossRef]
- Majchrzak, L.; Waligóra, H.; Zawieja, B.; Idziak, R.; Szulc, P. The Evaluation of Sweetcorn (Zea mays saccharata Sturt.) Infestation of Barnyardgrass (Echinochloa crus-galli) Depending on Weather Conditions and Crop Rotation. Agronomy 2024, 14, 776. [Google Scholar] [CrossRef]
- Ņečajeva, J.; Borodušķe, A.; Nikolajeva, V.; Seņkovs, M.; Fiļipovičs, M.; Jakobija, I.; Kalniņa, I.; Roga, A.; Skinderskis, E.; Gudrā, D.; et al. Characterisation and dynamics of the fungal communities of Avena fatua and Echinochloa crus-galli seeds in soil seed bank. Weed Res. 2024, 64, 310–320. [Google Scholar] [CrossRef]
- Tétard-Jones, C.; Edwards, R. Potential roles for microbial endophytes in herbicide tolerance in plants. Pest Manag. Sci. 2016, 72, 203–209. [Google Scholar] [CrossRef]
- Luu, T.A.; Phi, Q.T.; Nguyen, T.T.H.; Dinh, M.V.; Pham, B.N.; Do, Q.T. Antagonistic activity of endophytic bacteria isolated from weed plant against stem end rot pathogen of pitaya in Vietnam. Egypt. J. Biol. Pest Control 2021, 31, 14. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Li, H.; Ma, Y.; Zeng, D.; Du, L.; Jin, D. Characterization and genomic analysis of a bensulfuron methyl-degrading endophytic bacterium Proteus sp. CD3 isolated from barnyard grass (Echinochloa crus-galli). Front. Microbiol. 2022, 13, 1032001. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Cho, K.S. Isolation and characterization of a plant growth-promoting rhizobacterium, Serratia sp. SY5. J. Microbiol. Biotechnol. 2009, 19, 1431–1438. [Google Scholar] [PubMed]
- Wang, L.; Xi, N.; Lang, D.; Zhou, L.; Zhang, Y.; Zhang, X. Potential biocontrol and plant growth promotion of an endophytic bacteria isolated from Glycyrrhiza uralensis seeds. Egypt. J. Biol. Pest Control 2022, 32, 55. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Siddique, S.; Naveed, M.; Yaseen, M.; Shahbaz, M. Exploring potential of seed endophytic bacteria for enhancing drought stress resilience in maize (Zea mays L.). Sustainability 2022, 14, 673. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.; Pan, S.; Li, L.; Li, X. Characterization and metabolism effect of seed endophytic bacteria associated with peanut grown in south China. Front. Microbiol. 2019, 10, 2659. [Google Scholar] [CrossRef]
- Xin, G.; Zhang, G.; Kang, J.W.; Staley, J.T.; Doty, S.L. A diazotrophic, indole-3-acetic acid-producing endophyte from wild cottonwood. Biol. Fertil. Soils 2009, 45, 669–674. [Google Scholar] [CrossRef]
- Abdallah, R.B.; Mejdoub-Trabelsi, B.; Nefzi, A.; Jabnoun-Khiareddine, H.; Daami-Remadi, M. Isolation of endophytic bacteria from Withania somnifera and assessment of their ability to suppress Fusarium wilt disease in tomato and to promote plant growth. J. Plant Pathol. Microbiol. 2016, 7, 2–11. [Google Scholar] [CrossRef]
- Subramanian, P.; Mageswari, A.; Kim, K.; Lee, Y.; Sa, T. Psychrotolerant endophytic Pseudomonas sp. strains OB155 and OS261 induced chilling resistance in tomato plants (Solanum lycopersicum Mill.) by activation of their antioxidant capacity. Mol. Plant-Microbe Interact. 2015, 28, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 41564. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wu, Y.; Li, F.; Tang, W.; Gong, W.; Han, X.; White, J.F.; Ji, X.; Li, H. Seed endophytes and their roles in host plant stress resistance. J. Soil Sci. Plant Nutr. 2023, 23, 2927–2937. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, N.; Edrada-Ebel, R.; Ebel, R.; Proksch, P. Isolation, characterization, and bioactivity of endophytic fungi of Tylophora indica. World J. Microbiol. Biotechnol. 2011, 27, 571–577. [Google Scholar] [CrossRef]
- Vinodkumar, S.; Nakkeeran, S.; Renukadevi, P.; Mohankumar, S. Diversity and antiviral potential of rhizospheric and endophytic Bacillus species and phyto-antiviral principles against tobacco streak virus in cotton. Agric. Ecosyst. Environ. 2018, 267, 42–51. [Google Scholar]
- Kobayashi, D.Y.; El-Barrad, N.E.H. Selection of bacterial antagonists using enrichment cultures for the control of summer patch disease in Kentucky bluegrass. Curr. Microbiol. 1996, 32, 106–110. [Google Scholar] [CrossRef]
- Pappalettere, L.; Bartolini, S.; Toffanin, A. Enhancement of Tomato Seed Germination and Growth Parameters through Seed Priming with Auxin-Producing Plant Growth Promoting Bacteria Strains. Seeds 2024, 3, 479–492. [Google Scholar] [CrossRef]
- Relman, D.A. Universal bacterial 16S rDNA amplification and sequencing. In Diagnostic Molecular Microbiology, 1st ed.; ASM Press: Rockville, MD, USA, 1993; pp. 489–495. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Andrews, S. FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 10 July 2024).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [CrossRef]
- Cheng, S.; Jiang, J.-W.; Tan, L.-T.; Deng, J.-X.; Liang, P.-Y.; Su, H.; Sun, Z.-X.; Zhou, Y. Plant Growth-Promoting Ability of Mycorrhizal Fusarium Strain KB-3 Enhanced by Its IAA Producing Endohyphal Bacterium, Klebsiella aerogenes. Front. Microbiol. 2022, 13, 855399. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Shin, S.H.; Lim, Y.; Lee, S.E.; Yang, N.W.; Rhee, J.H. CAS agar diffusion assay for the measurement of siderophores in biological fluids. J. Microbiol. Methods 2001, 44, 89–95. [Google Scholar] [CrossRef]
- Liang, P.; Jiang, J.; Sun, Z.; Li, Y.; Yang, C.; Zhou, Y. Klebsiella michiganensis: A nitrogen-fixing endohyphal bacterium from Ustilago maydis. AMB Express 2023, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Rana, K.L.; Sheikh, I.; Kumar, V.; Yadav, A.N.; Dhaliwal, H.S.; Saxena, A.K. Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, a drought-adaptive phosphorus-solubilizing bacterium. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 785–795. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, M.; Zhao, Q.; Wang, F.; Gao, J.; Sheng, H.; An, L. Complete genome sequence of Sphingomonas sp. Cra20, a drought resistant and plant growth promoting rhizobacteria. Genomics 2020, 112, 3648–3657. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; He, Z.; Zhang, Z.; Xu, Y.; Li, Z.; Peng, Y.; Deng, N.; Chen, Y. Integration and potential application ability of culturable functional microorganism in oil tea Camellia. Indian J. Microbiol. 2021, 61, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Li, Q.; Cui, R.; Wang, Z.; Wang, Y.; Zhang, Y.-Z.; Ding, W.; Shen, X. Deciphering the root endosphere microbiome of the desert plant Alhagi sparsifolia for drought resistance-promoting bacteria. Appl. Environ. Microbiol. 2020, 86, e02863-19. [Google Scholar] [CrossRef]
- Shahid, M.; Hameed, S.; Imran, A.; Ali, S.; van Elsas, J.D. Root colonization and growth promotion of sunflower (Helianthus annuus L.) by phosphate solubilizing Enterobacter sp. Fs-11. World J. Microbiol. Biotechnol. 2012, 28, 2749–2758. [Google Scholar] [CrossRef]
- Saranya, K.; Sundaramanickam, A.; Shekhar, S.; Meena, M.; Sathishkumar, R.S.; Balasubramanian, T. Biosorption of multi-heavy metals by coral associated phosphate solubilising bacteria Cronobacter muytjensii KSCAS2. J. Environ. Manag. 2018, 222, 396–401. [Google Scholar] [CrossRef]
- Schmid, M.; Iversen, C.; Gontia, I.; Stephan, R.; Hofmann, A.; Hartmann, A.; Jha, B.; Eberl, L.; Riedel, K.; Lehner, A. Evidence for a plant-associated natural habitat for Cronobacter spp. Res. Microbiol. 2009, 160, 608–614. [Google Scholar] [CrossRef]
- Yu, T.F.; Xu, Z.S.; Guo, J.K.; Wang, Y.X.; Abernathy, B.; Fu, J.D.; Chen, X.; Zhou, Y.-B.; Chen, M.; Ye, X.-G.; et al. Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene SeCspA. Sci. Rep. 2017, 7, 44050. [Google Scholar] [CrossRef]
- Massa, F.; Defez, R.; Bianco, C. Exploitation of plant growth promoting bacteria for sustainable agriculture: Hierarchical approach to link laboratory and field experiments. Microorganisms 2022, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.P.; Dietel, K.; Rändler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef] [PubMed]
- Azizoglu, U. Bacillus thuringiensis as a biofertilizer and biostimulator: A mini-review of the little-known plant growth-promoting properties of Bt. Curr. Microbiol. 2019, 76, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.R.; Bonatelli, M.L.; Batista, B.D.; Teixeira-Silva, N.S.; Mondin, M.; dos Santos, R.C.; Bento, J.M.S.; Hayashibara, C.A.A.; Azevedo, J.L.; Quecine, M.C. Bacillus thuringiensis RZ2MS9, a tropical plant growth-promoting rhizobacterium, colonizes maize endophytically and alters the plant’s production of volatile organic compounds during co-inoculation with Azospirillum brasilense Ab-V5. Environ. Microbiol. Rep. 2021, 13, 812–821. [Google Scholar] [CrossRef]
- Padda, K.P.; Puri, A.; Chanway, C.P. Plant growth promotion and nitrogen fixation in canola (Brassica napus) by an endophytic strain of Paenibacillus polymyxa and its GFP-tagged derivative in a long-term study. Botany 2016, 94, 1209–1217. [Google Scholar] [CrossRef]
- Manjunatha, B.S.; Nivetha, N.; Krishna, G.K.; Elangovan, A.; Pushkar, S.; Chandrashekar, N.; Aggarwal, C.; Asha, A.D.; Chinnusamy, V.; Raipuria, R.K.; et al. Plant growth-promoting rhizobacteria Shewanella putrefaciens and Cronobacter dublinensis enhance drought tolerance of pearl millet by modulating hormones and stress-responsive genes. Physiol. Plant. 2022, 174, e13676. [Google Scholar] [CrossRef]
- Eida, A.A.; Bougouffa, S.; L’Haridon, F.; Alam, I.; Weisskopf, L.; Bajic, V.B.; Saad, M.M.; Hirt, H. Genome insights of the plant-growth promoting bacterium Cronobacter muytjensii JZ38 with volatile-mediated antagonistic activity against Phytophthora infestans. Front. Microbiol. 2020, 11, 369. [Google Scholar] [CrossRef]
- Gao, X.; Luan, J.; Wang, L.; Li, H.; Wang, Q.; Wang, Z.; Jin, Z.; Yu, F. Effect of the plant growth promoting rhizobacterium, Cronobacter sp. Y501, for enhancing drought tolerance in maize (Zea mays L.). J. Soil Sci. Plant Nutr. 2023, 23, 2786–2797. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.-J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Krause, A.G.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Zhang, X.; Lang, D.; Zhang, X. Growth-promoting bacteria alleviate drought stress of, G. uralensis through improving photosynthesis characteristics and water status. J. Plant Interact. 2019, 14, 580–589. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Pinedo, I.; Ledger, T.; Greve, M.; Poupin, M.J. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015, 6, 466. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The influence of humic acids and nitrophenols on metabolic compounds and pesticide behavior in wheat under biotic stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Chaudhary, P.; Agri, U.; Chaudhary, A.; Kumar, A.; Kumar, G. Endophytes and their potential in biotic stress management and crop production. Front. Microbiol. 2022, 13, 933017. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Webster, S.; He, S.Y. Growth–defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef] [PubMed]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]




| Species | Strain ID | Source | Location | Accession Numbers | Base Size (bp) | Scaffold N50 | No. of Scaffolds | Percent G + C (%) | No. of Genes | No. Proteins | No. Plasmids | Plasmid Size (bp) | ANI with BC-14 (%) | DDH with BC-14 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. condimenti | LMG 26250T | spiced meat | Slovakia | GCF_001277255.1 | 4,499,482 | 4,347,991 | 2 | 56 | 4242 | 4059 | 1 | 164,790 | 88.77 | 80.8 |
| C. dublinensis | BC-14 | barnyard grass | China | JBIPQX000000000.1 | 4,491,274 | 805,486 | 21 | 58.09 | 4236 | 4058 | 1 | 151,085 | 100 | 100 |
| C. dublinensis | LMG 23823T | milk powder plant | Ireland | GCF_001277235.1 | 4,628,405 | 4,431,067 | 2 | 58 | 4379 | 4224 | 1 | 203,534 | 97.34 | 87.9 |
| C. dublinensis | CFSA A137 | noodle | China | GCF_032598745.1 | 4,768,277 | 4,532,538 | 3 | 57.5 | 4590 | 4315 | 2 | 174,783/60,956 | 98.07 | 89.7 |
| C. dublinensis | CFSA A84 | noodle | China | GCF_029227785.1 | 4,733,612 | 4,526,300 | 4 | 57.5 | 4561 | 4347 | 3 | 162,169/38,910/6233 | 98.12 | 91.6 |
| C. malonaticus | LMG 23826T | breast abscess | America | GCF_001277215.2 | 4,473,761 | 4,294,639 | 3 | 57 | 4292 | 4090 | 2 | 126,501/52,758 | 89.67 | 78.7 |
| C. muytjensii | ATCC 51329T | - | France | GCF_001277195.1 | 4,364,114 | 4,364,114 | 1 | 57.5 | 4100 | 3934 | 0 | 0 | 92.49 | 81.6 |
| C. sakazakii | CS-931T | feces | Mexico | GCF_003516125.1 | 4,437,993 | 4,267,208 | 3 | 57 | 4228 | 4050 | 2 | 119,197/51,588 | 89.62 | 76.5 |
| C. turicensis | LMG23827T | blood culture | Switzerland | GCF_041222865.12 | 4,554,702 | 4,384,662 | 3 | 57.5 | 4329 | 4137 | 2 | 147,683/22,357 | 89.78 | 83.2 |
| C. universalis | NCTC 9529T | water | - | GCF_001277175.1 | 4,436,873 | 4,307,096 | 2 | 57 | 4210 | 4039 | 1 | 136,454 | 90.16 | 80.3 |
| Characteristic | Result | Characteristic | Result | Characteristic | Result |
|---|---|---|---|---|---|
| β-galactosidase | − | Urease | + | Mannitol oxidation | + |
| Arginine dehydrogenase | + | Gelatin liquefaction | + | Inositol oxidation | − |
| Lysine decarboxylase | + | Sorbitol oxidation | + | Glucose oxidase | + |
| Ornithine decarboxylation | + | Rhamnose oxidation | + | Amygdalin oxidation | + |
| Citric acid utilization | + | Sucrose oxidation | + | Arabinose oxidation | + |
| H2S production | − | Melibiose oxidation | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Wang, Q.; Yang, D.; He, Q.; Deng, J.; Zhou, Y.; Zhang, L.; Jiang, J. A Seed Endophytic Bacterium Cronobacter dublinensis BC-14 Enhances the Growth and Drought Tolerance of Echinochloa crus-galli. Microorganisms 2024, 12, 2544. https://doi.org/10.3390/microorganisms12122544
Cheng S, Wang Q, Yang D, He Q, Deng J, Zhou Y, Zhang L, Jiang J. A Seed Endophytic Bacterium Cronobacter dublinensis BC-14 Enhances the Growth and Drought Tolerance of Echinochloa crus-galli. Microorganisms. 2024; 12(12):2544. https://doi.org/10.3390/microorganisms12122544
Chicago/Turabian StyleCheng, Sheng, Qingling Wang, Dashan Yang, Quanlong He, Jianxin Deng, Yi Zhou, Lin Zhang, and Jianwei Jiang. 2024. "A Seed Endophytic Bacterium Cronobacter dublinensis BC-14 Enhances the Growth and Drought Tolerance of Echinochloa crus-galli" Microorganisms 12, no. 12: 2544. https://doi.org/10.3390/microorganisms12122544
APA StyleCheng, S., Wang, Q., Yang, D., He, Q., Deng, J., Zhou, Y., Zhang, L., & Jiang, J. (2024). A Seed Endophytic Bacterium Cronobacter dublinensis BC-14 Enhances the Growth and Drought Tolerance of Echinochloa crus-galli. Microorganisms, 12(12), 2544. https://doi.org/10.3390/microorganisms12122544





