Factors for Treatment Failure After Fecal Microbiota Transplantation in Clostridioides difficile Infection
Abstract
1. Introduction
2. Materials and Methods
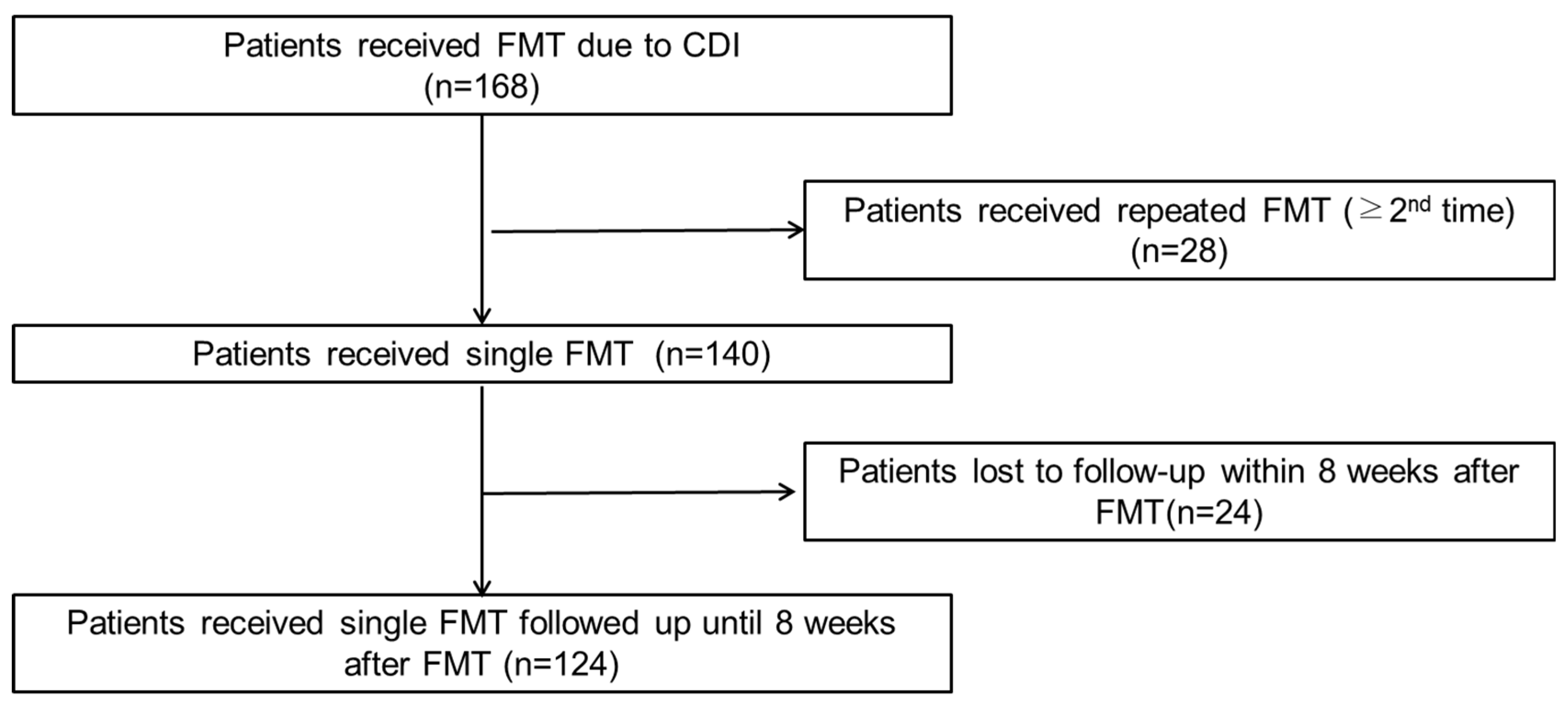
2.1. Patient Selection
2.2. Data Collection
2.3. Antibiotic Administration
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations
4.1.1. Study Design Limitations
4.1.2. Single-Center Bias
4.1.3. Data Collection Constraints
4.1.4. Sample Size Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 29–30, 100642. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Allegretti, A.S.; Phelps, E.; Xu, H.; Fischer, M.; Kassam, Z. Classifying fecal microbiota transplantation failure: An Observational study examining timing and characteristics of fecal microbiota transplantation failures. Clin. Gastroenterol. Hepatol. 2018, 16, 1832–1833. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Kahn, S.; Kashyap, P.; Laine, L.; Rubin, D.; Atreja, A.; Moore, T.; Wu, G. Update on fecal microbiota transplantation 2015: Indications, methodologies, mechanisms, and outlook. Gastroenterology 2015, 149, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.; Neamtu, B.; Mihalache, M.; Boicean, A.; Banciu, A.; Banciu, D.D.; Moga, D.F.C.; Birlutiu, V. Fecal microbiota transplant in severe and non-severe Clostridioides difficile infection. Is there a role of FMT in primary severe CDI. J. Clin. Med. 2021, 10, 5822. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Mehta, S.R.; Kassam, Z.; Kelly, C.R.; Kao, D.; Xu, H.; Fischer, M. Risk Factors that predict the failure of multiple fecal microbiota transplantations for Clostridioides difficile infection. Dig. Dis. Sci. 2021, 66, 213–217. [Google Scholar] [CrossRef]
- Brown, J.R.; Flemer, B.; Joyce, S.A.; Zulquernain, A.; Sheehan, D.; Shanahan, F.; O’Toole, P.W. Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol. 2018, 18, 131. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Peery, A.F.; Kelly, C.R.; Kao, D.; Vaughn, B.P.; Lebwohl, B.; Singh, S.; Imdad, A.; Altayar, O. AGA Clinical practice guideline on fecal microbiota-based therapies for select gastrointestinal diseases. Gastroenterology 2024, 166, 409–434. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Brandt, L.J.; Aroniadis, O.C.; Mellow, M.; Kanatzar, A.; Kelly, C.; Park, T.; Stollman, N.; Rohlke, F.; Surawicz, C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am. J. Gastroenterol. 2012, 107, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Plechot, K.; Gohil, S.; Le, J. Clostridium difficile: Diagnosis and the consequence of over diagnosis. Infect. Dis. Ther. 2021, 10, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Whang, D.H. Diagnostic algorithm for the rapid and cost-effective detection of Clostridioides difficile infection: Comparison between C. DIFF QUIK CHEK COMPLETE and VIDAS GDH & toxin assay. Lab. Med. Qual. Assur. 2020, 42, 130–139. [Google Scholar] [CrossRef]
- Sehgal, K.; Zandvakili, I.; Tariq, R.; Pardi, D.S.; Khanna, S. Systematic review and meta-analysis: Efficacy of vancomycin taper and pulse regimens in Clostridioides difficile infection. Expert Rev. Anti. Infect. Ther. 2022, 20, 577–583. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kao, D.; Sitko, J.; Fischer, M.; Kassam, Z. Early antibiotic use after fecal microbiota transplantation increases risk of treatment failure. Clin. Infect. Dis. 2018, 66, 134–135. [Google Scholar] [CrossRef]
- Singh, P.; Alm, E.J.; Kelley, J.M.; Cheng, V.; Smith, M.; Kassam, Z.; Nee, J.; Iturrino, J.; Lembo, A. Effect of antibiotic pretreatment on bacterial engraftment after fecal microbiota transplant (FMT) in IBS-D. Gut Microbes 2022, 14, 2020067. [Google Scholar] [CrossRef]
- Mamo, Y.; Woodworth, M.H.; Wang, T.; Dhere, T.; Kraft, C.S. Durability and long-term clinical outcomes of fecal microbiota transplant treatment in patients with recurrent Clostridium difficile infection. Clin. Infect. Dis. 2018, 66, 1705–1711. [Google Scholar] [CrossRef]
- Warraich, F.; Sohail, S.H.; Knee, A.; Smith, J.; Schlecht, H.; Skiest, D. Factors associated with fecal microbiota transplant failure in the treatment of recurrent Clostridioides difficile infection: A single-center retrospective study. Cureus 2023, 15, e45118. [Google Scholar] [CrossRef]
- Beran, A.; Sharma, S.; Ghazaleh, S.; Lee-Smith, W.; Aziz, M.; Kamal, F.; Acharya, A.; Adler, D.G. Predictors of fecal microbiota transplant failure in Clostridioides difficile Infection: An updated meta-analysis. J. Clin. Gastroenterol. 2023, 57, 389–399. [Google Scholar] [CrossRef]
- Peri, R.; Aguilar, R.C.; Tüffers, K.; Erhardt, A.; Link, A.; Ehlermann, P.; Angeli, W.; Frank, T.; Storr, M.; Glück, T.; et al. The impact of technical and clinical factors on fecal microbiota transfer outcomes for the treatment of recurrent Clostridioides difficile infections in Germany. United Eur. Gastroenterol. J. 2019, 7, 716–722. [Google Scholar] [CrossRef]
- Khanna, S.; Pardi, D.S.; Kelly, C.R.; Kraft, C.S.; Dhere, T.; Henn, M.R.; Lombardo, M.J.; Vulic, M.; Ohsumi, T.; Winkler, J.; et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J. Infect. Dis. 2016, 214, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Sipe, B.W.; Rogers, N.A.; Cook, G.K.; Robb, B.W.; Vuppalanchi, R.; Rex, D.K. Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: Description of a protocol with high success rate. Aliment. Pharmacol. Ther. 2015, 42, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, J.H.; Park, S.H.; Cha, B.; Kwon, K.S.; Kim, H.; Shin, Y.W. Efficacy and safety of fecal microbiota transplantation for clearance of multidrug-resistant organisms under multiple comorbidities: A prospective comparative trial. Biomedicines 2022, 10, 2404. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, J.; Park, S.H.; Cha, B.; Hong, J.T.; Lee, D.H.; Kwon, K.S. Role of probiotics in preventing carbapenem-resistant Enterobacteriaceae colonization in the intensive care unit: Risk factors and microbiome analysis study. Microorganisms 2023, 11, 2970. [Google Scholar] [CrossRef]
- Hecker, M.T.; Ho, E.; Donskey, C.J. Fear of failure: Engaging patients in antimicrobial stewardship after fecal transplantation for recurrent Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 2017, 38, 127–129. [Google Scholar] [CrossRef]
- Chiou, C.Y.; Chiou, H.L. Aminoglycosides and C. difficile colitis. N. Engl. J. Med. 1990, 322, 338. [Google Scholar] [CrossRef]
- Teng, C.; Reveles, K.R.; Obodozie-Ofoegbu, O.O.; Frei, C.R. Clostridium difficile infection risk with important antibiotic classes: An analysis of the FDA adverse event reporting system. Int. J. Med. Sci. 2019, 16, 630–635. [Google Scholar] [CrossRef]
- Papanicolas, L.E.; Warner, M.; Wesselingh, S.L.; Rogers, G.B. Protect commensal gut bacteria to improve antimicrobial stewardship. Clin. Microbiol. Infect. 2020, 26, 814–815. [Google Scholar] [CrossRef]
- Shin, J.H.; Hays, R.A.; Warren, C.A. Hospitalized older patients with Clostridioides difficile infection refractory to conventional antibiotic therapy benefit from fecal microbiota transplant. Adv. Geriatr. Med. Res. 2021, 3, e210012. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Kwon, K.T.; Jeong, S.J.; Moon, C.; Kim, B.; Kiem, S.; Kim, H.S.; Heo, E.; Kim, S.W. Guidelines on implementing antimicrobial stewardship programs in Korea. Infect. Chemother. 2021, 53, 617–659. [Google Scholar] [CrossRef]
| Characteristics | n (%) |
|---|---|
| Age, years [median (IQR)] | 75 (62–81) |
| Sex | |
| Male | 59 (47.6) |
| Female | 65 (52.4) |
| Diagnosis at admission | |
| Colitis or ileus | 92 (74.2) |
| Pneumonia | 11 (8.9) |
| Urinary tract infection | 11 (20.4) |
| Others | 26 (21.0) |
| DM | |
| Uncomplicated DM | 31 (25.0) |
| Complicated DM | 3 (2.40) |
| Liver disease, mild | 2 (1.60) |
| HIV | 1 (0.8) |
| Malignancy | |
| Any leukemia, lymphoma, or localized solid tumor | 8 (6.5) |
| Metastatic solid tumor | 2 (1.6) |
| Chronic kidney disease | 27 (21.8) |
| Congestive heart failure | 11 (8.9) |
| AMI | 3 (2.4) |
| PAOD | 4 (3.2) |
| COPD | 7 (5.6) |
| Cerebral vascular accident | 12 (22.2) |
| Hemiplegia | 13 (10.5) |
| Rheumatic disease | 2 (1.6) |
| Dementia | 23 (18.5) |
| Peptic ulcer disease | 12 (9.7) |
| Age-adjusted Charlson Comorbidity Index | 5 (3–6) |
| Characteristics | n (%) |
|---|---|
| Initial CDI diagnosis | |
| GDH | 48 (38.7) |
| Toxin B PCR | 16 (12.9) |
| GDH + Toxin B PCR | 60 (48.4) |
| FMT method | |
| Colonoscopy | 99 (79.8) |
| Duodenoscopy | 11 (8.9) |
| Colonoscopy + Duodenoscopy | 14 (11.3) |
| Antibiotics | |
| Non-CDI antibiotics before FMT | 79 (63.7) |
| Non-CDI antibiotics after FMT (≤7 days) | 43 (34.7) |
| Non-CDI antibiotics after FMT (>7 days) | 53 (42.7) |
| CDI antibiotics before FMT (recurrent or refractory CDI) | 36 (29) |
| Oral vancomycin duration, median (interquartile range), days | 13 (5–21) |
| Oral metronidazole duration, median (interquartile range), days | 5 (3–9) |
| CDI antibiotics (oral vancomycin) during ongoing FMT | 33 (26.6) |
| FMT Outcome | |
| Symptom resolution within 7 days after FMT | 93 (75) |
| Variable | Treatment Failure | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| n/total (%) | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Non-CDI antibiotics after FMT (>7 days) | 26/39 (66.7) | 4.30 (1.92–9.63) | <0.01 | 2.62 (1.08–6.36) | 0.03 |
| Non-CDI antibiotics after FMT (≤7 days) | 17/39 (43.6) | 1.76 (0.80–3.84) | 0.16 | ||
| Non-CDI antibiotics before FMT | 33/39 (84.6) | 4.66 (1.77–12.29) | 0.001 | 3.03 (1.12–8.19) | 0.03 |
| Aminoglycoside after FMT | 10/39 (25.6) | 4.54 (1.51–13.61) | 0.007 | 3.69 (1.14–12.00) | 0.03 |
| Aztreonam after FMT | 6/39 (15.4) | 15.27 (1.77–131.77) | 0.013 | 8.28 (0.9–82.70) | 0.06 |
| CDI antibiotics (oral vancomycin) during FMT | 13/39 (33.3) | 1.63 (0.71–3.74) | 0.253 | ||
| Male | 18/39 (46.2) | 0.92 (0.43–1.97) | 0.829 | ||
| Age-related CCI | 1.05 (0.91–1.21) | 0.549 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Lee, J.-H.; Lee, S.; Shin, J.; Cha, B.; Hong, J.-T.; Kwon, K.S. Factors for Treatment Failure After Fecal Microbiota Transplantation in Clostridioides difficile Infection. Microorganisms 2024, 12, 2539. https://doi.org/10.3390/microorganisms12122539
Park S-H, Lee J-H, Lee S, Shin J, Cha B, Hong J-T, Kwon KS. Factors for Treatment Failure After Fecal Microbiota Transplantation in Clostridioides difficile Infection. Microorganisms. 2024; 12(12):2539. https://doi.org/10.3390/microorganisms12122539
Chicago/Turabian StylePark, Soo-Hyun, Jung-Hwan Lee, Suhjoon Lee, Jongbeom Shin, Boram Cha, Ji-Taek Hong, and Kye Sook Kwon. 2024. "Factors for Treatment Failure After Fecal Microbiota Transplantation in Clostridioides difficile Infection" Microorganisms 12, no. 12: 2539. https://doi.org/10.3390/microorganisms12122539
APA StylePark, S.-H., Lee, J.-H., Lee, S., Shin, J., Cha, B., Hong, J.-T., & Kwon, K. S. (2024). Factors for Treatment Failure After Fecal Microbiota Transplantation in Clostridioides difficile Infection. Microorganisms, 12(12), 2539. https://doi.org/10.3390/microorganisms12122539





