Susceptibility of Tambaqui (Colossoma macropomum) to Nile Tilapia-Derived Streptococcus agalactiae and Francisella orientalis
Abstract
1. Introduction
2. Materials and Methods
2.1. S. agalactiae and F. orientalis Strains
2.2. Fish and Experimental Infections
2.3. Bacterial Identification by MALDI-TOF MS
2.4. Histological Examination
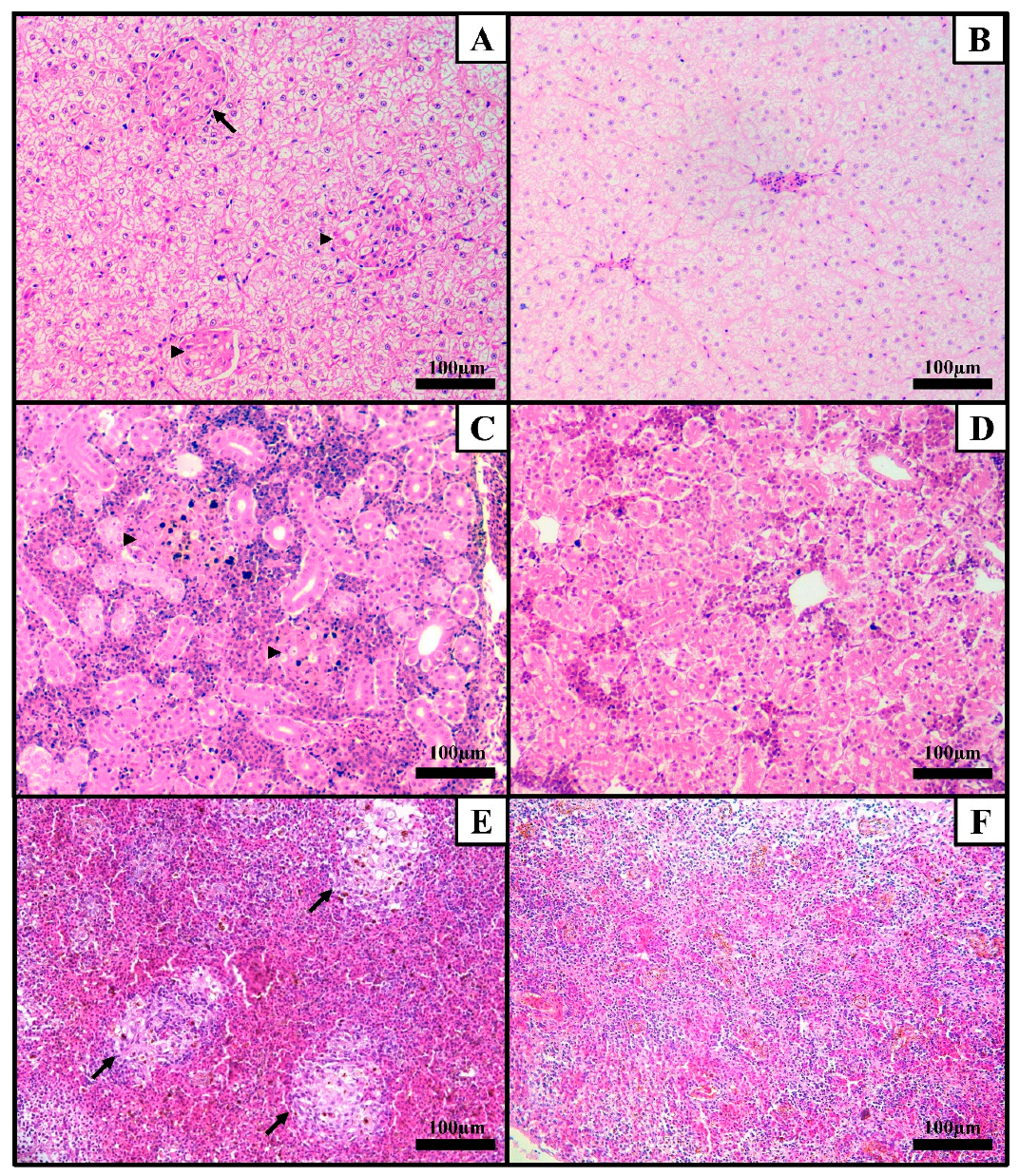
3. Results
3.1. Streptococcus agalactiae Experimental Infection
3.2. Francisella orientalis Experimental Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Val, A.L.; Oliveira, A.M. Colossoma Macropomum—A Tropical Fish Model for Biology and Aquaculture. J. Exp. Zool. A Ecol. Integr. Physiol. 2021, 335, 761–770. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Produção Da Pecuária Municipal 2023; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2024.
- Peixe, B.R. Anuário Brasileiro Da Piscicultura 2024; Associação Brasileira de Piscicultura: São Paulo, Brazil, 2024. [Google Scholar]
- EMBRAPA. Agentes Patogênicos de Tambaquis Cultivados, com Destaque para Registros em Rio Preto da Eva, AM; EMBRAPA: Manaus, Brazil, 2016; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/156602/1/Doc-127-fechado.pdf (accessed on 21 September 2024).
- Gallani, S.U.; Valladão, G.M.R.; Assane, I.M.; de Oliveira Alves, L.; Kotzent, S.; Hashimoto, D.T.; Pilarski, F. Motile Aeromonas Septicemia in Tambaqui Colossoma Macropomum: Pathogenicity, Lethality and New Insights for Control and Disinfection in Aquaculture. Microb. Pathog. 2020, 149, 104512. [Google Scholar] [CrossRef] [PubMed]
- Yunis-Aguinaga, J.; Sotil, G.; Morey, G.A.M.; Fernandez-Espinel, C.; Flores-Dominick, V.; Rengifo-Marin, G.; da Silva Claudiano, G.; Medina-Morillo, M. Susceptibility of the Cultured Amazonian Fish, Colossoma Macropomum, to Experimental Infection with Aeromonas Species from Ornamental Fish. Microb. Pathog. 2024, 186, 106461. [Google Scholar] [CrossRef] [PubMed]
- Mielke, T.D.; Francisco, C.J.; Dorella, F.A.; Figueiredo, H.C.P.; Tavares, G.C.; Gallani, S.U. The Strategic Use of Water Additives for Tambaqui Colossoma Macropomum Transport: New Insights of Bacteriosis and Productivity Approach. Aquaculture 2022, 558, 738406. [Google Scholar] [CrossRef]
- Reis, F.Y.T.; Rocha, V.P.; Janampa-Sarmiento, P.C.; Costa, H.L.; Egger, R.C.; Passos, N.C.; de Assis, C.H.S.; Carneiro, S.P.; Santos, Á.F.; Silva, B.A.; et al. Edwardsiella tarda in Tambaqui (Colossoma macropomum): A Pathogenicity, Antimicrobial Susceptibility, and Genetic Analysis of Brazilian Isolates. Animals 2023, 13, 2910. [Google Scholar] [CrossRef]
- Mian, G.F.; Godoy, D.T.; Leal, C.A.G.; Yuhara, T.Y.; Costa, G.M.; Figueiredo, H.C.P. Aspects of the Natural History and Virulence of S. Agalactiae Infection in Nile Tilapia. Vet. Microbiol. 2009, 136, 180–183. [Google Scholar] [CrossRef]
- Sebastião, F.A.; Pilarski, F.; Kearney, M.T.; Soto, E. Molecular Detection of Francisella Noatunensis Subsp. Orientalis in Cultured Nile Tilapia (Oreochromis niloticus L.) in Three Brazilian States. J. Fish. Dis. 2017, 40, 1731–1735. [Google Scholar] [CrossRef]
- Leal, C.A.G.; Silva, B.A.; Colombo, S.A. Susceptibility Profile and Epidemiological Cut-off Values Are Influenced by Serotype in Fish Pathogenic Streptococcus Agalactiae. Antibiotics 2023, 12, 1726. [Google Scholar] [CrossRef]
- Tavares-Dias, M.; Martins, M.L. An Overall Estimation of Losses Caused by Diseases in the Brazilian Fish Farms. J. Parasit. Dis. 2017, 41, 913–918. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Lu, M.; Deng, G.; Jiang, X.; Tian, Y.; Quan, Y.; Jian, Q. Identification and Molecular Typing of Streptococcus Agalactiae Isolated from Pond-Cultured Tilapia in China. Fish. Sci. 2011, 77, 623–632. [Google Scholar] [CrossRef]
- Zhang, Z. Research Advances on Tilapia Streptococcosis. Pathogens 2021, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- De Queiróz, G.A.; Silva, T.M.F.E.; Leal, C.A.G. Duration of Protection and Humoral Immune Response in Nile Tilapia (Oreochromis niloticus L.) Vaccinated against Streptococcus Agalactiae. Animals 2024, 14, 1744. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zou, Q.; Han, S.; Shi, J.; Yan, H.; Hu, D.; Yi, Y. Omics Analysis Revealed the Possible Mechanism of Streptococcus Disease Outbreak in Tilapia under High Temperature. Fish Shellfish Immunol. 2023, 134, 108639. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Ren, Y.; Wang, Y.; Pan, H.; Liang, D.; Bei, W.; Chang, O.; Wang, Q.; Shi, C. Epidemiological Characteristics of Streptococcus Agalactiae in Tilapia in China from 2006 to 2020. Aquaculture 2022, 549, 737724. [Google Scholar] [CrossRef]
- Ramirez-Paredes, J.G.; Larsson, P.; Thompson, K.D.; Penman, D.J.; Busse, H.J.; Öhrman, C.; Sjödin, A.; Soto, E.; Richards, R.H.; Adams, A.; et al. Reclassification of Francisella Noatunensis Subsp. Orientalis Ottem et al. 2009 as Francisella Orientalis Sp. Nov., Francisella Noatunensis Subsp. Chilensis Subsp. Nov. and Emended Description of Francisella Noatunensis. Int. J. Syst. Evol. Microbiol. 2020, 70, 2034–2048. [Google Scholar] [CrossRef]
- Chong, R. Francisellosis in Fish: An Emerging Challenge. Microbiol. Aust. 2016, 37, 112. [Google Scholar] [CrossRef]
- Carreon, M.M.; de Oliveira Viadanna, P.H.; Hirano, L.Q.L.; Fernandez-Alarcon, M.F.; de Castro, I.P.; Junqueira Junior, D.G.; Silva, H.O.; Costa, F.A.A.; Lima, A.M.C. Francisella Noatunensis Subsp. Orientalis Outbreak in Nile Tilapia Juveniles Cultivated in Net Cages in the Araguari River Basin, Brazil. Res. Soc. Dev. 2021, 10, e40101119332. [Google Scholar] [CrossRef]
- Barony, G.M.; Tavares, G.C.; Pereira, F.L.; Carvalho, A.F.; Dorella, F.A.; Leal, C.A.G.; Figueiredo, H.C.P. Large-Scale Genomic Analyses Reveal the Population Structure and Evolutionary Trends of Streptococcus Agalactiae Strains in Brazilian Fish Farms. Sci. Rep. 2017, 7, 13538. [Google Scholar] [CrossRef] [PubMed]
- Assis, G.B.N.; Pereira, F.L.; Zegarra, A.U.; Tavares, G.C.; Leal, C.A.; Figueiredo, H.C.P. Use of MALDI-TOF Mass Spectrometry for the Fast Identification of Gram-Positive Fish Pathogens. Front. Microbiol. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Pereira, F.L.; Tavares, G.C.; de Carvalho, A.F.; Rosa, J.C.C.; Rezende, C.P.; Leal, C.A.G.; Figueiredo, H.C.P. Effects of Temperature Changes in the Transcriptional Profile of the Emerging Fish Pathogen Francisella noatunensis Subsp. Orientalis. Microb. Pathog. 2019, 133, 103548. [Google Scholar] [CrossRef]
- Gonçalves, L.A.; de Castro Soares, S.; Pereira, F.L.; Dorella, F.A.; de Carvalho, A.F.; de Freitas Almeida, G.M.; Leal, C.A.G.; Azevedo, V.; Figueiredo, H.C.P. Complete Genome Sequences of Francisella noatunensis subsp. Orientalis Strains FNO12, FNO24 and FNO190: A Fish Pathogen with Genomic Clonal Behavior. Stand. Genomic Sci. 2016, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Marcusso, P.F.; Aguinaga, J.Y.; Claudiano, G.D.S.; Eto, S.F.; Fernandes, D.C.; Mello, H.; Marinho Neto, F.D.A.; Salvador, R.; de Moraes, J.R.E.; de Moraes, F.R. Influence of Temperature on Streptococcus Agalactiae Infection in Nile Tilapia. Braz. J. Vet. Res. Anim. Sci. 2015, 52, 57. [Google Scholar] [CrossRef]
- Soto, E.; Abrams, S.B.; Revan, F. Effects of Temperature and Salt Concentration on Francisella Noatunensis Subsp. Orientalis Infections in Nile Tilapia Oreochromis Niloticus. Dis. Aquat. Organ. 2012, 101, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bullock, G.L.; Hsu, T.C.; Schotts, E.B., Jr. Columnaris Disease of Fishes; US Fish & Wildlife Publications: Shepherdstown, WV, USA, 1986; Volume 129. [Google Scholar]
- Dell, R.B.; Holleran, S.; Ramakrishnan, R. Sample Size Determination. ILAR J. 2002, 43, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.L.; Cardoso, L.; Furtado, W.E.; Tancredo, K.R.; Lehmann, N.B.; Figueredo, A.B.; Steckert, L.D.; Addam, K.; Pádua, S.B.; Ferreira, T.H. Histopathology Guide for Freshwater Fish, 1st ed.; Federal University of Santa Catarina: Florianópolis, Brazil, 2018. [Google Scholar]
- Fournie, J.W.; Krol, R.M.; Hawkins, W.E. Fixation of Fish Tissues. In The Laboratory Fish; Elsevier: Amsterdam, The Netherlands, 2000; pp. 569–578. [Google Scholar]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; McGrow-Hill Book Company: New York, NY, USA, 1968. [Google Scholar]
- Soto, E.; Zayas, M.; Tobar, J.; Illanes, O.; Yount, S.; Francis, S.; Dennis, M.M. Laboratory-Controlled Challenges of Nile Tilapia (Oreochromis niloticus) with Streptococcus agalactiae: Comparisons between Immersion, Oral, Intracoelomic and Intramuscular Routes of Infection. J. Comp. Pathol. 2016, 155, 339–345. [Google Scholar] [CrossRef]
- Iregui, C.A.; Comas, J.; Vásquez, G.M.; Verján, N. Experimental Early Pathogenesis of Streptococcus agalactiae Infection in Red Tilapia Oreochromis spp. J. Fish Dis. 2016, 39, 205–215. [Google Scholar] [CrossRef]
- De Oliveira, T.F.; Queiroz, G.A.; Teixeira, J.P.; Figueiredo, H.C.P.; Leal, C.A.G. Recurrent Streptoccoccus agalactiae Infection in Nile Tilapia (Oreochromis niloticus) Treated with Florfenicol. Aquaculture 2018, 493, 51–60. [Google Scholar] [CrossRef]
- Da Paixão, A.E.M.; dos Santos, J.C.; Pinto, M.S.; Pereira, D.S.P.; de Oliveira Ramos, C.E.C.; Cerqueira, R.B.; Navarro, R.D.; da Silva, R.F. Effect of Commercial Probiotics (Bacillus subtilis and Saccharomyces cerevisiae) on Growth Performance, Body Composition, Hematology Parameters, and Disease Resistance against Streptococcus agalactiae in Tambaqui (Colossoma Macropomum). Aquac. Int. 2017, 25, 2035–2045. [Google Scholar] [CrossRef]
- Owatari, M.S.; Jesus, G.F.A.; Cardoso, L.; Lehmann, N.B.; Martins, M.L.; Mouriño, J.L.P. Can Histology and Haematology Explain Inapparent Streptococcus Agalactiae Infections and Asymptomatic Mortalities on Nile Tilapia Farms? Res. Vet. Sci. 2020, 129, 13–20. [Google Scholar] [CrossRef]
- Palang, I.; Withyachumnarnkul, B.; Senapin, S.; Sirimanapong, W.; Vanichviriyakit, R. Brain Histopathology in Red Tilapia Oreochromis Sp. Experimentally Infected with Streptococcus agalactiae Serotype III. Microsc. Res. Tech. 2020, 83, 877–888. [Google Scholar] [CrossRef]
- Soto, E.; Wang, R.; Wiles, J.; Green, C.; Plumb, J.; Hawke, J.; Soto, E. Characterization of Isolates of Streptococcus Agalactiae from Diseased Farmed and Wild Marine Fish from the U.S. Gulf Coast, Latin America, and Thailand. J. Aquat. Anim. Health 2015, 27, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarro, F.J.; Martínez-Morcillo, F.J.; de Oliveira, S.; Candel, S.; Cabas, I.; García-Ayala, A.; Martínez-Menchón, T.; Corbalán-Vélez, R.; Mesa-del-Castillo, P.; Cayuela, M.L.; et al. Hydrogen Peroxide in Neutrophil Inflammation: Lesson from the Zebrafish. Dev. Comp. Immunol. 2020, 105, 103583. [Google Scholar] [CrossRef] [PubMed]
- Adigun, R.; Basit, H.; Murray, J. Cell Liquefactive Necrosis; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Janda, J.M.; Abbott, S.L.; McIver, C.J. Plesiomonas Shigelloides Revisited. Clin. Microbiol. Rev. 2016, 29, 349–374. [Google Scholar] [CrossRef]
- Rather, M.A.; Willayat, M.M.; Wani, S.A.; Hussain, S.A.; Shah, S.A. Enterotoxin Gene Profile and Molecular Epidemiology of Aeromonas Species from Fish and Diverse Water Sources. J. Appl. Microbiol. 2019, 127, 921–931. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. The Nature and Consequences of Co-infections in Tilapia: A Review. J. Fish Dis. 2020, 43, 651–664. [Google Scholar] [CrossRef]
- Chen, S.L. Genomic Insights Into the Distribution and Evolution of Group B Streptococcus. Front. Microbiol. 2019, 10, 1447. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-Access Bacterial Population Genomics: BIGSdb Software, the PubMLST.Org Website and Their Applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, C.M.; Crumlish, M.; Fontaine, M.C.; Pollock, J.; Foster, G.; Dagleish, M.P.; Turnbull, J.F.; Zadoks, R.N. Human Streptococcus Agalactiae Strains in Aquatic Mammals and Fish. BMC Microbiol. 2013, 13, 41. [Google Scholar] [CrossRef]
- Kawasaki, M.; Delamare-Deboutteville, J.; Bowater, R.O.; Walker, M.J.; Beatson, S.; Ben Zakour, N.L.; Barnes, A.C. Microevolution of Streptococcus agalactiae ST-261 from Australia Indicates Dissemination via Imported Tilapia and Ongoing Adaptation to Marine Hosts or Environment. Appl. Environ. Microbiol. 2018, 84, e00859-18. [Google Scholar] [CrossRef]
- Godoy, D.T.; Carvalho-Castro, G.A.; Leal, C.A.G.; Pereira, U.P.; Leite, R.C.; Figueiredo, H.C.P. Genetic Diversity and New Genotyping Scheme for Fish Pathogenic Streptococcus Agalactiae. Lett. Appl. Microbiol. 2013, 57, 476–483. [Google Scholar] [CrossRef]
- Chideroli, R.T.; Amoroso, N.; Mainardi, R.M.; Suphoronski, S.A.; de Padua, S.B.; Alfieri, A.F.; Alfieri, A.A.; Mosela, M.; Moralez, A.T.P.; de Oliveira, A.G.; et al. Emergence of a New Multidrug-Resistant and Highly Virulent Serotype of Streptococcus Agalactiae in Fish Farms from Brazil. Aquaculture 2017, 479, 45–51. [Google Scholar] [CrossRef]
- Soto, E.; Primus, A.E.; Pouder, D.B.; George, R.H.; Gerlach, T.J.; Cassle, S.E.; Johnson, T.; Boyd, S.; Handsel, T.; Yanong, R.P.E. Identification of Francisella noatunensis in Novel Host Species French Grunt (Haemulon flavolineatum) and Caesar Grunt (Haemulon carbonarium). J. Zoo Wildl. Med. 2014, 45, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Camus, A.C.; Dill, J.A.; McDermott, A.J.; Clauss, T.M.; Berliner, A.L.; Boylan, S.M.; Soto, E. FRANCISELLA NOATUNENSIS Subsp. Orientalis Infection in Indo-Pacific Reef Fish Entering the United States through the Ornamental Fish Trade. J. Fish Dis. 2013, 36, 681–684. [Google Scholar] [CrossRef]
- López-Crespo, R.; Martínez-Chavarría, L.; Lugo-García, A.; Romero-Romero, L.; García-Márquez, L.; Reyes-Matute, A. Outbreak of Francisellosis (Francisella noatunensis Subsp. Orientalis) in Cultured Neon Jewel Cichlids Hemichromis bimaculatus from Morelos, Mexico. Dis. Aquat. Organ. 2019, 137, 125–130. [Google Scholar] [CrossRef]
- Chang, C.-H.; Poudyal, S.; Pulpipat, T.; Wang, P.-C.; Chen, S.-C. Pathological Manifestations of Francisella orientalis in the Green Texas Cichlid (Herichthys cyanoguttatus). Animals 2021, 11, 2284. [Google Scholar] [CrossRef] [PubMed]
- Pulpipat, T.; Lin, K.; Chen, Y.; Wang, P.; Chen, S. Molecular Characterization and Pathogenicity of Francisella noatunensis Subsp. Orientalis Isolated from Cultured Tilapia (Oreochromis Sp.) in Taiwan. J. Fish Dis. 2019, 42, 643–655. [Google Scholar] [CrossRef]
- Soto, E.; Griffin, M.; Wiles, J.; Hawke, J.P. Genetic Analysis and Antimicrobial Susceptibility of Francisella noatunensis Subsp. Orientalis (Syn. F. asiatica) Isolates from Fish. Vet. Microbiol. 2012, 154, 407–412. [Google Scholar] [CrossRef]
- Colquhoun, D.J.; Duodu, S. Francisella Infections in Farmed and Wild Aquatic Organisms. Vet. Res. 2011, 42, 47. [Google Scholar] [CrossRef]
- Dong, H.T.; Nguyen, V.V.; Kayansamruaj, P.; Gangnonngiw, W.; Senapin, S.; Pirarat, N.; Nilubol, D.; Rodkhum, C. Francisella noatunensis Subsp. Orientalis Infects Striped Catfish (Pangasianodon hypophthalmus) and Common Carp (Cyprinus carpio) but Does Not Kill the Hosts. Aquaculture 2016, 464, 190–195. [Google Scholar] [CrossRef]
- Xu, M.; Li, F.; Chen, B.; Deng, Y.; Chen, D.; Geng, Y.; Ouyang, P.; Huang, X. Isolation, Identification and Histopathological Observation of Francisella noatunensis Subsp. Orientalis from Nile Tilapia (Oreochromis niloticus). Aquaculture 2025, 595, 741532. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune System and Immune Responses in Fish and Their Role in Comparative Immunity Study: A Model for Higher Organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, S.; Pulpipat, T.; Wang, P.C.; Chen, S.C. Comparison of the Pathogenicity of Francisella orientalis in Nile Tilapia (Oreochromis niloticus), Asian Seabass (Lates calcarifer) and Largemouth Bass (Micropterus salmoides) through Experimental Intraperitoneal Infection. J. Fish Dis. 2020, 43, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.; Kidd, S.; Mendez, S.; Marancik, D.; Revan, F.; Hiltchie, D.; Camus, A. Francisella noatunensis Subsp. Orientalis Pathogenesis Analyzed by Experimental Immersion Challenge in Nile Tilapia, Oreochromis niloticus (L.). Vet. Microbiol. 2013, 164, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Rajme-Manzur, D.; Gollas-Galván, T.; Vargas-Albores, F.; Martínez-Porchas, M.; Hernández-Oñate, M.Á.; Hernández-López, J. Granulomatous Bacterial Diseases in Fish: An Overview of the Host’s Immune Response. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 261, 111058. [Google Scholar] [CrossRef] [PubMed]
- Volkman, H.E.; Clay, H.; Beery, D.; Chang, J.C.W.; Sherman, D.R.; Ramakrishnan, L. Tuberculous Granuloma Formation Is Enhanced by a Mycobacterium Virulence Determinant. PLoS Biol. 2004, 2, 1946–1956. [Google Scholar] [CrossRef]
- Brudal, E.; Ulanova, L.S.O.; Lampe, E.; Rishovd, A.-L.; Griffiths, G.; Winther-Larsen, H.C. Establishment of Three Francisella Infections in Zebrafish Embryos at Different Temperatures. Infect. Immun. 2014, 82, 2180–2194. [Google Scholar] [CrossRef]
- Vásquez-Machado, G.; Barato-Gómez, P.; Iregui-Castro, C. Morphological Characterization of the Adherence and Invasion of Streptococcus Agalactiae to the Intestinal Mucosa of Tilapia Oreochromis Sp.: An In Vitro Model. J. Fish Dis. 2019, 42, 1223–1231. [Google Scholar] [CrossRef]

| Group | Inoculum | Water Temperature | No. of Fish |
|---|---|---|---|
| GSA | SA95 strain (1 × 107 CFU fish−1) + BHI broth | 28 °C | 6 |
| GCSA | Sterile BHI broth | 28 °C | 6 |
| GFO | FNO12 strain (3.4 × 107 CFU fish−1) + MHB | 22 °C | 6 |
| GCFO | Sterile MHB | 22 °C | 6 |
| Bacteria | Fish | Death | Bacterial Reisolation | Histological Alterations | |||||
|---|---|---|---|---|---|---|---|---|---|
| Brain | Kidney | Spleen | Brain | Kidney | Liver | Spleen | |||
| Streptococcus agalactiae | 1 | Yes | +* | +* | NA | − | − | + | + |
| 2 | Yes | +* | +* | NA | + | − | − | + | |
| 3 | Yes | + | +* | NA | − | + | + | + | |
| 4 | Yes | + | + | NA | + | − | + | + | |
| 5 | Yes | + | + | NA | − | − | + | + | |
| 6 | No | − | − | NA | NA | NA | NA | NA | |
| Total | 5/6 | 5/6 | 5/6 | NA | 2/5 | 1/5 | 4/5 | 5/5 | |
| Francisella orientalis | 1 | No | NA | − | + | − | − | − | + |
| 2 | No | NA | + | + | − | − | + | − | |
| 3 | No | NA | − | + | − | + | + | − | |
| 4 | No | NA | + | + | − | − | + | + | |
| 5 | No | NA | + | − | − | − | + | − | |
| 6 | No | NA | − | + | − | + | + | − | |
| Total | 0/6 | NA | 3/6 | 5/6 | 0/6 | 2/6 | 5/6 | 2/6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, F.Y.T.; Rocha, V.P.; Janampa-Sarmiento, P.C.; Santos, Á.F.; Leibowitz, M.P.; Luz, R.K.; Pierezan, F.; Gallani, S.U.; Tavares, G.C.; Figueiredo, H.C.P. Susceptibility of Tambaqui (Colossoma macropomum) to Nile Tilapia-Derived Streptococcus agalactiae and Francisella orientalis. Microorganisms 2024, 12, 2440. https://doi.org/10.3390/microorganisms12122440
Reis FYT, Rocha VP, Janampa-Sarmiento PC, Santos ÁF, Leibowitz MP, Luz RK, Pierezan F, Gallani SU, Tavares GC, Figueiredo HCP. Susceptibility of Tambaqui (Colossoma macropomum) to Nile Tilapia-Derived Streptococcus agalactiae and Francisella orientalis. Microorganisms. 2024; 12(12):2440. https://doi.org/10.3390/microorganisms12122440
Chicago/Turabian StyleReis, Francisco Yan Tavares, Victória Pontes Rocha, Peter Charrie Janampa-Sarmiento, Ágna Ferreira Santos, Márcia Pimenta Leibowitz, Ronald Kennedy Luz, Felipe Pierezan, Sílvia Umeda Gallani, Guilherme Campos Tavares, and Henrique César Pereira Figueiredo. 2024. "Susceptibility of Tambaqui (Colossoma macropomum) to Nile Tilapia-Derived Streptococcus agalactiae and Francisella orientalis" Microorganisms 12, no. 12: 2440. https://doi.org/10.3390/microorganisms12122440
APA StyleReis, F. Y. T., Rocha, V. P., Janampa-Sarmiento, P. C., Santos, Á. F., Leibowitz, M. P., Luz, R. K., Pierezan, F., Gallani, S. U., Tavares, G. C., & Figueiredo, H. C. P. (2024). Susceptibility of Tambaqui (Colossoma macropomum) to Nile Tilapia-Derived Streptococcus agalactiae and Francisella orientalis. Microorganisms, 12(12), 2440. https://doi.org/10.3390/microorganisms12122440








