Claroideoglomus etunicatum and Bacillus thuringiensis Affect the Growth of the Invasive Plant Ageratina adenophora and Its Defense Against the Specialist Herbivore Procecidochares utilis
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil, Plants, and Insect Preparation
2.2. Claroideoglomus etunicatum and Bacillus thuringiensis
2.3. Experimental Design
2.4. Measurement
2.4.1. Colonization of AMF and Density of Bacillus
2.4.2. Biomass and Root Growth Characteristics
2.4.3. Nutrient Concentrations
2.4.4. Antioxidant Enzyme Activities and Defense Hormones
2.4.5. Secondary Metabolites Concentrations
2.4.6. Duration of Development of Procecidochares utilis
2.5. Statistical Analysis
3. Results
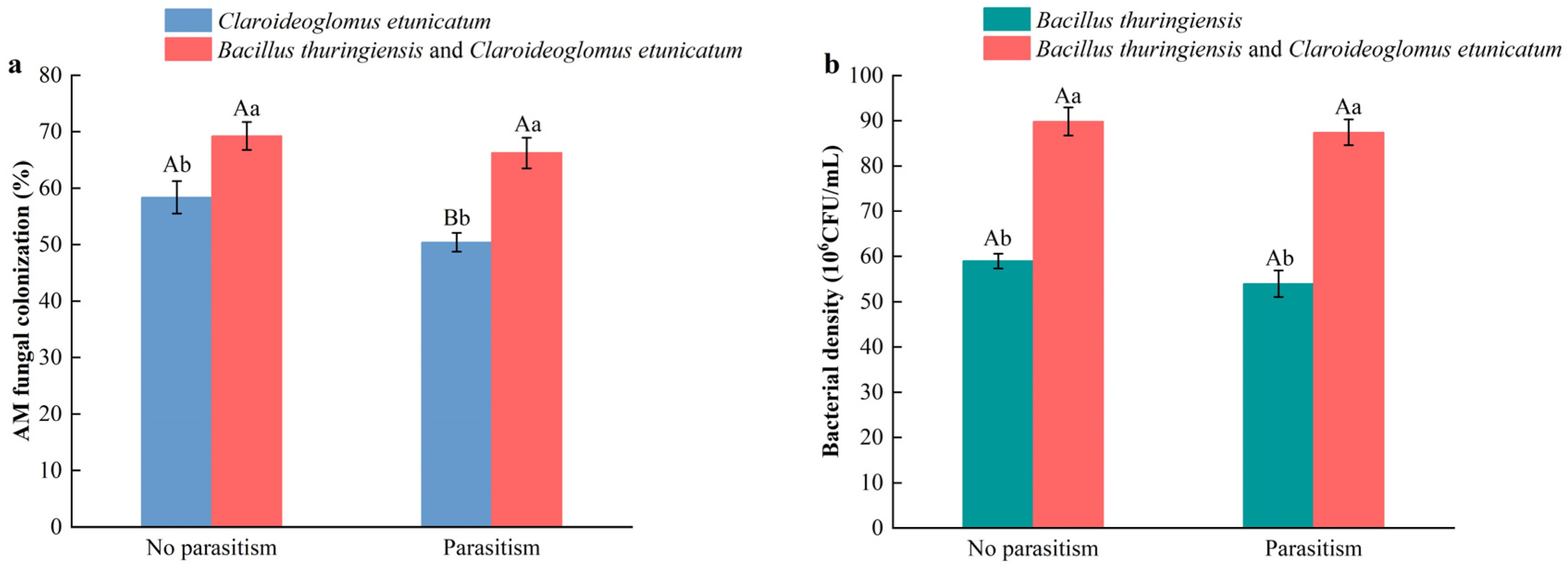
3.1. Effects of Inoculum and Parasitism by Procecidochares utilis on AMF Colonization and Bacillus Density of Ageratina adenophora
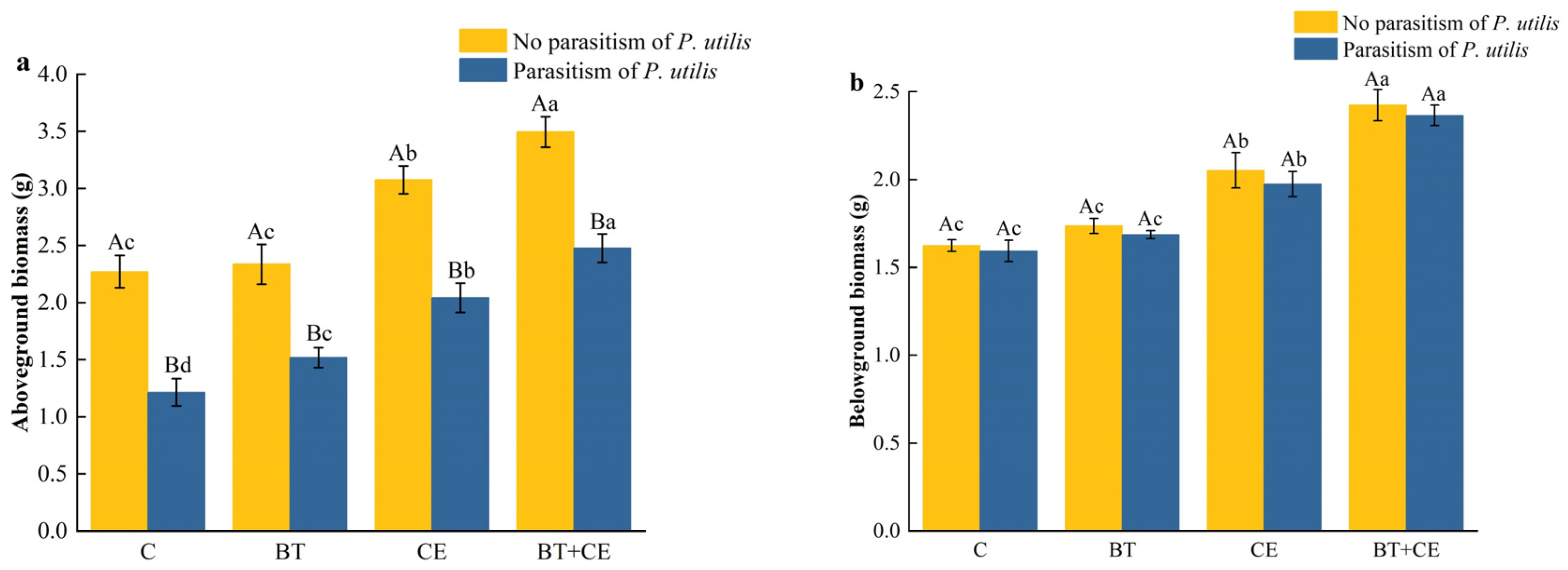
3.2. Effect of Inocula and Parasitism by Procecidochares utilis on the Growth of Ageratina adenophora
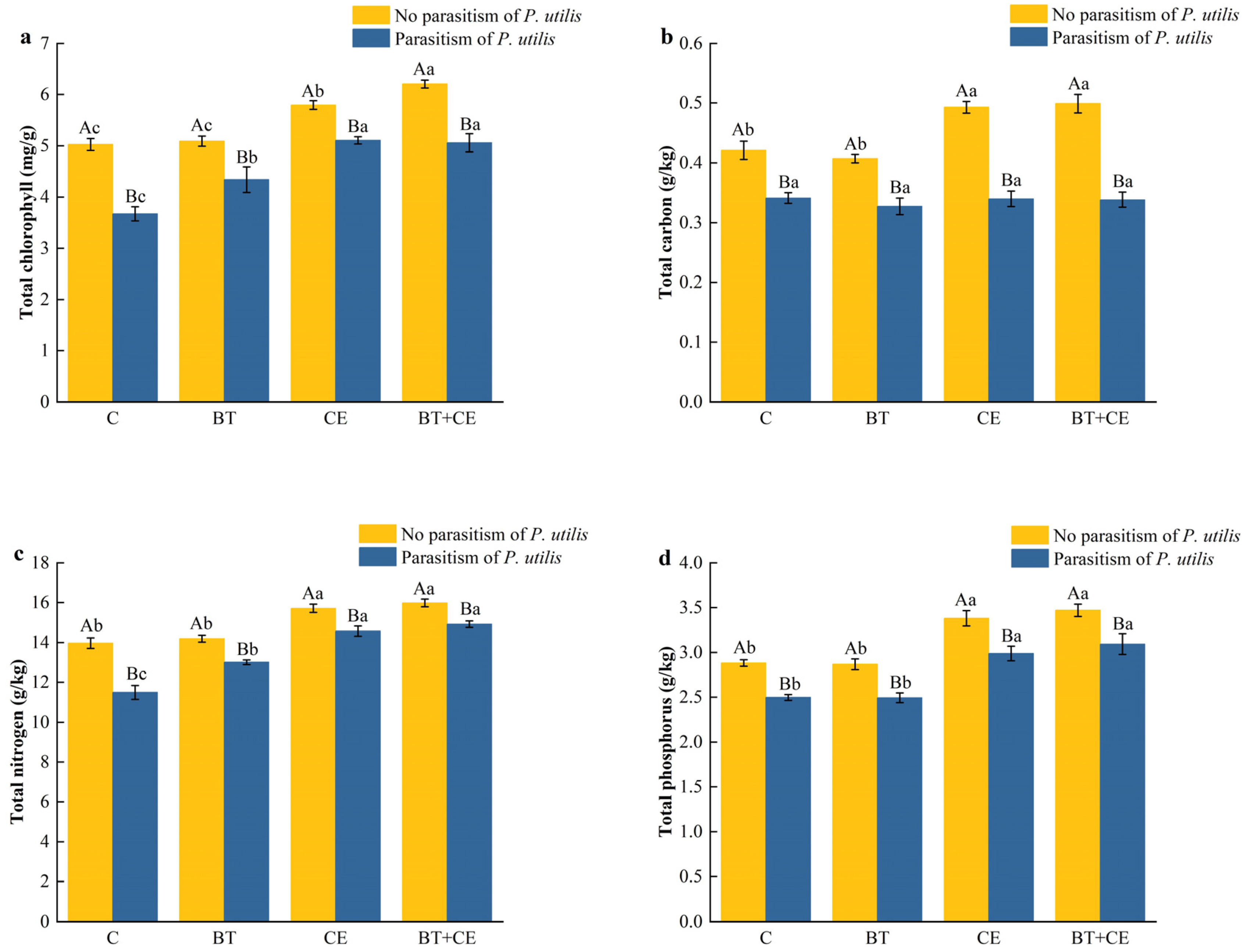
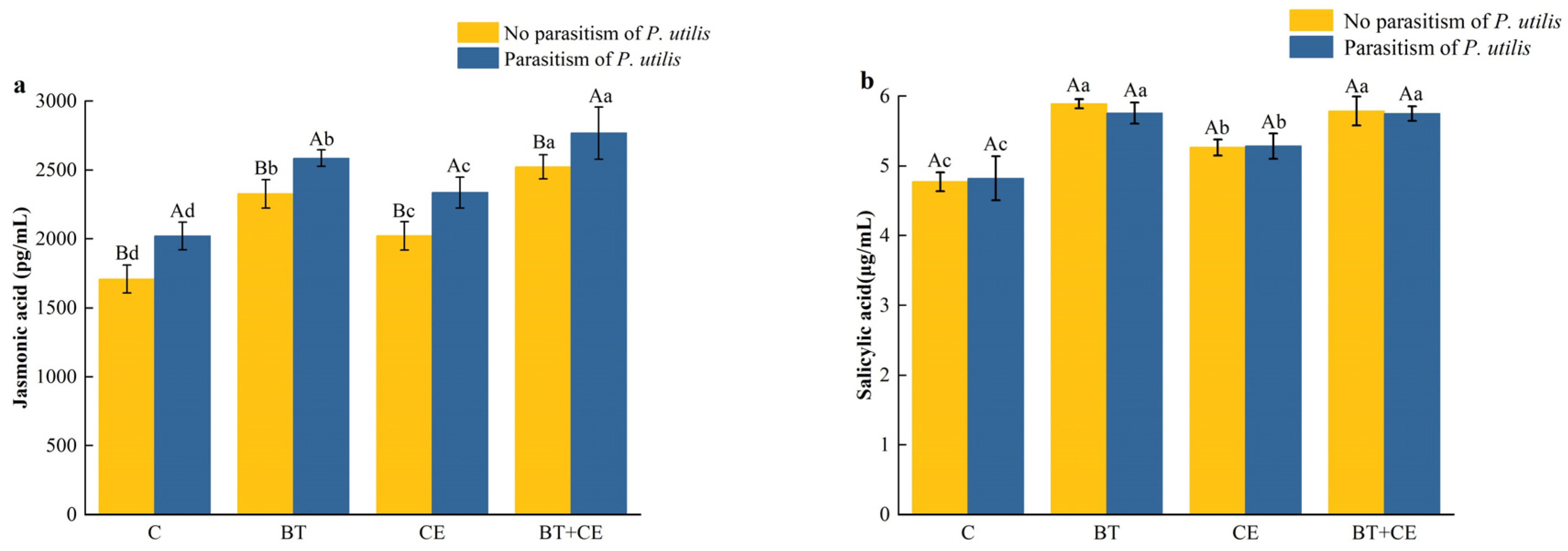
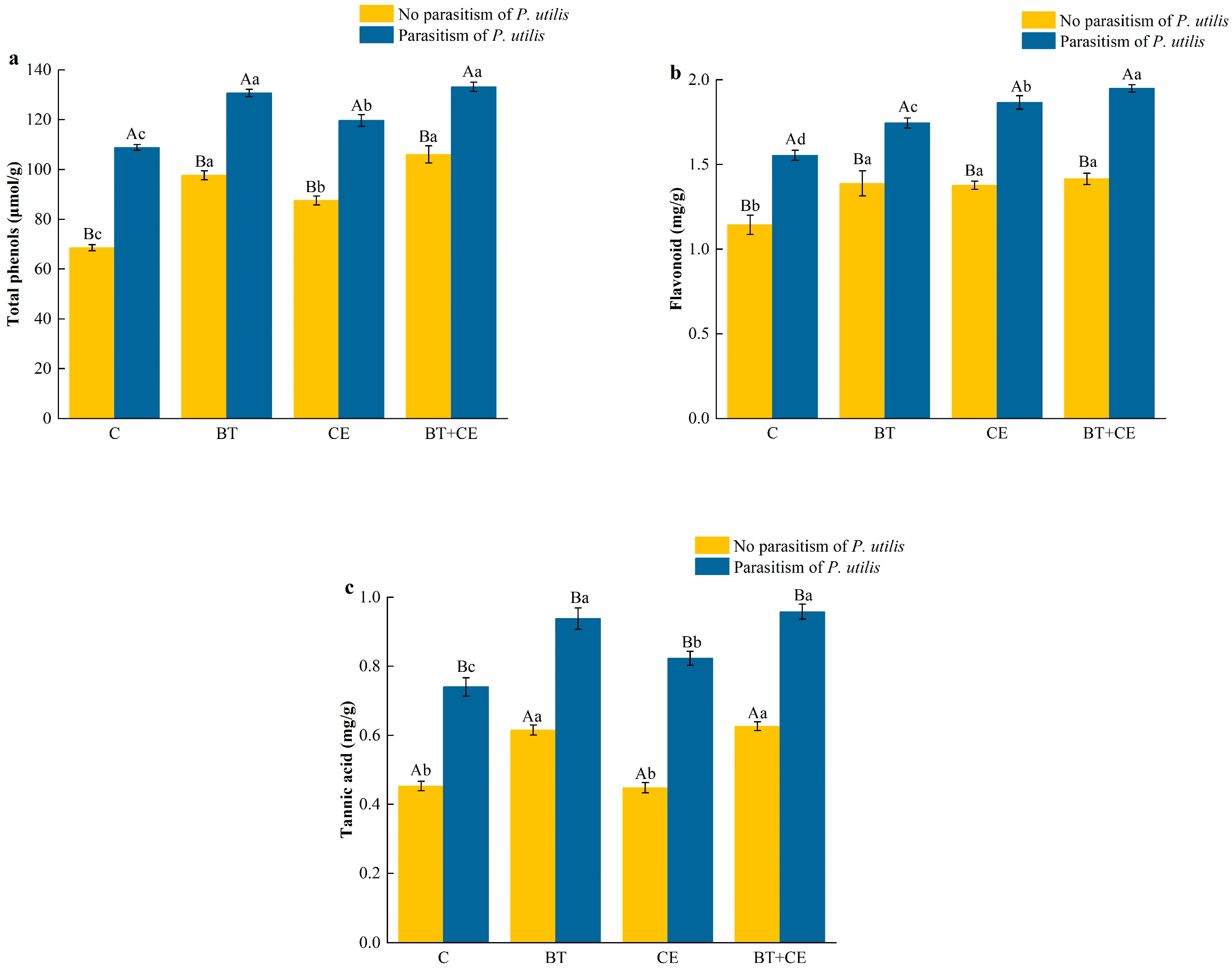
3.3. Effect of Inocula and Parasitism by Procecidochares utilis on the Defense of Ageratina adenophora
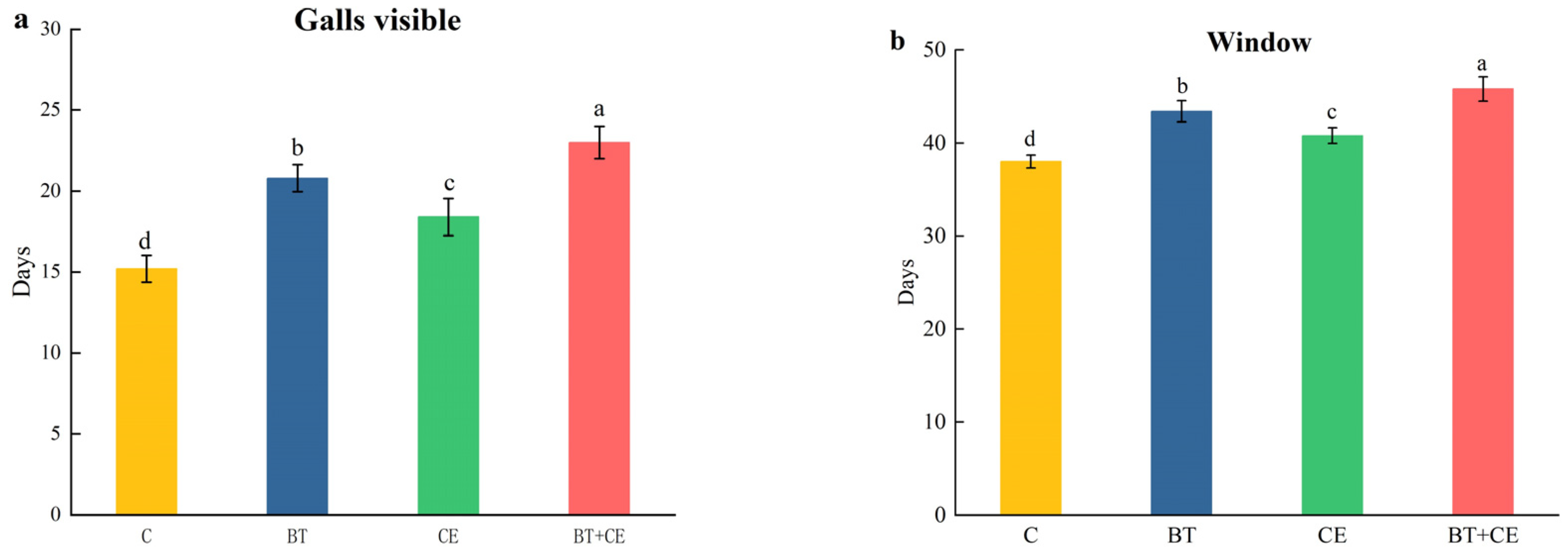
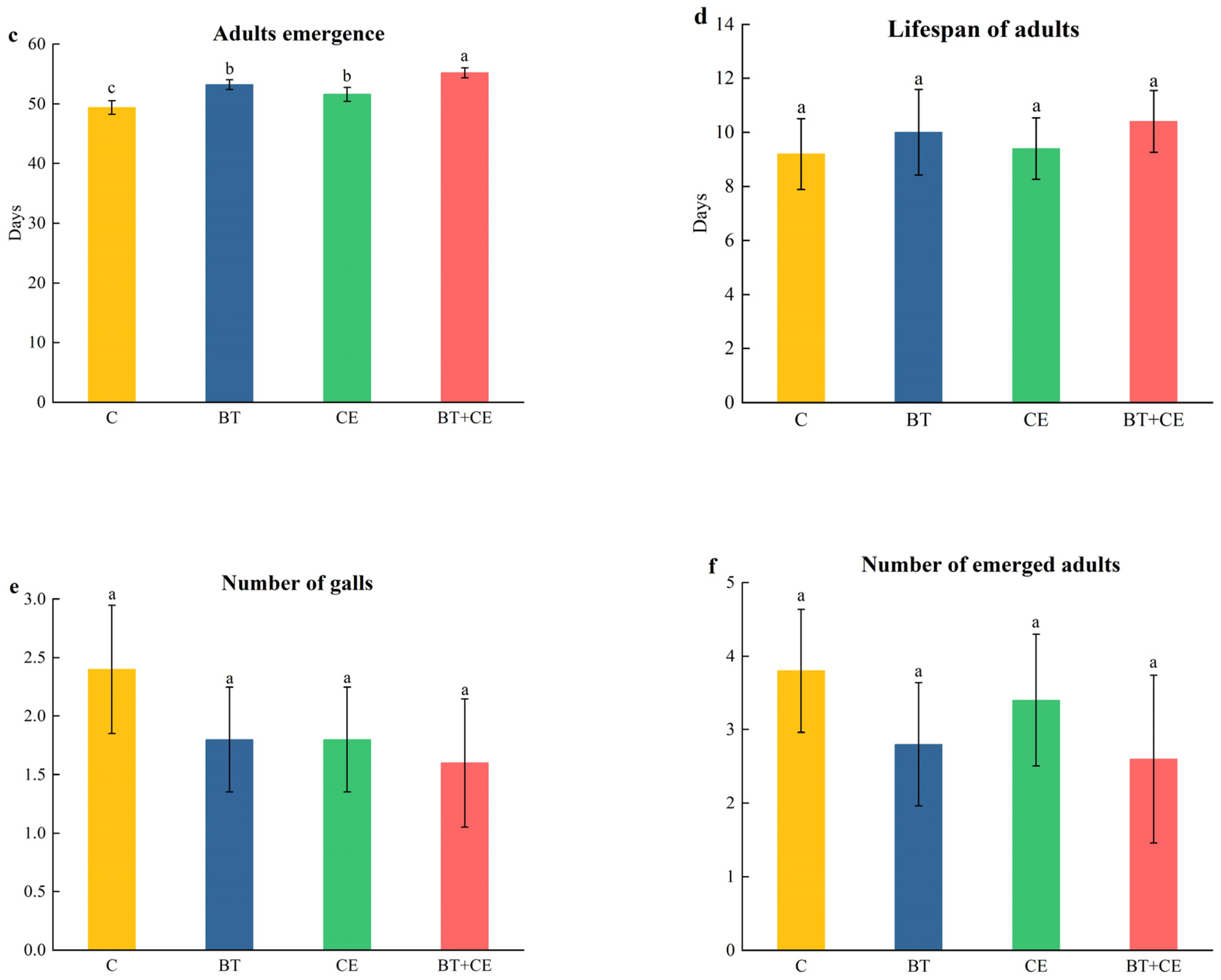
3.4. Development Time of Procecidochares utilis Reared on Ageratina adenophora with Inocula
3.5. PCAs of Patterns of Ageratina adenophora’s Response to Inoculation with Bacillus thuringiensis or/and Claroideoglomus etunicatum and Parasitism of Procecidochares utilis
3.6. Correlation of Root Colonization rate of Claroideoglomus etunicatum and the Density of Bacillus thuringiensis with Duration of Development of Procecidochares utilis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Mose, D.; Pergl, J.; Pysek, P.; Kleunen, M.; Weber, E.; Winter, M.; et al. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, A.; Stevens, C.J.; Peltzer, D.A.; Ostle, N.J.; Orwin, K.H. Belowground competition drives invasive plant impact on native species regardless of nitrogen availability. Oecologia 2018, 186, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.A.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H.; Stevens, J.R.; Cobbold, S.M. Plant-soil feedbacks: A meta-analytical review. Ecol. Lett. 2008, 11, 980–992. [Google Scholar] [CrossRef]
- Dickie, I.A.; Bufford, J.L.; Cobb, R.C.; Desprez-Loustau, M.L.; Grelet, G.; Hulme, P.E.; Klironomos, J.; Makiola, A.; Nuñez, M.A.; Pringle, A.; et al. The emerging science of linked plant-fungal invasions. New Phytol. 2017, 215, 1314–1332. [Google Scholar] [CrossRef]
- Cortois, R.; Schroeder-Georgi, T.; Weigelt, A.; van der Putten, W.H.; De Deyn, G.B.; Marcel, H. Plant-soil feedbacks: Role of plant functional group and plant traits. J. Ecol. 2016, 104, 1608–1617. [Google Scholar] [CrossRef]
- Tang, J.Q.; Guo, X.C.; Lu, X.Y.; Liu, M.C.; Zhang, H.Y.; Feng, Y.L.; Kong, D.L. A review on the effects of invasive plants on mycorrhizal fungi of native plants and their underlying mechanisms. Chin. J. Plant Ecol. 2020, 44, 1095. [Google Scholar] [CrossRef]
- Fang, K.; Wang, Y.Z.; Zhang, H.B. Differential effects of plant growth-promoting bacteria on invasive and native plants. S. Afr. J. Bot. 2019, 124, 94–101. [Google Scholar] [CrossRef]
- Zhang, F.J.; Li, Q.; Chen, F.X.; Xu, H.Y.; Inderjit Wan, F.H. Arbuscular mycorrhizal fungi facilitate growth and competitive ability of an exotic species Flaveria bidentis. Soil. Biol. Biochem. 2017, 115, 275–284. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, G.; Declerck, S. Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 2018, 12, 2339–2351. [Google Scholar] [CrossRef]
- Chen, X.; Li, Q.; Wang, Y.; Chen, F.X.; Zhang, X.Y.; Zhang, F.J. Bacillus promotes invasiveness of exotic Flaveria bidentis by increasing its nitrogen and phosphorus uptake. J. Plant Ecol. 2022, 15, 596–609. [Google Scholar] [CrossRef]
- Zhang, F.J.; Sun, J.R.; Wang, C.; Li, C.; Chen, F.; Xu, H.; Chen, X. Bacillus benefits the competitive growth of Ambrosia artemisiifolia by increasing available nutrient levels. Front. Plant Sci. 2023, 13, 1069016. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Siemann, E.; He, M.; Wei, H.; Shao, X.; Ding, J.Q. Warming benefits a native species competing with an invasive congener in the presence of a biocontrol beetle. New Phytol. 2016, 211, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.M.; Kao-Kniffin, J.; Kessler, A. Shifts in plant-microbe interactions over community succession and their effects on plant resistance to herbivores. New Phytol. 2020, 226, 1144–1157. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Brunel, C.; van Kleunen, M. Soil-microorganism-mediated invasional meltdown in plants. Nat. Ecol. Evol. 2020, 4, 1612–1621. [Google Scholar] [CrossRef]
- Lu, X.; He, M.; Ding, J.; Siemann, E. Latitudinal variation in soil biota: Testing the biotic interaction hypothesis with an invasive plant and a native congener. ISME J. 2018, 12, 2811–2822. [Google Scholar] [CrossRef]
- Wang, M.; Ruan, W.; Kostenko, O.; Carvalho, S.; Hannula, S.E.; Mulder, P.P.J.; Bu, F.; van der Putten, W.H.; Bezemer, T.M. Removal of soil biota alters soil feedback effects on plant growth and defense chemistry. New Phytol. 2019, 221, 1478–1491. [Google Scholar] [CrossRef]
- Allen, W.J.; Waller, L.P.; Barratt, B.I.P.; Dickie, I.A.; Tylianakis, J.M. Exotic plants accumulate and share herbivores yet dominate communities via rapid growth. Nat. Commun. 2021, 12, 2696. [Google Scholar] [CrossRef]
- Gao, L.L.; Wei, C.Q.; He, Y.F.; Tang, X.F.; Chen, W.; Xu, H.; Wu, Y.Q.; Wilschut, R.A.; Lu, X.M. Aboveground herbivory can promote exotic plant invasion through intra-and interspecific aboveground–belowground interactions. New Phytol. 2023, 237, 2347–2359. [Google Scholar] [CrossRef]
- Kempel, A.; Nater, P.; Fischer, M.; van Kleunen, M. Plant-microbe-herbivore interactions in invasive and non-invasive alien plant species. Funct. Ecol. 2013, 27, 498–508. [Google Scholar] [CrossRef]
- Jiao, X.; Takishita, Y.; Zhou, G.; Smith, D.L. Plant associated rhizobacteria for biocontrol and plant growth enhancement. Front. Plant Sci. 2021, 12, 634796. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Siemann, E.; Harvey, J.A.; Ding, J.Q.; Biere, A. Effects of soil biota on growth, resistance and tolerance to herbivory in Triadica sebifera plants. Geoderma 2021, 402, 115191. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Delgado-Baquerizo, M.; Travers, S.K.; Val, J.; Oliver, I.; Hamonts, K.; Singh, B.K. Competition drives the response of soil microbial diversity to increased grazing by vertebrate herbivores. Ecology 2017, 98, 1922–1931. [Google Scholar] [CrossRef]
- Frew, A.; Antunes, P.M.; Cameron, D.D.; Hartley, S.E.; Johnson, S.N.; Rillig, M.C.; Bennett, A.E. Plant herbivore protection by arbuscular mycorrhizas: A role for fungal diversity? New Phytol. 2022, 233, 1022–1031. [Google Scholar] [CrossRef]
- Cozzolino, V.; Monda, H.; Savy, D.; Di Meo, V.; Vinci, G.; Smalla, K. Cooperation among phosphate-solubilizing bacteria, humic acids and arbuscular mycorrhizal fungi induces soil microbiome shifts and enhances plant nutrient uptake. Chem. Biol. Technol. Agric. 2021, 8, 31. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate-solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Wang, L.; Pokharel, S.S.; Chen, F.J. Arbuscular mycorrhizal fungi alter the food utilization, growth, development and reproduction of armyworm (Mythimna separata) fed on Bacillus thuringiensis maize. PeerJ 2019, 7, e7679. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, H.; Zhang, W.T.; Han, B.; Zhou, H.K.; Lu, X.M.; Deng, Y.F.; Liu, K.S.; Shao, X.Q. Arbuscular mycorrhizal fungi alter the interaction effects between Bacillus and Rhizobium on root morphological traits of Medicago ruthenica L. J. Soil Sci. Plant Nutr. 2023, 23, 2868–2877. [Google Scholar] [CrossRef]
- Liu, R.; Dai, M.; Wu, X.; Li, M.; Liu, X. Suppression of the root-knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] on tomato by dual inoculation with arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria. Mycorrhiza 2012, 22, 289–296. [Google Scholar]
- Xun, F.F.; Xie, B.M.; Liu, S.S.; Guo, C.H. Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ. Sci. Pollut. R. 2015, 22, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.L.; Gao, T.T.; Zhang, D.N.; Ding, K.; Li, C.; Ma, F. Functions of arbuscular mycorrhizal fungi in horticultural crops. Sci. Hortic. 2022, 303, 111219. [Google Scholar] [CrossRef]
- Elisée, E.D.; Jacques, B.; Mohamed, H.; Colin, F. The effects of an arbuscular mycorrhizal fungus and rhizobium symbioses on soybean aphid mostly fail to propagate to the third trophic level. Microorganisms 2022, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Du, E.W.; Chen, X.; Li, Q.; Chen, F.X.; Xu, H.Y.; Zhang, F.J. Rhizoglomus intraradices and associated Brevibacterium frigoritolerans enhance the competitive growth of Flaveria bidentis. Plant Soil. 2020, 453, 281–295. [Google Scholar] [CrossRef]
- Du, E.W.; Jia, Y.N.; Wu, C.P.; Chen, X.; Zhang, F.J. Arbuscular mycorrhizal fungi and Bacillus promote Flaveria bidentis invasion success by inhibiting the growth of native species under different soil nutrient levels. Plant Soil 2024, 500, 147–160. [Google Scholar] [CrossRef]
- Datta, A.; Schweiger, O.; Kühn, I. Niche expansion of the invasive plant species Ageratina adenophora despite evolutionary constraints. J. Biogeogr. 2019, 46, 1306–1315. [Google Scholar] [CrossRef]
- Tang, S.C.; Pan, Y.M.; Wei, C.Q.; Li, X.Q.; Lü, S.H. Testing of an integrated regime for effective and sustainable control of invasive crofton weed (Ageratina adenophora) comprising the use of natural inhibitor species, activated charcoal, and fungicide. Weed Biol. Manag. 2019, 19, 9–18. [Google Scholar] [CrossRef]
- Gui, F.R.; Wan, F.H.; Guo, J.Y. Determination of the population genetic structure of the invasive weed Ageratina adenophora using ISSR-PCR markers. Russ. J. Plant Physiol. 2009, 56, 410–416. [Google Scholar] [CrossRef]
- Poudel, A.S.; Jha, P.K.; Shrestha, B.B.; Muniappan, R.; Novak, K. Biology and management of the invasive weed Ageratina adenophora (Asteraceae): Current state of knowledge and future research needs. Weed Res. 2019, 59, 79–92. [Google Scholar] [CrossRef]
- Ren, Z.H.; Okyere, S.K.; Wen, J.; Xie, L.; Cui, Y.J.; Wang, S.; Wang, J.C.; Cao, S.Z.; Shen, L.H.; Ma, X.P.; et al. An overview: The toxicity of Ageratina adenophora on animals and its possible interventions. Int. J. Mol. Sci. 2021, 22, 11581. [Google Scholar] [CrossRef]
- Gao, X.; Sun, Y.Y.; Diao, X.H.; Zhao, Y.P.; Esteban, R.; Yang, G.Q. Increased free amino acid contents of the invasive weed Ageratina adenophora and the effects on its specialist herbivore Procecidochares utilis. Weed Biol. Manag. 2020, 20, 61–68. [Google Scholar] [CrossRef]
- Buccellato, L.; Fisher, J.T.; Witkowski, E.T.; Byrne, M.J. The effects of a stem gall fly and a leaf pathogen on the reproductive output of Crofton weed, Ageratina adenophora (Asteraceae), in greenhouse and field trials. Biol. Control. 2021, 152, 104453. [Google Scholar] [CrossRef]
- Li, L.Q.; Zhang, M.S.; Liang, Z.P.; Xiao, B.; Wan, F.H.; Liu, W.X. Arbuscular mycorrhizal fungi enhance invasive plant, Ageratina adenophora growth and competition with native plants. Chin. J. Ecol. 2016, 35, 79–86. [Google Scholar]
- Du, E.W.; Chen, Y.P.; Li, Y.H.; Sun, Z.X.; Gui, F.R. Rhizospheric Bacillus-facilitated effects on the growth and competitive ability of the invasive plant Ageratina adenophora. Front. Plant Sci. 2022, 13, 882255. [Google Scholar] [CrossRef]
- Du, E.W.; Chen, Y.P.; Li, Y.; Li, Y.H.; Sun, Z.X.; Hao, R.S.; Gui, F.R. The effect of Septoglomus constrictum and Bacillus cereus on the competitive growth of Ageratina adenophora. Front. Microbiol. 2023, 14, 1131797. [Google Scholar] [CrossRef]
- Du, E.W.; Chen, Y.P.; Li, Y.H.; Zhang, F.J.; Sun, Z.X.; Hao, R.S.; Gui, F.R. Effect of arbuscular mycorrhizal fungi on the responses of Ageratina adenophora to Aphis gossypii herbivory. Front. Plant Sci. 2022, 13, 1015947. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Yang, G.Q. Ecological Adaptation Mechanism of Ageratina adenophpra and Its Specialist Herbivore Procecidochares Utilis Mediated by Bacilluscereus; Yangzhou University: Yangzhou, China, 2021. [Google Scholar]
- Daniels, B.A.; Skipper, H.D. Methods for the recovery and quantitative estimation of propagules from soil. In Methods and Principles of Mycorrhizal Research; Schenck, N.C., Ed.; American Phytopathological Society: St Paul, MN, USA, 1982; pp. 133–151. [Google Scholar]
- Liu, R.J.; Chen, Y.L. Mycorrhizology, 1st ed.; Science Press: Beijing, China, 2007. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Oser, B.L. Hawks Physiological Chemistry; Tata McGraw-Hill Publishing Company: New Delhi, India, 1971. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for quantitation of micro-gram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Determination of Total nitrogen in plant material 1. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Isaac, R.A.; Johnson, W.C. High speed analysis of agricultural samples using inductively coupled plasma-atomic emission spectroscopy. Spectrochim. Acta B 1983, 38, 277–282. [Google Scholar] [CrossRef]
- Graham, H.D. Stabilization of the Prussian blue color in the determination of polyphenols. J. Agric. Food Chem. 1992, 40, 801–805. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Pang, B.P.; Gao, J.P.; Zhou, X.R. Relationship between host plant preference of Liriomyza huidobrensis (Blanchard) (Diptera: Agromyzidae) and secondary plant compounds and trichomes of host foliage. Acta Entomol. Sin. 2006, 49, 810–815. [Google Scholar]
- Lin, Y.; He, S.Q.; Lu, Z.H.; Gao, Y.L.; Duan, Y.R.; Li, Z.Y.; Chen, B.; Gui, F.R. Influence of Aphis gossypii feeding on defense strategy of native and introduced populations of Ageratina adenophora. Arthropod-Plant Interact. 2020, 14, 345–356. [Google Scholar] [CrossRef]
- Hamada, E.A.; Ahmed, M.A.W.; Muhammad, S.S. Plant-soil feedback and plant invasion: Effect of soil conditioning on native and invasive Prosopis species using the plant functional trait approach. Front. Plant Sci. 2024, 2024, 1321950. [Google Scholar]
- Fahey, C.; Flory, S.L. Soil microbes alter competition between native and invasive plants. J. Ecol. 2022, 110, 404–414. [Google Scholar] [CrossRef]
- Xia, Y.; Dong, M.H.; Yu, L.; Kong, L.D.; Seviour, R.; Kong, Y.H. Compositional and functional profiling of the rhizosphere microbiomes of the invasive weed Ageratina adenophora and native plants. PeerJ 2021, 9, e10844. [Google Scholar] [CrossRef]
- Sardans, J.; Bartrons, M.; Margalef, O.; Gargallo-Garriga, A.; Janssens, I.A.; Ciais, P. Plant invasion is associated with higher plant-soil nutrient concentrations in nutrient-poor environments. Glob. Chang. Biol. 2016, 23, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.J.; Liao, H.X.; Chen, B.M.; Peng, S.L. Arbuscular mycorrhizal fungi are a double-edged sword in plant invasion controlled by phosphorus concentration. New Phytol. 2020, 226, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Q.; Wang, L.T.; Meng, Y.L.; Jiao, S.N.; Yin, J.L.; Xu, H.Y.; Zhang, F.J. Nitrogen uptake, not transfer of carbon and nitrogen by CMN, explains the effect of AMF on the competitive interactions between Flaveria bidentis and native species. Front. Ecol. Evol. 2021, 9, 1–11. [Google Scholar] [CrossRef]
- Bernaola, L.; Stout, M.J. The effect of mycorrhizal seed treatments on rice growth, yield, and tolerance to insect herbivores. J. Pest Sci. 2021, 94, 375–392. [Google Scholar] [CrossRef]
- Fateme, S.; Shahnaz SNoghabi Ebrahim, S.; Guy, S. Arbuscular Mycorrhizal Fungi Inducing Tomato Plant Resistance and Its Role in Control of Bemisia tabaci Under Greenhouse Conditions. Neotrop. Entomol. 2024, 53, 424–438. [Google Scholar]
- Currie, A.F.; Murray, P.J.; Gange, A.C. Is a specialist root-feeding insect affected by arbuscular mycorrhizal fungi? Appl. Soil. Ecol. 2011, 47, 77–83. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, Q.X.; Zhao, Y.P.; Diao, Y.H.; Gui, F.R.; Yang, G.Q. Beneficial rhizobacterium provides positive plant–soil feedback effects to Ageratina adenophora. J. Integr. Agric. 2021, 20, 1327–1335. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Mao, Y.; Jiang, W.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. Effects of Trichoderma asperellum 6S-2 on apple tree growth and replanted soil microbial environment. J. Fungi 2022, 8, 63. [Google Scholar] [CrossRef]
- Sun, C.F.; Li, Q.; Han, L.L.; Chen, X.; Zhang, F.J. The effects of allelochemicals from root exudates of Flaveria bidentis on two Bacillus species. Front. Plant Sci. 2022, 13, 1001208. [Google Scholar] [CrossRef]
- Cappellari, L.D.R.; Chiappero, J.; Palermo, T.B.; Giordano, W.F.; Banchio, E. Impact of Soil Rhizobacteria Inoculation and Leaf-Chewing Insect Herbivory on Mentha piperita Leaf Secondary Metabolites. J. Chem. Ecol. 2020, 46, 619–630. [Google Scholar] [CrossRef]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria activates cadinene synthase genes and induces systemic resistance in cotton against beet armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular Mycorrhizal Fungi in Conferring Tolerance to Biotic Stresses in Plants. J. Plant Growth Regul. 2022, 41, 1429–1444. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant. 2021, 172, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Gupta, A.K.; Taggar, G.K. Induced resistance by oxidative shifts in pigeonpea (Cajanus cajan L.) following Helicoverpa armigera (hübner) herbivory. Pest. Manag. Sci. 2015, 71, 770–782. [Google Scholar] [CrossRef]
- Balog, A.; Loxdale, H.D.; Bálint, J.; Benedek, K.; Szabó, K.A.; Jánosi-Rancz, K.T.; Domokos, E. The arbuscular mycorrhizal fungus Rhizophagus irregularis affects arthropod colonization on sweet pepper in both the field and greenhouse. J. Pest Sci. 2017, 90, 935–946. [Google Scholar] [CrossRef]
- Maurya, A.K.; Kelly, M.P.; Mahaney, S.M.; Gomez, S.K. Arbuscular mycorrhizal symbiosis alters plant gene expression and aphid weight in a tripartite interaction. J. Plant Interact. 2018, 13, 294–305. [Google Scholar] [CrossRef]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for plant growth promotion and stress resilience: What have we learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef]
- Jiang, D.; Tan, M.T.; Wu, S.; Zheng, L.; Wang, Q.; Wang, G.R.; Yan, S.C. Defense responses of arbuscular mycorrhizal fungus-colonized poplar seedlings against gypsy moth larvae: A multiomics study. Hortic. Res. 2021, 8, 245–258. [Google Scholar] [CrossRef]
- Valenzuela-Soto, J.H.; Estrada-Hernández, M.G.; Ibarra-Laclette, E.; Délano-Frier, J.P. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly bemisia tabaci development. Planta 2010, 231, 397. [Google Scholar] [CrossRef]
- Farhan, M.; Pan, J.; Hussain, H.; Zhao, J.; Yang, H.; Ahmad, I.; Zhang, S. Aphid-Resistant plant secondary metabolites: Types, insecticidal mechanisms, and prospects for utilization. Plants 2024, 13, 2332. [Google Scholar] [CrossRef]
- Tian, B.; Pei, Y.; Huang, W.; Ding, J.; Siemann, E. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. ISME J. 2021, 15, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Botir, K.; José David, P.R.; Tatiana, P.; Peter, S. Interrelated effects of mycorrhiza and free-living nitrogen fixers cascade up to aboveground herbivores. Ecol. Evol. 2015, 5, 3756–3768. [Google Scholar]
- Bunn, R.A.; Ramsey, P.W.; Lekberg, Y. Do nativeand invasive plants differ in their interactions with arbuscular mycorrhizal fungi? A meta-analysis. J. Ecol. 2015, 103, 1547–1556. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 191–248. [Google Scholar]
- Richard, S.A.; Marcia, T.; Doris, Z.D. Effect of co-inoculation with growth-promoting bacteria and arbuscular mycorrhizae on rrowth of Persea americana seedlings infected with Phytophthora cinnamomi. Microorganisms 2024, 12, 721. [Google Scholar]
- Elisée, E.D.; Mohamed, H.; Colin, F. Influence on soybean aphid by the tripartite interaction between soybean, a rhizobium bacterium, and an arbuscular mycorrhizal fungus. Microorganisms 2022, 10, 1196. [Google Scholar] [CrossRef]
- Yin, W.D.; Zhou, L.F.; Yang, K.W.; Fang, J.Y.; Biere, A.; Callaway, R.M.; Wu, M.K.; Yu, H.W.; Shi, Y.; Ding, J.Q. Rapid evolutionary trade-offs between resistance to herbivory and tolerance to abiotic stress in an invasive plant. Ecol. Lett. 2023, 26, 942–954. [Google Scholar] [CrossRef]

| Inoculation Treatment | Parasitism Treatment | Root Length (m) | Root Surface Area (cm2) | Root Diameter (mm) | Root Volume (cm3) |
|---|---|---|---|---|---|
| C | No parasitism | 7.97 ± 0.17 Ac | 597.29 ± 15.59 Ac | 0.42 ± 0.02 Ac | 3.02 ± 0.27 Ac |
| Parasitism | 7.92 ± 0.23 Ad | 584.38 ± 17.28 Ad | 0.41 ± 0.02 Ad | 2.93 ± 0.18 Ad | |
| BT | No parasitism | 8.11 ± 0.19 Ac | 612.69 ± 13.86 Ac | 0.42 ± 0.01 Ac | 2.91 ± 0.23 Ac |
| Parasitism | 8.41 ± 0.19 Ac | 654.69 ± 18.31 Ac | 0.48 ± 0.02 Ac | 3.72 ± 0.14 Ac | |
| CE | No parasitism | 10.33 ± 0.49 Ab | 902.67 ± 14.27 Ab | 0.78 ± 0.04 Ab | 5.01 ± 0.32 Ab |
| Parasitism | 9.85 ± 0.28 Ab | 887.74 ± 19.58 Ab | 0.75 ± 0.04 Ab | 5.03 ± 0.21 Ab | |
| BT + CE | No parasitism | 11.61 ± 0.76 Aa | 955.82 ± 26.72 Aa | 0.82 ± 0.02 Aa | 5.49 ± 0.29 Aa |
| Parasitism | 11.07 ± 0.59 Aa | 959.86 ± 25.60 Aa | 0.80 ± 0.02 Aa | 5.33 ± 0.21 Aa |
| Inoculation Treatment | Parasitism Treatment | Soluble Sugar (μg/mg) | Soluble Protein (μg/mg) | Starch (μg/mg) |
|---|---|---|---|---|
| C | No parasitism | 10.41 ± 0.24 Ac | 6.25 ± 0.17 Ac | 8.66 ± 0.11 Ac |
| Parasitism | 8.09 ± 0.23 Bc | 3.18 ± 0.09 Bc | 5.88 ± 0.31 Bc | |
| BT | No parasitism | 10.48 ± 0.46 Ac | 6.53 ± 0.26 Ac | 8.36 ± 0.36 Ac |
| Parasitism | 9.04 ± 0.14 Bb | 4.77 ± 0.14 Bb | 7.41 ± 0.09 Bb | |
| CE | No parasitism | 11.42 ± 0.12 Ab | 7.33 ± 0.24 Ab | 9.58 ± 0.13 Ab |
| Parasitism | 9.14 ± 0.14 Bb | 4.77 ± 0.09 Bb | 7.39 ± 0.13 Bb | |
| BT + CE | No parasitism | 12.39 ± 0.29 Aa | 8.81 ± 0.11 Aa | 10.13 ± 0.46 Aa |
| Parasitism | 9.74 ± 0.12 Ba | 5.18 ± 0.09 Ba | 7.84 ± 0.11 Ba |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, E.; Li, P.; Zhao, W.; Luo, R.; Chen, Y.; Lu, M.; Sun, Z.; Gui, F. Claroideoglomus etunicatum and Bacillus thuringiensis Affect the Growth of the Invasive Plant Ageratina adenophora and Its Defense Against the Specialist Herbivore Procecidochares utilis. Microorganisms 2024, 12, 2438. https://doi.org/10.3390/microorganisms12122438
Du E, Li P, Zhao W, Luo R, Chen Y, Lu M, Sun Z, Gui F. Claroideoglomus etunicatum and Bacillus thuringiensis Affect the Growth of the Invasive Plant Ageratina adenophora and Its Defense Against the Specialist Herbivore Procecidochares utilis. Microorganisms. 2024; 12(12):2438. https://doi.org/10.3390/microorganisms12122438
Chicago/Turabian StyleDu, Ewei, Pengcun Li, Wenyuan Zhao, Rongchao Luo, Yaping Chen, Minghong Lu, Zhongxiang Sun, and Furong Gui. 2024. "Claroideoglomus etunicatum and Bacillus thuringiensis Affect the Growth of the Invasive Plant Ageratina adenophora and Its Defense Against the Specialist Herbivore Procecidochares utilis" Microorganisms 12, no. 12: 2438. https://doi.org/10.3390/microorganisms12122438
APA StyleDu, E., Li, P., Zhao, W., Luo, R., Chen, Y., Lu, M., Sun, Z., & Gui, F. (2024). Claroideoglomus etunicatum and Bacillus thuringiensis Affect the Growth of the Invasive Plant Ageratina adenophora and Its Defense Against the Specialist Herbivore Procecidochares utilis. Microorganisms, 12(12), 2438. https://doi.org/10.3390/microorganisms12122438






