Effect of Warming on Soil Fungal Community Along Altitude Gradients in a Subalpine Meadow
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Sample Collection
2.4. Analysis of Grass and Biochemical Properties
2.5. Soil DNA Extraction and Illumina MiSeq Sequencing
2.6. Processing of Sequencing Data
2.7. Statistical Analysis
3. Results
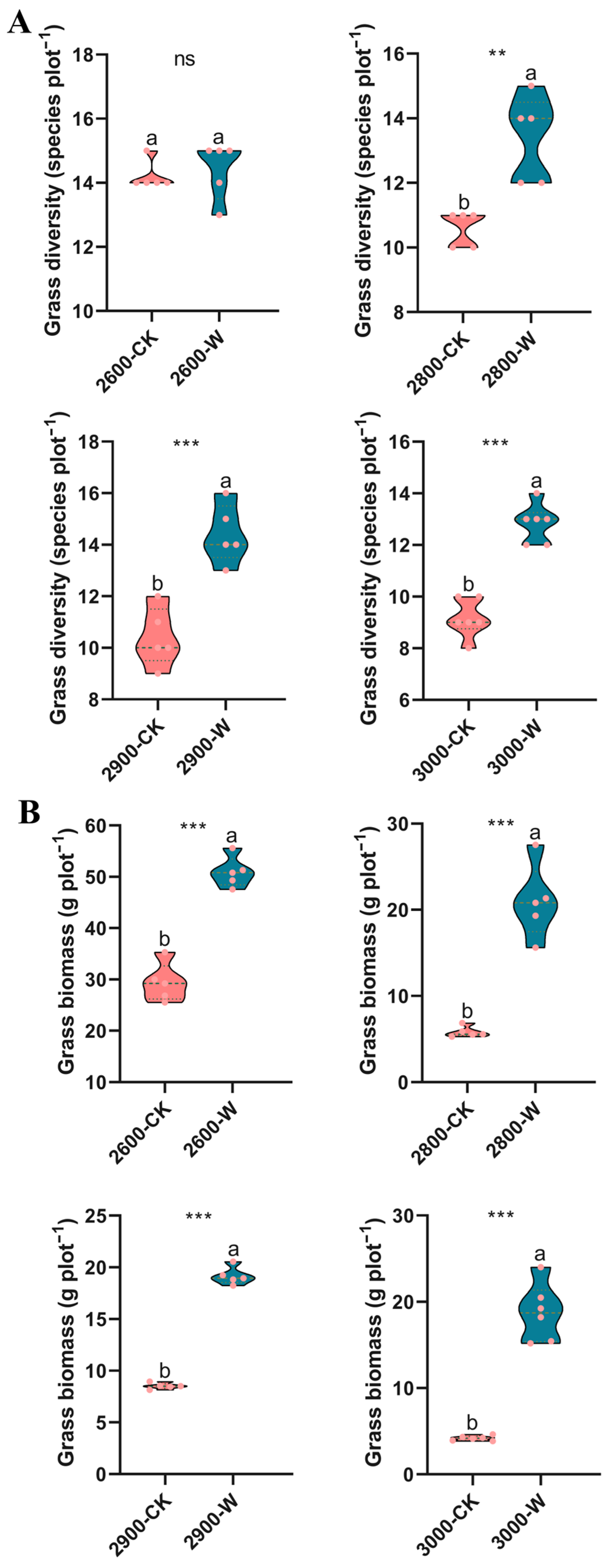
3.1. Soil and Grass Properties
3.2. Soil Fungal Alpha-Diversity
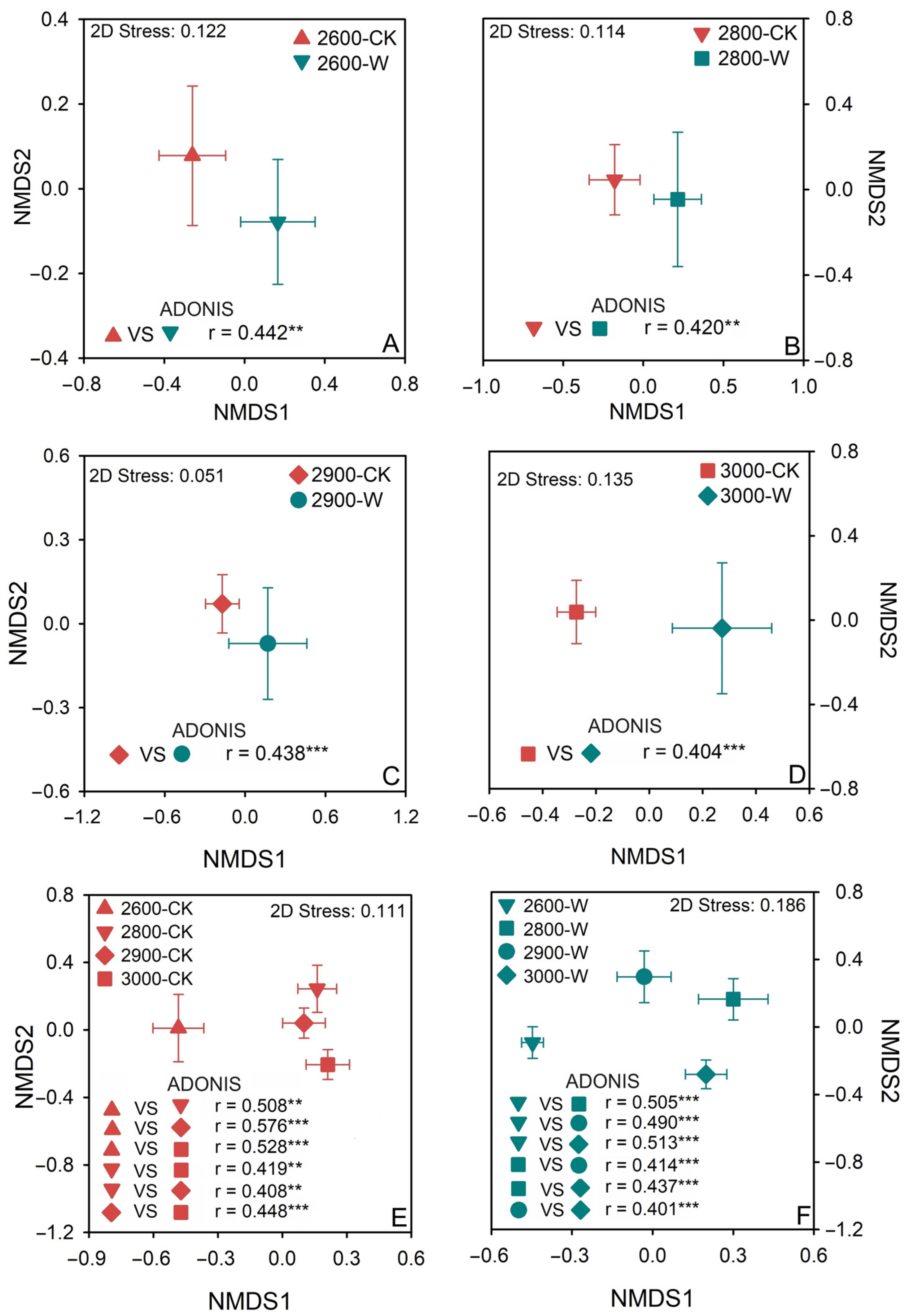
3.3. Soil Fungal Community Composition
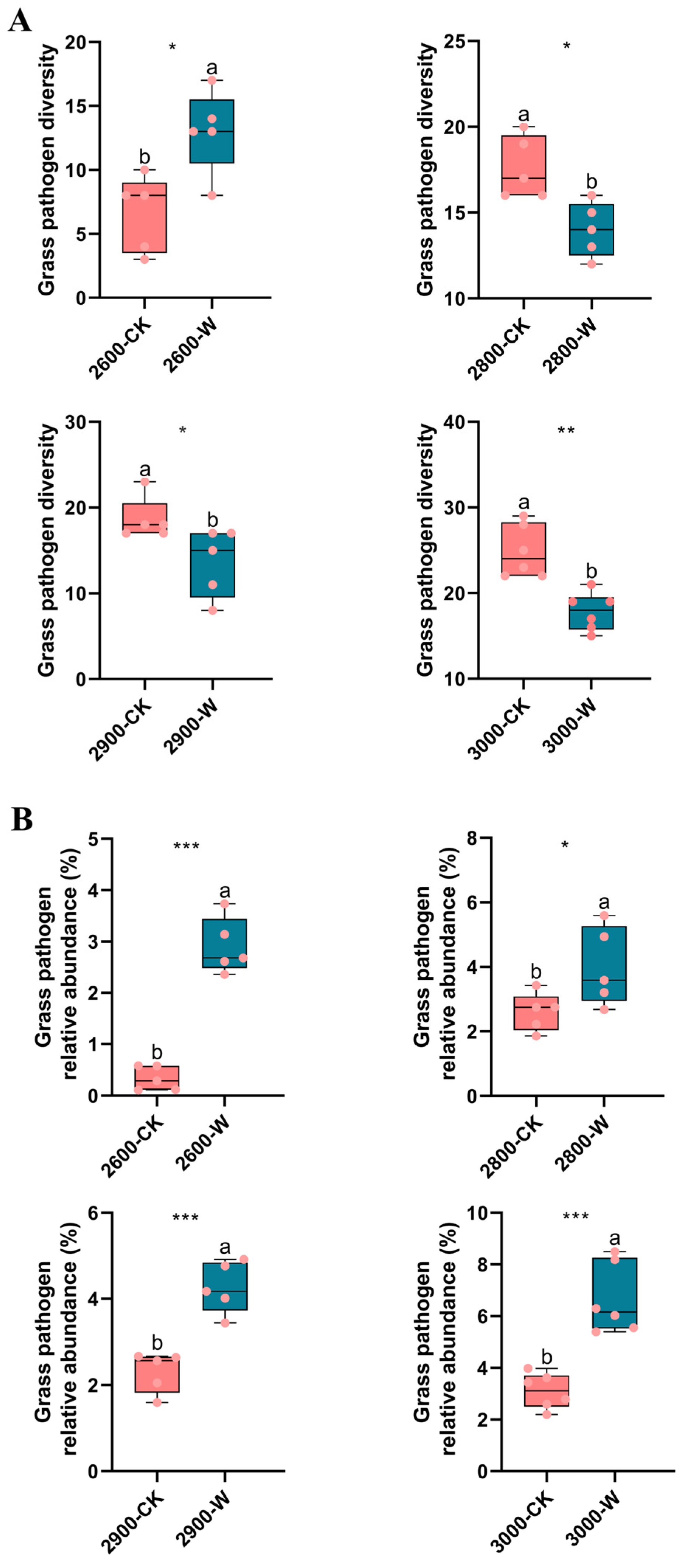
3.4. Grass Pathogens and Soil Saprotrophs
3.5. Soil Fungal Network Analysis
3.6. Soil Fungal Community Assembly Processes
4. Discussion
5. Conclusions
5.1. The Effect of Warming on Aboveground Grass and Soil Organic Carbon Content
5.2. The Effect of Warming on Belowground Fungal Community
5.3. The Effect of Warming on Soil Pathogen
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Guillén Bolaños, T.; Bindi, M.; Brown, S.; Zhou, G. The human imperative of stabilizing global climate change at 1.5 °C. Science 2019, 365, eaaw6974. [Google Scholar] [CrossRef] [PubMed]
- Armstrong McKay, D.I.; Staal, A.; Abrams, J.F.; Winkelmann, R.; Sakschewski, B.; Loriani, S.; Lenton, T.M. Exceeding 1.5 °C global warming could trigger multiple climate tipping points. Science 2022, 377, eabn7950. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Philos. Trans. Royal Soc. B Biol. Sci. 2020, 375, 20190104. [Google Scholar] [CrossRef] [PubMed]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Iglesias, A.; Xoplaki, E. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Markers for global climate change and its impact on social, biological and ecological systems: A review. Am. J. Clim. Chang. 2020, 9, 159. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Wanek, W. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Prudent, M.; Dequiedt, S.; Sorin, C.; Girodet, S.; Nowak, V.; Duc, G.; Maron, P.A. The diversity of soil microbial communities matters when legumes face drought. Plant Cell Environ. 2020, 43, 1023–1035. [Google Scholar] [CrossRef]
- French, S.; Levy-Booth, D.; Samarajeewa, A.; Shannon, K.E.; Smith, J.; Trevors, J.T. Elevated temperatures and carbon dioxide concentrations: Effects on selected microbial activities in temperate agricultural soils. World J. Microbiol. Biotechnol. 2009, 25, 1887–1900. [Google Scholar] [CrossRef]
- García, F.C.; Bestion, E.; Warfield, R.; Yvon-Durocher, G. Changes in temperature alter the relationship between biodiversity and ecosystem functioning. Proc. Natl. Acad. Sci. USA 2018, 115, 10989–10994. [Google Scholar] [CrossRef]
- Marín, C.; Godoy, R.; Valenzuela, E.; Schloter, M.; Wubet, T.; Boy, J.; Gschwendtner, S. Functional land-use change effects on soil fungal communities in Chilean temperate rainforests. J. Soil Sci. Plant Nutr. 2017, 17, 985–1002. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, R.; Yan, W.; Zhong, Y. Divergent response of soil microbes to environmental stress change under different plant communities in the Loess Plateau. Catena 2023, 230, 107240. [Google Scholar] [CrossRef]
- Lubetkin, K.C.; Westerling, A.L.R.; Kueppers, L.M. Climate and landscape drive the pace and pattern of conifer encroachment into subalpine meadows. Ecol. Appl. 2017, 27, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Jäger, H.; Peratoner, G.; Tappeiner, U.; Tasser, E. Grassland biomass balance in the European Alps: Current and future ecosystem service perspectives. Ecosyst. Serv. 2020, 45, 101163. [Google Scholar] [CrossRef]
- Dunne, J.A.; Harte, J.; Taylor, K.J. Subalpine meadow flowering phenology responses to climate change: Integrating experimental and gradient methods. Ecol. Monogr. 2003, 73, 69–86. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Zi, H.; Hu, L.; Ade, L.; Wang, G.; Lerdau, M. The effect of simulated warming on root dynamics and soil microbial community in an alpine meadow of the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2017, 116, 30–41. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Qi, X.; Zeng, Y.; Guo, X.; Zhuang, G.; Ma, A. Soil bacterial communities associated with multi-nutrient cycling under long-term warming in the alpine meadow. Front. Microbiol. 2023, 14, 1136187. [Google Scholar] [CrossRef]
- Han, F.; Yu, C.; Fu, G. Warming alters elevation distributions of soil bacterial and fungal communities in alpine grasslands. Glob. Ecol. Conserv. 2022, 39, e02306. [Google Scholar] [CrossRef]
- Bai, X.; Du, P.; Guo, S.; Zhang, P.; Lin, C.; Tang, P.; Zhang, C. Monitoring land cover change and disturbance of the mount wutai world cultural landscape heritage protected area, based on remote Sensing time—Series images from 1987 to 2018. Remote Sens. 2019, 11, 1332. [Google Scholar] [CrossRef]
- Hao, A.; Luo, Z.; Chen, X. Elevational patterns of warming effects on plant community and topsoil properties: Focus on subalpine meadows ecosystem. J. Mt. Sci. 2024, 21, 146–159. [Google Scholar] [CrossRef]
- Luo, S.Z.; Zhang, J.H.; Zhang, H.F.; Zheng, Q.R.; Gao, Y.P.; Li, M.H. Warming Stimulated Soil Respiration in a Subalpine Meadow in North China. Wuhan Univ. J. Nat. Sci. 2023, 28, 77–87. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, Y.J.; Zhu, J.T.; Zeng, H.; Chang, W.J.; Cong, N.; Chen, N. Responses of community characteristics and productivity to a warming gradient in a Kobresia pygmaea meadow of Tibetan Plateau. Acta Ecol. Sin. 2019, 39, 474–485. [Google Scholar] [CrossRef]
- Li, C.; Peng, F.; Xue, X.; You, Q.; Lai, C.; Zhang, W.; Cheng, Y. Productivity and quality of alpine grassland vary with soil water availability under experimental warming. Front. Plant Sci. 2018, 9, 398944. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yu, J.; Liang, W.; Lu, C.; Zhu, J.; Li, Q. The interactive effects of elevated ozone and wheat cultivars on soil microbial community composition and metabolic diversity. Appl. Soil Ecol. 2015, 87, 11–18. [Google Scholar] [CrossRef]
- Gerenfes, D.; Giorgis, A.; Negasa, G. Comparison of organic matter determination methods in soil by loss on ignition and potassium dichromate method. Int. J. Hortic. Food Sci. 2022, 4, 49–53. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Organic and total c, n (h, o, s) analysis. Handbook of soil analysis: Mineralogical, organic and inorganic methods. In Handbook of Soil Analysis: Mineralogical Organic and Inorganic Methods; Springer Nature: Berlin/Heidelberg, Germany, 2006; pp. 327–370. [Google Scholar] [CrossRef]
- Qaswar, M.; Ahmed, W.; Jing, H.; Hongzhu, F.; Xiaojun, S.; Xianjun, J.; Zhang, H. Soil carbon (C), nitrogen (N) and phosphorus (P) stoichiometry drives phosphorus lability in paddy soil under long-term fertilization: A fractionation and path analysis study. PLoS ONE 2019, 14, e0218195. [Google Scholar] [CrossRef] [PubMed]
- Barrow, N.J.; Shaw, T.C. Sodium bicarbonate as an extractant for soil phosphate III. Effects of the buffering capacity of a soil for phosphate. Geoderma 1976, 16, 273–283. [Google Scholar] [CrossRef]
- Vandenbruwane, J.; De Neve, S.; Qualls, R.G.; Salomez, J.; Hofman, G. Optimization of dissolved organic nitrogen (DON) measurements in aqueous samples with high inorganic nitrogen concentrations. Sci. Total Environ. 2007, 386, 103–113. [Google Scholar] [CrossRef]
- Tylova-Munzarova, E.; Lorenzen, B.; Brix, H.; Votrubova, O. The effects of NH4+ and NO3− on growth, resource allocation and nitrogen uptake kinetics of Phragmites australis and Glyceria maxima. Aquat. Bot. 2005, 81, 326–342. [Google Scholar] [CrossRef]
- Chen, B.; Du, K.; Sun, C.; Vimalanathan, A.; Liang, X.; Li, Y.; Shao, Y. Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Caporaso, J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Duchesnay, É. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Ferrer, A.; Heath, K.D.; Mosquera, S.L.; Suaréz, Y.; Dalling, J.W. Assembly of wood-inhabiting archaeal, bacterial and fungal communities along a salinity gradient: Common taxa are broadly distributed but locally abundant in preferred habitats. FEMS Microbiol. Ecol. 2022, 98, fiac040. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Awasthi, M.K.; Yang, J.; Tian, Y.; Li, H.; Cao, S.; Ravindran, B. Bacterial community dynamics and co-occurrence network patterns during different stages of biochar-driven composting. Bioresour. Technol. 2023, 384, 129358. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.; Schreiner, E.G.; Silsbee, D.G. Climate, geography, and tree establishment in subalpine meadows of the Olympic Mountains, Washington, USA. Arct. Alp. Res. 1995, 27, 217–225. [Google Scholar] [CrossRef]
- Saito, M.; Kato, T.; Tang, Y. Temperature controls ecosystem CO2 exchange of an alpine meadow on the northeastern Tibetan Plateau. Glob. Chang. Biol. 2009, 15, 221–228. [Google Scholar] [CrossRef]
- Kwaku, E.A.; Dong, S.; Shen, H.; Li, W.; Sha, W.; Su, X.; Zhao, Z. Biomass and species diversity of different alpine plant communities respond differently to nitrogen deposition and experimental warming. Plants 2021, 10, 2719. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Q.; Dong, S.; Liu, S.; Wang, X.; Su, X.; Zhao, H. Effects of grazing and climate warming on plant diversity, productivity and living state in the alpine rangelands and cultivated grasslands of the Qinghai-Tibetan Plateau. Rangel. J. 2015, 37, 57–65. [Google Scholar] [CrossRef]
- Wang, S.; Duan, J.; Xu, G.; Wang, Y.; Zhang, Z.; Rui, Y.; Wang, W. Effects of warming and grazing on soil N availability, species composition., and ANPP in an alpine meadow. Ecology 2012, 93, 2365–2376. [Google Scholar] [CrossRef]
- Wangchuk, K.; Darabant, A.; Nirola, H.; Wangdi, J.; Gratzer, G. Climate warming decreases plant diversity but increases community biomass in high-altitude grasslands. Rangel. Ecol. Manag. 2021, 75, 51–57. [Google Scholar] [CrossRef]
- Wen, J.; Qin, R.; Zhang, S.; Yang, X.; Xu, M. Effects of long-term warming on the aboveground biomass and species diversity in an alpine meadow on the Qinghai-Tibetan Plateau of China. J. Arid. Land 2020, 12, 252–266. [Google Scholar] [CrossRef]
- Qin, H.; Niu, L.; Wu, Q.; Chen, J.; Li, Y.; Liang, C.; Shen, Y. Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil 2017, 420, 407–421. [Google Scholar] [CrossRef]
- Huang, Q.; Jiao, F.; Huang, Y.; Li, N.; Wang, B.; Gao, H.; An, S. Response of soil fungal community composition and functions on the alteration of precipitation in the grassland of Loess Plateau. Sci. Total Environ. 2021, 751, 142273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xie, X.; Jiang, Y.; Li, J.; Fu, Q.; Qiu, Y.; Chen, H. Effects of simulated warming on soil microbial community diversity and composition across diverse ecosystems. Sci. Total Environ. 2024, 911, 168793. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Delgado-Baquerizo, M.; Bing, H.; Wang, Y.; Wang, J.; Chen, J.; Chang, R. Warming-induced shifts in alpine soil microbiome: An ecosystem-scale study with environmental context-dependent insights. Environ. Res. 2024, 255, 119206. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiong, J.; Hu, C.; Shi, Y.; Wang, K.; Zhang, D. Temperature sensitivity of soil bacterial community along contrasting warming gradient. Appl. Soil Ecol. 2015, 94, 40–48. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Bååth, E.; Reischke, S.; Salinas, N.; Meir, P. Adaptation of soil microbial growth to temperature: Using a tropical elevation gradient to predict future changes. Glob. Chang. Biol. 2019, 25, 827–838. [Google Scholar] [CrossRef]
- Fu, G.; Li, T.; Zha, X. Temperature sensitivity quandary of soil microbial community structure in Tibetan alpine grasslands: A meta-analysis and field experiment. Sci. Total Environ. 2024, 955, 176961. [Google Scholar] [CrossRef]
- Jiang, S.; Ling, N.; Ma, Z.; He, X.; He, J.S. Short-term warming increases root-associated fungal community dissimilarities among host plant species on the Qinghai-Tibetan Plateau. Plant Soil 2021, 466, 597–611. [Google Scholar] [CrossRef]
- D’Alò, F.; Baldrian, P.; Odriozola, I.; Morais, D.; Větrovský, T.; Zucconi, L.; Onofri, S. Composition and functioning of the soil microbiome in the highest altitudes of the Italian Alps and potential effects of climate change. FEMS Microbiol. Ecol. 2022, 98, fiac025. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Shen, J.; Xu, F.; Su, J. Effects of plant diversity and soil properties on soil fungal community structure with secondary succession in the Pinus yunnanensis forest. Geoderma 2020, 379, 114646. [Google Scholar] [CrossRef]
- Guo, Q.; Gong, L. Compared with pure forest., mixed forest alters microbial diversity and increases the complexity of interdomain networks in arid areas. Microbiol. Spectr. 2024, 12, e02642-23. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Zhuang, X.; Wu, J.; Cui, M.; Lv, D.; Liu, C.; Zhuang, G. Ascomycota members dominate fungal communities during straw residue decomposition in arable soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef] [PubMed]
- Geethanjali, P.; Jayashankar, M. A review on litter decomposition by soil fungal community. IOSR J. Pharm. Biol. Sci. 2016, 11, 1–3. [Google Scholar] [CrossRef]
- Qiao, Y.F.; Miao, X.C.; Burger, M.; Miao, S.J. Shift of fungal community composition in response to exogenous C application associated with soil properties after 10-year field experiment in black soil of China. J. Soils Sediments 2022, 22, 2281–2289. [Google Scholar] [CrossRef]
- Mäkelä, M.R.; Donofrio, N.; de Vries, R.P. Plant biomass degradation by fungi. Fungal Genet. Biol. 2014, 72, 2–9. [Google Scholar] [CrossRef]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the roots of plant-interacting fungi. Fungal Biol. Rev. 2020, 34, 100–113. [Google Scholar] [CrossRef]
- Howard, N.; Pressel, S.; Kaye, R.S.; Daniell, T.J.; Field, K.J. The potential role of Mucoromycotina ‘fine root endophytes’ in plant nitrogen nutrition. Physiol. Plant. 2022, 174, e13715. [Google Scholar] [CrossRef]
- Virtanen, R.; Luoto, M.; Rämä, T.; Mikkola, K.; Hjort, J.; Grytnes, J.A.; Birks, H.J.B. Recent vegetation changes at the high-latitude tree line ecotone are controlled by geomorphological disturbance, productivity and diversity. Glob. Ecol. Biogeogr. 2010, 19, 810–821. [Google Scholar] [CrossRef]
- Crous, P.W.; Braun, U.; McDonald, B.A.; Lennox, C.L.; Edwards, J.; Mann, R.C.; Groenewald, J.Z. Redefining genera of cereal pathogens: Oculimacula, Rhynchosporium and Spermospora. Fungal Syst. Evol. 2021, 7, 67–98. [Google Scholar] [CrossRef]
- Aiello, D.; Vitale, A.; Polizzi, G.; Voglmayr, H. Ochraceocephala foeniculi gen. et sp. nov., a new pathogen causing crown rot of fennel in Italy. MycoKeys 2020, 66, 1–30. [Google Scholar] [CrossRef]
- Bakhshi, M.; Arzanlou, M.; Zare, R.; Groenewald, J.Z.; Crous, P.W. New species of Septoria associated with leaf spot diseases in Iran. Mycologia 2019, 111, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.S.J.; Barber, P.A.; Alvarado, P.; Groenewald, J.Z. Fungal Planet description sheets: 558–624. Persoonia 2017, 38, 240. [Google Scholar] [CrossRef] [PubMed]
- Vallad, G.E.; Goodman, R.M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Hawksworth, D.L.; Hyde, K.D.; Jones, E.G.; Maharachchikumbura, S.S.; Manamgoda, D.S.; Xu, J.C. Epitypification and neotypification: Guidelines with appropriate and inappropriate examples. Fungal Divers. 2014, 69, 57–91. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Yuan, D.; Lu, J.; Li, H.; Luo, S.; Zhang, J.; Xiang, X. Effect of Warming on Soil Fungal Community Along Altitude Gradients in a Subalpine Meadow. Microorganisms 2024, 12, 2527. https://doi.org/10.3390/microorganisms12122527
Yin J, Yuan D, Lu J, Li H, Luo S, Zhang J, Xiang X. Effect of Warming on Soil Fungal Community Along Altitude Gradients in a Subalpine Meadow. Microorganisms. 2024; 12(12):2527. https://doi.org/10.3390/microorganisms12122527
Chicago/Turabian StyleYin, Jing, Dandan Yuan, Jing Lu, He Li, Shuzheng Luo, Jianhua Zhang, and Xingjia Xiang. 2024. "Effect of Warming on Soil Fungal Community Along Altitude Gradients in a Subalpine Meadow" Microorganisms 12, no. 12: 2527. https://doi.org/10.3390/microorganisms12122527
APA StyleYin, J., Yuan, D., Lu, J., Li, H., Luo, S., Zhang, J., & Xiang, X. (2024). Effect of Warming on Soil Fungal Community Along Altitude Gradients in a Subalpine Meadow. Microorganisms, 12(12), 2527. https://doi.org/10.3390/microorganisms12122527





