Abstract
Enterococcus faecalis is used as a probiotic in animal and human food supplements. Atmospheric and room temperature plasma (ARTP) systems have frequently been used to screen for effective mutant probiotics. In this study, E. faecalis was treated with ARTP, and high-yielding digestive enzyme mutant strains were obtained by measuring the activities of α-amylase, lipase, and neutral protease. A total of 833 mutant strains were obtained after 40–60 s of ARTP treatment, and after screening for digestive enzyme activity, EF-448, EF-798, and EF-804 were obtained. The three strains demonstrated an 180% increase in α-amylase activity, a 30% increase in lipase activity, and a more than 40% increase in neutral protease activity. Furthermore, the enzyme activities remained stable after nine generations. In addition, the strains exhibited high auto-aggregation capacity (over 91%) and high cell hydrophobicity (over 93%). After exposure to simulated intestinal fluid for 6 h, the survival rates of EF-448 and EF-798 were 85.71% and 82.32%, respectively. Moreover, the three mutant strains retained antioxidant capacity and DPPH free radical scavenging ability, and there was no hemolysis. A safety experiment has shown that there is no mortality of Macrobrachium rosenbergii within 14 days after receiving injections of mutant strains at different concentrations. In conclusion, this study obtained three mutant strains with high production of digestive enzymes and stable inheritance through ARTP mutagenesis of E. faecalis, providing an efficient microbial resource.
1. Introduction
In recent years, numerous studies have shown that probiotics are beneficial to humans and animals as food products or feed additives, including Lactobacillus plantarum, Bifidobacterium, Bacillus subtilis, and others [,]. For aquatic animals, probiotics as feed additives can promote growth, increase immunity, and improve the gut microbiota environment []. Enterococcus faecalis, as a dominant bacterial strain in human and animal intestines, naturally colonizes and plays an important role in maintaining the homeostasis of the intestinal environment. It has been proven to participate in the digestion, absorption, and metabolism of nutrients. Owing to its potent probiotic characteristics, E. faecalis has garnered significant interest in the feed sector []. Unlike mammals, crustaceans possess short intestines and lack robust mechanical digestive capabilities. Consequently, their digestion mostly depends on the organism’s secretion of digestive enzymes []. The application of exogenous enzymes in aquaculture can improve growth performance and boost the health of aquatic organisms []. Furthermore, the use of digestive enzyme supplements in the baseline diet of animals might improve feed consumption and promote their growth performance [,].
Atmospheric and Room Temperature Plasma (ARTP) is a whole-cell mutagenesis tool based on high-frequency atmospheric pressure luminescent discharge plasma [,]. The principle of mutagenesis of ARTP, briefly, can produce a variety of mutant strains by generating active high-energy particles capable of altering a cell’s structure and permeability, leading to DNA damage and forcing the cell to activate the save our souls (SOS) repair mechanism [,]. These active agents attack the O-H, O-P, and N-C glycosidic bonds in the structure of the DNA chain, thereby disrupting the molecular structure of the DNA and leading to single-stranded DNA breaks. Double-stranded DNA breaks occur when the breakpoints in different single-stranded regions are in close proximity with each other []. Currently, ARTP mutagenesis has been used in 24 bacterial and 14 fungus species successfully []. Studies have demonstrated that ARTP can affect the genetic material and protein structure of micro-organisms. It could increase the chitosan production capacity of Bacillus [,], the surfactant production of Bacillus subtilis [], the acid tolerance of Lactobacillus acidophilus [], the antimicrobial capacity of Brevibacillus sp. SPR19, and the tolerance and adsorption capacity of Bacillus velezensis for hexavalent chromium [,].
The effects of ARTP on the characteristics, genetic stability analysis, and safety evaluation of E. faecalis for high digestive enzyme activities in aquatic animals remain poorly understood. Therefore, in this study, ARTP was used to mutate E. faecalis to screen for strains with enhanced capabilities for producing digestive enzymes, including α-amylase, protease, and lipase. Furthermore, the safety, genetic stability, and tolerance of the mutant strains also will be investigated. This will provide a theoretical basis and stable and efficient strain resources for further in-depth research in the feed probiotics industry.
2. Materials and Methods
2.1. Strain and Culture Conditions
E. faecalis was provided by the Freshwater Fisheries Research Center of Chinese Academy of Fishery Sciences (Wuxi, China). The strain was stored in 50% glycerol at −80 °C. E. faecalis was cultured at 37 °C for 24 h in De Man, Rogosa Sharpe (MRS) (Qingdao Hi-tech Industrial Park Hope Bio-technology Co., Ltd., Qingdao, China) solid medium. A single colony was transferred to MRS broth and cultivated at 180 rpm and 37 °C for 24 h. All media were sterilized at 121 °C for 20 min [].
2.2. Growth Curve
E. faecalis was inoculated (1%, v/v) in MRS broth and cultivated at 180 rpm and 37 °C for 24 h. A total volume of 200 μL of sample was absorbed to determine the value of OD600 at 0, 3, 6, 9, 12, 15, 18, 21, and 24 h []. The curve was plotted with time on the X-axis and OD600 on the Y-axis. The end of the logarithmic growth phase was taken as the optimum age for ARTP mutagenesis. Three replicates were performed.
2.3. Mutagenic Bacteria Suspension Preparation
E. faecalis was cultured in MRS solid medium at 37 °C for 24 h. Single colonies were transferred to MRS broth and cultivated at 180 rpm and 37 °C for 18 h. After dilution to 1.0 × 107 CFU/mL with sterile saline, 1.5 mL bacteria solution was taken and centrifuged at 5000 r/min for 10 min at 4 °C, and the pellet was washed three times with sterile saline and finally suspended in saline. We added 5% glycerol for moisturizing.
2.4. Mutagenesis by ARTP
The ARTP mutagenesis experiment was conducted according to the procedures outlined by Ma et al. []. Specifically, 10 μL of the bacterial suspension was transferred onto a sterile slide and then subjected to mutagenesis using an ARTP mutagenic apparatus (Wuxi Yuanqing Tianmu Biological Technology Co., Ltd., Wuxi, China). RF power was set to 100 W, the helium gas flow rate was 10 SLM, and the mutagenesis time was set to 0, 10, 20, 30, 40, 50, 60, 80, 100, and 120 s. The treated cells were serially diluted to the requisite concentration and inoculated onto MRS agar in order to assess cell viability. Lethality rate was calculated in accordance with the following formula:
Notes: A represents the colony number of the control group, while B represents the colony number of the mutant group.
Lethality rate = (A − B)/A × 100%
2.5. Pre-Screening and Re-Screening
2.5.1. Pre-Screening
The optimal irradiation time was selected according to the growth curve. A single colony was picked for activation after mutagenesis. After strain rejuvenation, single colonies of E. faecalis and its mutant strains were transferred to MRS broth, cultivated at 180 rpm and 37 °C for 18 h, and then centrifuged at 5000 r/min for 10 min. The α-amylase activity was quantified by the kit of Nanjing Jiancheng Bioengineering Institute (Nanjing, China. Kit code: C016-1-1). α-Amylase measurement principle: α-amylase hydrolyses starch to form glucose, maltose, and dextrin. If the substrate concentration is known and in excess, iodine solution is added to bind to the unhydrolyzed starch to form a blue complex, and the amount of starch hydrolyzed can be calculated from the shade of blue to calculate amylase activity. Three replicates were performed.
2.5.2. Re-Screening
The best 10% of the higher α-amylase yields was used to prepare the supernatant of the fermentation broth according to Section 2.5.1 for lipase and neutral protease activity. Lipase activities were quantified by the commercial detection kits of Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China. Kit code: BC2345). Neutral protease activity was quantified by the commercial detection kits of Suzhou Keming Biotechnology Co., Ltd. (Suzhou, China, Kit code: NPT-2-W). Lipase measurement principle: lipase catalyzed the hydrolysis of oil esters into fatty acids. The formation rate of fatty acids was determined by the copper soap method. Neutral proteases measurement principle: neutral proteases catalyst the hydrolysis of casein to produce tyrosine. Three replicates were performed.
2.5.3. Overall Rating
The overall rating was calculated according to the formula:
Overall rating (%) = (α-amylase activity/maximum α-amylase activity)
× 100 × 0.4 + (neutral protease activity/maximum neutral protease activity) ×
100 × 0.4 + (lipase activity/maximum lipase activity) × 100 × 0.2
× 100 × 0.4 + (neutral protease activity/maximum neutral protease activity) ×
100 × 0.4 + (lipase activity/maximum lipase activity) × 100 × 0.2
2.6. The Genetic Stability Analysis of the Mutant
The genetic stability experiment was performed according to Xu et al. []. E. faecalis was cultured in MRS solid medium at 37 °C for 24 h. Single colonies were transferred to a new MRS solid medium and cultivated at 37 °C for 24 h. Nine consecutive generations were inoculated, and the activities of digestive enzymes (α-amylase, neutral protease, and lipase) were determined at generation 1, 3, 6, and 9. Experiments were performed in triplicate.
2.7. Evaluation of Probiotic Characteristics
2.7.1. Auto-Aggregation of Selected Mutant Isolates
Auto-aggregation was carried out according to Angmo et al. with little modification []. E. faecalis and the three mutant isolates screened were inoculated in MRS broth at 180 rpm and 37 °C for 18 h. The cell suspensions were centrifuged at 4000 rpm for 10 min. The washed pellets were resuspended in PBS (0.1 M, pH 7.2). The OD600 of the bacterial suspension was adjusted to 1.0 ± 0.05. The absorbance was measured at 600 nm. Experiments were performed in triplicate. Samples were monitored with different time intervals (0, 2, 4, and 24 h), and the auto-aggregation percentage was calculated as follows:
Note: At denotes the absorbance at time t h, while A0 is that at time 0 h.
Auto-aggregation (%) = (1 − At/A0) × 100
2.7.2. Cell Surface Hydrophobicity of Selected Mutant Isolates
The hydrophobicity test was performed according to the methods described by Jin et al. []. Bacterial suspensions are prepared in the same way as in Section 2.7.1. The xylene (2 mL) and bacterial suspension (2 mL) were mixed with equal volumes, vortexed thoroughly for 3 min, and let to stand at 37 °C for 40 min to stratify. The aqueous phase was then separated with a pipette and the absorbance was measured at OD600. Experiments were performed in triplicate. The hydrophobicity of xylene was calculated as follows:
Notes: A0 and A are the absorbance (at OD600) before and after the treatment.
Hydrophobicity (%) = (1 − A/A0) × 100%
2.7.3. Tolerance Analysis of Screened Strains
The intestinal juice tolerance assessment was performed according to Shi et al. with little modification []. E. faecalis and the three mutant isolates screened were inoculated in an MRS liquid medium at 180 r/min and 37 °C for 18 h. The OD600 of the bacterial suspension was adjusted to 1.0 ± 0.05 with sterile PBS. The suspension was inoculated into a test tube containing 5 mL of simulated intestinal fluid at an inoculum volume of 10%, shaken and mixed well, and then placed in a static water bath at 37 °C for 6 h. Samples were taken at 0, 2, 4, and 6 h of incubation, and the number of viable bacteria and the survival rate were determined. Three replicates per strain were prepared in parallel.
Notes: At is the number of surviving colonies after 0, 2, 4, and 6 h of incubation, and A0 is the number of surviving colonies at 0 h.
Survival rate (%) = (At/A0) × 100%
2.7.4. Determination of the Antioxidant Capacity of the Mutant Strains
An assay kit (Jiancheng Bioengineering Institute, Nanjing, China. Kit code: A015-3-1) was used to measure the level of total antioxidant capacity (T-AOC) in fermentation supernatants, following the instructions provided by the manufacturer. The measurement technique involves the reduction of Fe3+-TPTZ to create blue Fe2+-TPTZ by antioxidants under acidic circumstances. T-AOC of the sample was determined by measuring the absorbance at 593 nm.
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals of samples were determined by an assay kit (Jiancheng Bioengineering Institute, Nanjing, China. Kit code: A153-1-1). The measurement basis of this approach relies on the characteristics of the DPPH radical, which include having a single electron, exhibiting significant absorption at 517 nm, and causing a purple color in its alcoholic solution.
2.8. Safety Evaluation
2.8.1. Hemolytic Activity
The three strains were cultured at 37 °C for 18 h, and single colonies were picked and inoculated with a sterile inoculating ring on sterile Columbia blood solid medium (Qingdao Hi-tech Industrial Park Hope Bio-technology Co., Ltd., Qingdao, China) by the scribing method, which was set as the experimental group; Aeromonas hydrophila were cultured as the control group in a 28 °C incubator for 48 h, it was observed whether there was any hemolysis phenomenon around the strains, and photos were taken for the record.
2.8.2. Mutagenic Strains Safety Testing
The safety evaluation was evaluated referring to the methods described by Divisekera et al. with little modification []. A total of 210 Macrobrachium rosenbergii (3.5 ± 0.05 g) were acclimatized in the control environment for 7 days and were randomly divided into 7 groups. The experiment was performed in triplicate. All groups were injected with 100 μL of EF-448, EF-798, and EF-804 at the concentration of 1.0 × 108 and 1.0 × 1010 CFU/mL, respectively, and the control group was injected with 100 μL of sterile saline. Disease incidence and death were continuously monitored and recorded for 14 days. Autopsy was checked at the end of the experiment for signs of poisoning.
2.9. Statistics Analysis
The results are represented as the mean ± SEM. Statistical analysis of the data was performed using a two-tailed unpaired Student’s t-test with SPSS 20.0 software, and p < 0.05 was considered as a significantly difference.
3. Results
3.1. Growth Curve of E. faecalis
The growth curve of E. faecalis (Figure 1) showed that the strain grew rapidly from 0 to 18 h, and reached the decline phase at 24 h. Therefore, 18 h was chosen as the fermentation time for the seed solution.
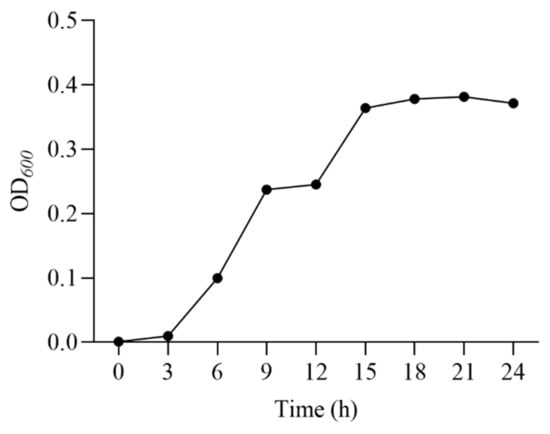
Figure 1.
Growth curve of E. faecalis.
3.2. ARTP Mutagenic Lethality Rate
The lethality of E. faecalis increased proportionally with the duration of mutagenesis (Figure 2), with the mortality of the strain reaching 53.78% after 10 s of ARTP treatment and reaching 99% when the mutagenesis time exceeded 80 s, essentially killing all cells. When the lethality was 85–95%, the strain was prone to mutation, so the optimal mutagenicity times selected in this study were 40 s, 50 s, and 60 s.
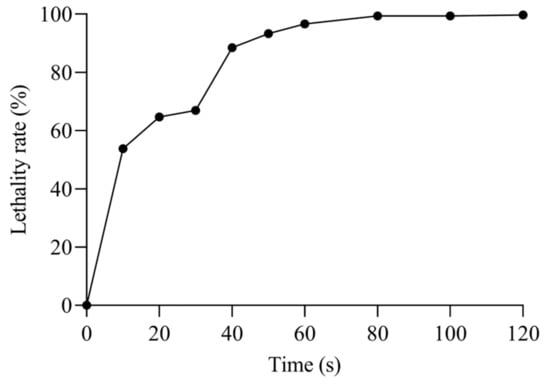
Figure 2.
Effect of ARTP treatment time on the lethality rate of E. faecalis.
3.3. Pre-Screening
A total of 833 single colonies (Figure 3), named EF-1 to EF-833, were selected. Among them, 698 strains showed a significant increase in α-amylase activity compared to the original strain (p < 0.05). Consequently, the top 10% of these strains were chosen for subsequent screening in this experiment, which amounted to 84 strains displaying an increase in α-amylase activity over 160% compared to that of the original strain.
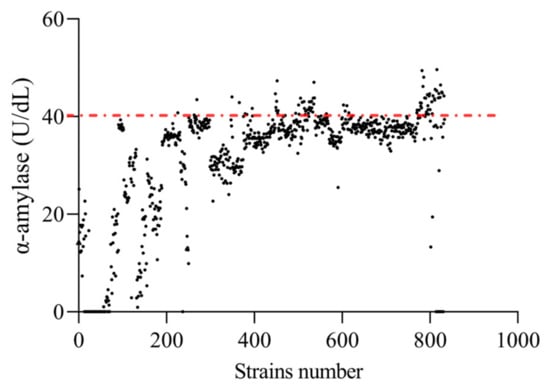
Figure 3.
α-amylase activity of mutant strains. Black dots are the α-amylase activity producing activity of each strain and the red line is the boundary for the top 10% of strains.
3.4. Re-Screening
Lipase and neutral protease activities were determined by re-screening, and the combined score was calculated by combining with α-amylase (Table 1). There were 72 mutant strains with higher digestive enzyme combined scores than the original strain, and the six strains (EF-513, EF-804, EF-494, EF-798, EF-783, and EF-448) with the higher content of digestive enzymes were selected for the genetic stability experiment compared to the original strain of E. faecalis (EF, p < 0.05).

Table 1.
Re-screening of potential probiotic enzyme production activity.
3.5. The Genetic Stability
There was a significant difference in the α-amylase activity of EF-783 and the lipase activity of EF-494, EF-513, and EF-783 from primary to ninth generation (p < 0.05, Figure 4A,B). The neutral protease activities of EF-513 and EF-783 were significantly different from primary to ninth generation (p < 0.05, Figure 4C). The activities of α-amylase, lipase, and neutral protease of EF-448, EF-798, and EF-804 were not significantly different and were consistently maintained (p > 0.05). Consequently, the strains EF-448, EF-798, and EF-804 were utilized to assess their performance.
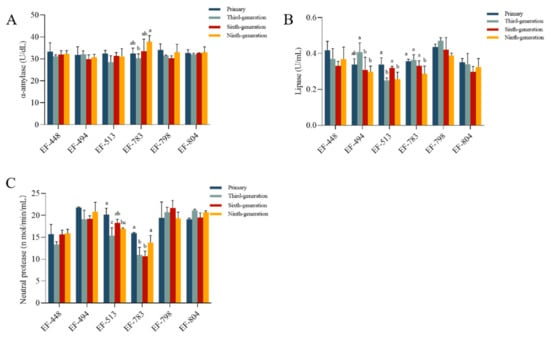
Figure 4.
(A) α-amylase, (B) lipase, and (C) neutral protease of genetic stability in the mutant strains. Bars with different letters for each mean value indicate statistically significant differences (p < 0.05).
3.6. Hydrophobicity and Auto-Aggregation Ability of Mutant Strains
3.6.1. Auto-Aggregation
Auto-aggregation rates of E. faecalis, EF-448, EF-798, and EF-804 showed similar trends at the different time points (2, 4, and 24 h), The data exhibited a progressive pattern and did not demonstrate a statistically significant distinction from that of different E. faecalis strains (p > 0.05, Table 2).

Table 2.
Auto-aggregation of different strains.
3.6.2. Hydrophobicity
Strains EF-448, EF-798, and EF-804 showed relatively high hydrophobicity ability and did not differ significantly from different E. faecalis strains (p > 0.05, Table 3).

Table 3.
Hydrophobicity of different strains.
3.7. Tolerance Analysis of Screened Strains
The survival of the strains in simulated intestinal fluid decreased with the increase in treatment duration. EF-448 and EF-798 still had high survival at 6 h, with 85.71% and 82.32%, respectively. EF-804 has relatively low survival rates, remaining at 50% at various times compared with others. It was shown that the three isolated strains were generally well tolerated by the simulated intestinal fluid (Figure 5).
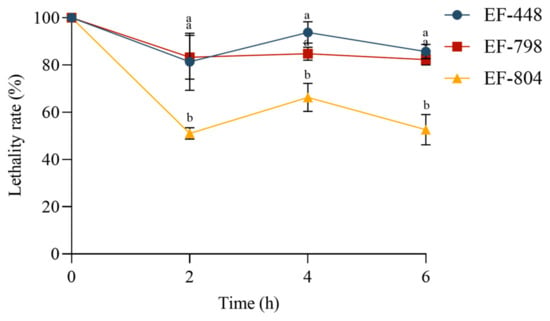
Figure 5.
Tolerance analysis of screened strains. Bars with different letters for each mean value indicate statistically significant differences (p < 0.05).
3.8. Antioxidant Capacity
There was no significant difference among T-AOC of the strains E. faecalis, EF-448, EF-798, and EF-804 in terms of the total antioxidant capacity and DPPH free radical scavenging ability (p > 0.05, Figure 6).
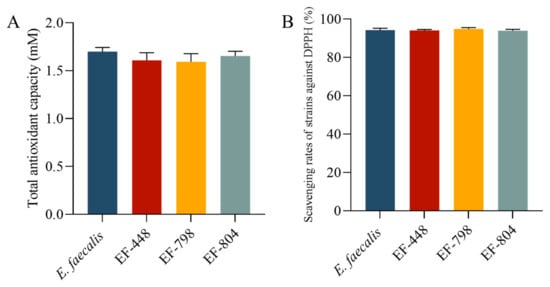
Figure 6.
(A) The antioxidant capacity and (B) scavenging rates of strains against the DPPH of antioxidant capacity of the strain.
3.9. Safety Evaluation
3.9.1. Hemolytic Activity
The results of strain hemolysis showed that none of the three strains, EF-448, EF-798, and EF-804, were hemolytic except for the positive control Aeromonas hydrophila which had β hemolysis (Figure 7).
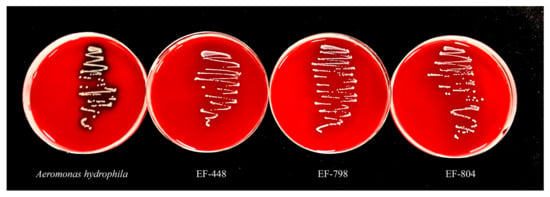
Figure 7.
Hemolytic activity.
3.9.2. Mutagenic Strains Safety Testing
The results showed that there was no mortality or intoxication in the experimental group in comparison with the control group (Table 4).

Table 4.
Mutagenic strains EF-494, EF-798 and EF-804 safety testing.
4. Discussion
The ARTP mutation breeding system has demonstrated the ease of use, high safety, and high genetic stability of mutants in the mutagenesis of various microorganisms using this new method. The effect of the plasma on the microorganisms depends on the operating conditions, such as power input, treatment distance, gas flow rate, and treatment time. The most critical of these is the treatment duration []. The lethality of E. faecalis increases with its increasing mutagenicity time due to DNA and protein damage caused by the injection of reactive ions into the cell, which generates multiple repair sites during the repair process, resulting in the creation of new mutants [,]. Mortality is directly proportional to treatment time. Therefore, determining the optimal time for mutagenesis is critical []. The mutations induced by ARTP are random, and there is no direct linear relationship between lethality and mutations. However, it was found that the strains were prone to mutations when the lethality was 85–95% [,]. Therefore, the optimal mutation times chosen in this study were 40 s, 50 s, and 60 s.
In recent years, as global fishmeal resources have become scarce and prices have continued to rise, the proportion of plant protein sources in feed has gradually increased. However, high a proportion of vegetable protein sources in feed leads to damage to the intestines and reduced digestive function and is harmful to animal health []. The addition of probiotics has been demonstrated to ameliorate intestinal barrier damage by modulating the composition of the gut microbiota []. The probiotics screened in this study as feed additives can colonize the digestive tract to increase digestive enzymes and enhance nutrient absorption. As fermentative strains, they can increase the digestibility of plant ingredients and reduce the negative effects of plant ingredients []. Carbohydrate metabolism in aquatic organisms is limited. Starch is the primary digestible polysaccharide found in plant feeds used in aquaculture and has a significant effect on the growth of these organisms. The growth process relies heavily on the presence of amylase, an enzyme that plays a vital role in starch digestion []. Consequently, enhancing the activity of starch enzymes can markedly augment feed availability by facilitating carbohydrate utilization. Protein is a vital ingredient for the growth, development, and sustenance of aquatic organisms. This feed element is the most expensive component, and its digestibility may be enhanced by augmenting protease activity, hence improving protein usage []. The fat is used to replace the protein and provide energy []. Therefore, the increase in neutral protease, lipase, and α-amylase activity in the E. faecalis contributed to the utilization of the feeding in this study.
Zhang et al. used ARTP to screen the mutant mut80 for a 90.54% and 143.10% increase in the amylase and protease activities of Bacillus licheniformis XS-4 []. The resequencing results showed that ARTP resulted in effective mutations in the amino acid metabolism genes ykvZ and alsT. Protease-related synthetic genes (aprX) were up-regulated, while the amylase gene (amyA) increased 11.26-fold. Zhao et al. screened Penicillium oxalicum for a strain with high production of starch-degrading enzymes by four rounds of EMS mutagenesis and two rounds of Co60-γ-ray mutagenesis and genetic engineering techniques as well as up-regulation of amylase-related synthetic genes (PoxGA15A, PoxAmy13A, POX_b02418, and PoxAmyR) []. ARTP mutagenesis treatment for 180 s increased the acid, neutral, and total protease activities of Aspergillus oryzae strain 3.042 by 54.7%, 17.3%, and 8.5%, respectively. However, the alkaline protease activity was reduced by 8.1% compared to the original strain []. This aligned with our observations. The three strains were screened with a 180% increase in α-amylase activity, a 30% increase in lipase, and a 40% increase in neutral protease. The reactive species in the helium RF APGD plasma jets could also cause changes in the molecular structure of lipase, leading to an increase in enzyme activity []. These results indicate that ARTP technology has emerged as a potent method for increasing enzyme synthesis.
The genetic stability of the mutant strain is crucial for industrial applications, since it indicates all potential changes at the genetic level. In the present study, the genetic stability of the mutant strain was investigated using continuous subcultures []. Even after nine rounds of fermentation, the digestive enzyme yields of EF-448, EF-798, and EF-804 remained high, indicating that the three mutant strains have good genetic stability. These findings were comparable to those of Zhang et al., who examined the genetic stability of the ARTP mutant by culturing the strain for six generations. Their results demonstrated that the chitosanase activity remained relatively constant throughout this period []. This may be due to the repair action of SOS, which is a highly error-tolerant process. Various mismatch sites are formed and stabilized during the repair process, resulting in genetic characteristics and mutants []. As a result, it is demonstrated that the ARTP mutation is a promising technique for creating extremely genetically stable mutants.
Adherence to intestinal epithelial cells is favorably correlated with probiotic auto-aggregation and hydrophobicity []. Consequently, probiotics with greater auto-aggregation and hydrophobicity have a greater ability to adhere to the cells lining the gut, thereby improving gut health [].
In this study, all strains screened were found to have high auto-aggregation capacity, and the auto-aggregation capacity after 24 h of incubation exceeded that after 2 and 4 h of incubation. which was similar to the results of Angmo et al. []. Furthermore, the auto-aggregation capacity exhibited a positive correlation with the duration of the experiment. The auto-aggregation capacity was enhanced by the rise in the aggregation-promoting component during incubation [].
Cell surface hydrophobicity is a various characteristic between different strains and is influenced by the glycoprotein molecules in the surface of microbial cells. As a result, noticeable variations in hydrophobicity can be found even among individuals of the same species []. At least 40% hydrophobicity is an essential prerequisite for probiotics []. EF-448, EF-798, and EF-804 had high cell surface hydrophobicity (90%) in this study. Three mutant isolates exhibited a ropy phenotype on MRS solid medium. After high-speed centrifugation of the liquid MRS medium, the supernatant still contained mucilaginous substances, presumed to be capsular polysaccharides (CPS). This substance is intimately associated with the cell and has a direct influence on the cell’s ability to perform auto-aggregation as well as its hydrophobicity [,]. There is a strong correlation between cell surface hydrophobicity and auto-aggregation, and hydrophobicity flanks the adhesive effect of the strain on the gut [].
Probiotics can adhere to the intestinal mucosa and mucus, produce antimicrobial substances to fight against pathogens, and interact with gut-associated lymphoid tissue (GALT) to inactivate harmful components of the intestinal contents []. In addition to adherence, the capacity to endure throughout the digestive system is also essential. The three strains tested in this study were well tolerated in simulated intestinal fluid (pH = 7.8).
Oxidative stress occurs when there is an imbalance between the formation of free radicals and the ability of cells to eliminate them, and oxidative stress is a key factor in the metabolic and physiological changes in organisms and in various diseases []. The antioxidant activity of probiotics may help to protect against oxidative damage by reducing free radicals and thus the oxidative stress response []. Glutathione is an antioxidant compound that can participate in the antioxidant mechanism of the intestinal mucosa and protect it from oxidation-induced tissue damage []. Yang et al. identified functional genes related to glutathione synthesis (gshAB) in the genome of mutant strains [].
Lou et al. demonstrated that E. faecalis exhibited a significant ability to scavenge DPPH free radicals (97%), which aligns with our findings []. This suggested that the three mutant isolates have the potential to be used as antioxidant strains in functional food and feed. Although it was found that the total antioxidant capacity and DPPH free radical scavenging ability of the strains were not altered after ARTP mutagenesis in the present study. Newly acquired probiotics need to be shown to be non-pathogenic over a period of time to determine their safety before they can be used. Jin et al. studied the hemolytic activity of 15 strains of Lactobacillus. All tested isolates were shown to be negative for a hemolytic reaction when grown in Columbia sheep blood agar []. This is consistent with our findings that all isolated strains had nonhemolytic activity. Hemolysis is usually associated with bacterial pathogenicity, but there is no universal correlation. Genetic analyses of virulence and pathogenicity are also required for a comprehensive assessment of strain pathogenicity. Future study should also focus on analysis safety at the genomic level and establishing its transcriptome, proteome, or metabolome for a fine selection of safe probiotic strains []. In this work, a probiotic was injected into M. rosenbergii, and no mortality was observed for 14 days. There were no safety concerns in either in vivo or in vitro safety studies of EF-448, EF-798, and EF-804. This has suggested that the mutagenic strains can be considered safe and suitable for use in production practices, allowing further evaluation of the potential role of the strains in vivo.
5. Conclusions
Ultimately, we utilized ARTP mutagenesis to acquire three genetically stable and exceptionally efficiency mutant strains, namely EF-448, EF-798, and EF-804. These strains exhibited a remarkable 180% increase in amylase activity, a 30% increase in lipase activity, and a 40% rise in neutral protease activity, respectively. Importantly, the mutant strain retains the superior hydrophobicity, auto-aggregation capacity, and comparative antioxidant capacity of the original strain. Furthermore, it exhibits a higher level of digestive enzyme production compared to the original strain.
Author Contributions
Data curation, M.Y., Z.L., Q.Z., X.Z., C.S. and B.L.; formal analysis, M.Y. and X.Z.; funding acquisition, B.L.; investigation, C.S. and A.W.; methodology, M.Y., Z.L., Q.Z., X.Z., C.S., B.L., A.W. and A.Z.; resources, C.S. and A.Z.; software, M.Y., Z.L., Q.Z., X.Z., B.L., A.W. and A.Z.; supervision, Q.Z. and A.W.; validation, A.Z.; writing—original draft, M.Y. and Z.L.; Writing—review and editing, C.S. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2023YFD2402000), China Agriculture Research System of MOF and MARA (CARS-48); Yancheng Fisheries’ High-Quality Development Topic (2022yc003); Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2023TD63, 2023JBFM13); Jiangsu Province Agricultural Science and Technology Independent Innovation Fund (CX(23)2008); Jiangsu Agricultural Industry Technology System (JATS [2023]470) and the “333 High Level Talent Project in Key Industry” of Jiangsu Province.
Institutional Review Board Statement
The care and use of animals followed the Animal Research Institute Committee guidelines of Freshwater Fisheries Research Center, Chinese Academy of Fishery Science, China. This study has been approved by the Committee of the Animal Research Institute of Freshwater Fisheries Research Center, Chinese Academy of Fishery Science, China (SYXK (Su) 2022-0031).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to thank the staff for their assistance during experiments at the Key Laboratory of Aquatic Animal Nutrition and Health, Freshwater Fisheries Research Center, Chinese Academy of Fishery Science; and Peng Shao (Yancheng Shangshui Environmental Biotechnology Engineering Co., Ltd.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ushakova, N.A.; Pravdin, V.G.; Kravtsova, L.Z.; Ponomarev, S.V.; Gridina, T.S.; Ponomareva, E.N.; Rudoy, D.V.; Chikindas, M.L. Complex bioactive supplements for aquaculture-evolutionary development of probiotic concepts. Probiotics Antimicrob. 2021, 13, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.X.; Li, M.; Hao, Q.; Olsen, R.E.; Ringo, E.; Yang, Y.L.; Zhang, Z.; Ran, C.; Zhou, Z.G. Effects of nuclease-treated fermentation product of Lactobacillus rhamnosus GCC-3 on growth, hepatic health and gut microbiota of zebrafish (Danio rerio) fed a high-fat diet. Aquacult. Rep. 2023, 29, 101529. [Google Scholar] [CrossRef]
- Hanifeh, M.; Spillmann, T.; Huhtinen, M.; Sclivagnotis, Y.S.; Gronthal, T.; Hynoenen, U. Ex-vivo adhesion of Enterococcus faecalis and Enterococcus faecium to the intestinal mucosa of healthy beagles. Animals 2021, 11, 3283. [Google Scholar] [CrossRef] [PubMed]
- Vogt, G. Synthesis of digestive enzymes, food processing, and nutrient absorption in decapod crustaceans: A comparison to the mammalian model of digestion. Zoology 2021, 147, 125945. [Google Scholar] [CrossRef]
- Zheng, C.C.; Wu, J.W.; Jin, Z.H.; Ye, Z.F.; Yang, S.; Sun, Y.Q.; Fei, H. Exogenous enzymes as functional additives in finfish aquaculture. Aquacult. Nutr. 2020, 26, 213–224. [Google Scholar] [CrossRef]
- Prakash, S.; Maas, R.M.; Horstmann, P.; Elbers, J.J.; Kokou, F.; Schrama, J.W.; Philip, A.J.P. Effect of dietary starch, amylase and ash on nutrient digestibility, faecal waste production and faecal characteristics of rainbow trout, (Oncorhynchus mykiss). Aquaculture 2024, 583, 740612. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.F.; Li, H.P.; Wang, L.Y.; Zhang, C.; Xing, X.H.; Bao, C.Y. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biotechnol. 2014, 98, 5387–5396. [Google Scholar] [CrossRef]
- Ottenheim, C.; Nawrath, M.; Wu, J.C. Microbial mutagenesis by atmospheric and room-temperature plasma (ARTP): The latest development. Bioresour. Bioprocess. 2018, 5, 12. [Google Scholar] [CrossRef]
- Sánchez-Osuna, M.; Cortés, P.; Lee, M.; Smith, A.T.; Barbé, J.; Erill, I. Non-canonical LexA proteins regulate the SOS response in the Bacteroidetes. Nucleic Acids Res. 2021, 49, 11050–11066. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, Y.; Sun, Y.J.; Jiang, J.C.; Tong, Y.J. Breeding of a thermostable xylanase-producing strain of Myceliophthora thermophila by atmospheric room temperature plasma (ARTP) mutagenesis. Front. Bioeng. Biotechnol. 2023, 10, 1095323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Li, Y.; Zhang, T.S.; Zhao, H. Increasing chitosanase production in Bacillus cereus by a novel mutagenesis and screen method. Bioengineered 2021, 12, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.J.; Zhang, X.; Zhai, Y.F.; Li, Y.; Shao, Z.H.; Liu, S.S.; Zhang, C.; Xing, X.H.; Zheng, H. Identification of the mutual gliding locus as a factor for gut colonization in non-native bee hosts using the ARTP mutagenesis. Microbiome 2024, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.N.; Dai, C.H.; Tang, Y.X.; Xu, X.T.; Umego, E.C.; He, R.H.; Ma, H.L. The selective breeding and mutagenesis mechanism of high-yielding surfactin Bacillus subtilis strains with atmospheric and room temperature plasma. J. Sci. Food Agric. 2022, 102, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Nyabako, B.A.; Fang, H.; Cui, F.J.; Liu, K.Y.; Tao, T.L.; Zan, X.Y.; Sun, W.J. Enhanced acid tolerance in Lactobacillus acidophilus by atmospheric and room temperature plasma (ARTP) coupled with adaptive laboratory evolution (ALE). Appl. Microbiol. Biotechnol. 2020, 191, 1499–1514. [Google Scholar] [CrossRef]
- Songnaka, N.; Nisoa, M.; Atipairin, A.; Wanganuttara, T.; Chinnawong, T. Enhanced antibacterial activity of Brevibacillus sp. SPR19 by atmospheric and room temperature plasma mutagenesis (ARTP). Sci. Pharm. 2022, 90, 23. [Google Scholar] [CrossRef]
- Bao, Z.J.; Wang, X.M.; Wang, Q.F.; Zou, L.; Peng, L.X.; Li, L.J.; Tu, W.Y.; Li, Q. A novel method of domestication combined with ARTP to improve the reduction ability of Bacillus velezensis to Cr (VI). J. Environ. 2023, 11, 109091. [Google Scholar] [CrossRef]
- Zhang, A.D.; Ma, Y.D.; Deng, Y.; Zhou, Z.W.; Cao, Y.; Yang, B.; Bai, J.; Sun, Q. Enhancing protease and amylase activities in Bacillus licheniformis XS-4 for traditional soy sauce fermentation using ARTP mutagenesis. Foods 2023, 12, 2381. [Google Scholar] [CrossRef]
- Ma, Y.F.; Yang, H.Q.; Chen, X.Z.; Sun, B.; Du, G.C.; Zhou, Z.M.; Song, J.N.; Fan, Y.; Shen, W. Significantly improving the yield of recombinant proteins in Bacillus subtilis by a novel powerful mutagenesis tool (ARTP): Alkaline α-amylase as a case study. Protein Expr. Purif. 2015, 114, 82–88. [Google Scholar] [CrossRef]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Jin, Y.M.; Luo, B.L.; Cai, J.J.; Yang, B.; Zhang, Y.; Tian, F.W.; Ni, Y.Q. Evaluation of indigenous lactic acid bacteria of raw mare milk from pastoral areas in Xinjiang, China, for potential use in probiotic fermented dairy products. J. Dairy Sci. 2021, 104, 5166–5184. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.H.; Li, X.F.; Fan, X.K.; Zeng, X.Q.; Zhang, T.; Wu, Z.; Wu, X.; Pan, D.D. The SlpX protein plays a crucial role in the intestinal juice tolerance of Lactobacillus acidophilus CICC6074. Food Biosci. 2024, 59, 103865. [Google Scholar] [CrossRef]
- Divisekera, D.; Samarasekera, J.; Hettiarachchi, C.; Maharjan, R.; Gooneratne, J.; Iqbal, C.M.; Gopalakrishnan, S.; Wahab, A.T.; Mazumdar, S.D. Oral toxicity evaluation of probiotic strains isolated from Finger millet [Eleusine coracana (L.) Gaertn.] in Wistar rat models (in vivo). Arch. Toxicol. 2021, 3, 91–102. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, X.; Jin, W.B.; Wang, F.; Tu, R.J.; Han, S.F.; Chen, H.Y.; Chen, C.; Xie, G.J.; Ma, F. Improving of lipid productivity of the oleaginous microalgae Chlorella pyrenoidosa via atmospheric and room temperature plasma (ARTP). Bioresour. Technol. 2017, 244, 1400–1406. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, J.; Wei, H.F.; Liu, Q.G.; Xu, W.H.; Bao, Y.H. Optimizing mycelial protein yield in Pleurotus djamor via ARTP mutagenesis and hybridization strategies. J. Biotechnol. 2024, 386, 64–71. [Google Scholar] [CrossRef]
- Shangguan, L.L.; Zhang, H.Y.; Liu, Z.X.; An, F.R.; Yang, Q.; Zhang, X.L.; Yao, L.; Yang, S.H.; Dai, J.; Chen, X. Improved glutamic acid production capacity of Corynebacterium glutamicum by the ARTP mutagenesis method. Fermentation 2023, 9, 599. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Z.Y.; Gui, X.; Xiang, W.; Jin, Y.B.; Chen, J.; Zhao, J. Multiomics comparative analysis of streptomyces mutants obtained by iterative atmosphere and room-temperature plasma mutagenesis. Front. Microbiol. 2021, 11, 630309. [Google Scholar] [CrossRef]
- Zhang, C.X.; Rahimnejad, S.; Wang, Y.R.; Lu, K.L.; Song, K.; Wang, L.; Mai, K.S. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture 2018, 483, 173–182. [Google Scholar] [CrossRef]
- Ding, F.F.; Zhou, N.N.; Luo, Y.; Wang, T.; Li, W.J.; Qiao, F.; Du, Z.Y.; Zhang, M.L. Probiotic Pediococcus pentosaceus restored gossypol-induced intestinal barrier injury by increasing propionate content in Nile tilapia. J. Anim. Sci. Biotechnol. 2024, 15, 54. [Google Scholar] [CrossRef]
- Doan, H.V.; Hoseinifar, S.H.; Ringo, E.; Esteban, M.A.; Dadar, M.; Dawood, M.A.O.; Faggio, C. Host-associated probiotics: A key factor in sustainable aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 16–24. [Google Scholar] [CrossRef]
- Wu, J.J.; Yu, T.; Wang, Q.J.; Zhang, C.J.; Fu, D.B.; Liu, W.; Jiang, M.; Xu, L.; Zhou, Y.; Wu, J.P. Effects of dietary microbial protease on growth performance, nutrient apparent digestibility, hepatic antioxidant capacity, protease activities and intestinal microflora in juvenile genetically improved farmed tilapia, Oreochromis niloticus. Aquacult. Rep. 2024, 34, 101894. [Google Scholar] [CrossRef]
- Mock, T.S.; Francis, D.S.; Jago, M.K.; Glencross, B.D.; Smullen, R.P.; Keast, R.S.; Turchini, G.M. The impact of dietary protein: Lipid ratio on growth performance, fatty acid metabolism, product quality and waste output in atlantic salmon (Salmo salar). Aquaculture 2019, 501, 191–201. [Google Scholar] [CrossRef]
- Zhao, S.; Tan, M.Z.; Wang, R.X.; Ye, F.T.; Chen, Y.P.; Luo, X.M.; Feng, J.X. Combination of genetic engineering and random mutagenesis for improving production of raw-starch-degrading enzymes in Penicillium oxalicum. Microb. Cell Fact. 2022, 21, 272. [Google Scholar] [CrossRef]
- Shu, L.; Si, X.G.; Yang, X.D.; Ma, W.Y.; Sun, J.L.; Zhang, J.; Xue, X.L.; Wang, D.P.; Gao, Q. Enhancement of Acid Protease Activity of Aspergillus oryzae Using Atmospheric and Room Temperature Plasma. Front. Microbiol. 2020, 11, 1418. [Google Scholar] [CrossRef]
- Li, H.P.; Wang, L.Y.; Li, G.; Jin, L.H.; Le, P.S.; Zhao, H.X.; Xing, X.H.; Bao, C.Y. Manipulation of lipase activity by the helium radio-frequency, atmospheric-pressure glow discharge plasma jet. Plasma Process. Polym. 2011, 8, 224–229. [Google Scholar] [CrossRef]
- Jiang, Y.; Shang, Y.-P.; Li, H.; Zhang, C.; Pan, J.; Bai, Y.-P.; Li, C.-X.; Xu, J.-H. Enhancing transglutaminase production of Streptomyces mobaraensis by iterative mutagenesis breeding with atmospheric and room-temperature plasma (ARTP). Bioresour. Bioprocess. 2017, 4, 37. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Zhou, Q.Q.; Zhang, X.F.; Wang, L.Y.; Chang, H.B.; Li, H.P.; Oda, Y.; Xing, X.H. Quantitative evaluation of DNA damage and mutation rate by atmospheric and room-temperature plasma (ARTP) and conventional mutagenesis. Appl. Microbiol. Biotechnol. 2015, 99, 5639–5646. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Ul-Alam, A.; Jahid, I.K. Characterization and evaluation lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef]
- Goh, Y.J.; Klaenhammer, T.R. Functional roles of aggregation-promoting-like factor in stress tolerance and adherence of Lactobacillus acidophilus NCFM. Appl. Environ. Microb. 2010, 76, 5005–5012. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Sadeghi, A.; Rahimi, D.; Purabdolah, H.; Shahryari, S. Postbiotic and anti-aflatoxigenic capabilities of Lactobacillus kunkeei as the potential probiotic LAB isolated from the natural honey. Probiotics Antimicrob. Proteins 2021, 13, 343–355. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Siddiq, M.; Zhao, H.B.; Zhu, J.; Yan, L.; Shao, D.Y.; Xu, X.G.; Shi, J.L. Identification, characterization, and probiotic potential of Lactobacillus rhamnosus isolated from human milk. LWT-Food Sci. Technol. 2017, 84, 271–280. [Google Scholar] [CrossRef]
- Yang, J.F.; Sun, Y.; Lei, X.Y.; Zhao, L.X.; Luo, R.; Liu, W.J. Evaluation of novel isolates of Lacticaseibacillus rhamnosus Probio-M9 derived through space mutagenesis. Food Biosci. 2023, 52, 102456. [Google Scholar] [CrossRef]
- Xiao, L.Y.; Yang, Y.; Han, S.; Rui, X.; Ma, K.; Zhang, C.L.; Wang, G.X.; Li, W. Effects of genes required for exopolysaccharides biosynthesis in Lacticaseibacillus paracasei S-NB on cell surface characteristics and probiotic properties. Int. J. Biol. Macromol. 2023, 224, 292–305. [Google Scholar] [CrossRef]
- Cuffia, F.; George, G.; Godoy, L.; Vinderola, G.; Reinheimer, J.; Burns, P. In vivo study of the immunomodulatory capacity and the impact of probiotic strains on physicochemical and sensory characteristics: Case of pasta filata soft cheeses. Food Res. Int. 2019, 125, 108606. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Kasahara, K. The emerging role of oxidative stress in complications of COVID-19 and potential therapeutic approach to diminish oxidative stress. Respir. Med. 2021, 187, 106605. [Google Scholar] [CrossRef]
- Ghiasi, F.; Hashemi, S.M.B.; Abedi, E. Effective enhancement of food oxidative stability induced by Lactobacillus strains: In vitro activity. Food Control 2023, 153, 109912. [Google Scholar] [CrossRef]
- Cárdenas, N.; Laiño, J.E.; Delgado, S.; Jiménez, E.; del Valle, M.J.; de Giori, G.S.; Sesma, F.; Mayo, B.; Fernández, L.; LeBlanc, J.G.; et al. Relationships between the genome and some phenotypical properties of Lactobacillus fermentum CECT 5716, a probiotic strain isolated from human milk. Appl. Microbiol. Biotechnol. 2015, 99, 4343–4353. [Google Scholar] [CrossRef]
- Lou, H.B.; Wang, J.; Wang, Y.P.; Gao, Y.D.; Wang, W. Comprehensive assessment of Enterococcus faecalis SN21-3: Probiotic features and safety evaluation for potential animal use. Food Biosci. 2024, 58, 103688. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Behare, P.V.; Yadav, H.; Srivastava, A.K. Emerging pre-clinical safety assessments for potential probiotic strains: A review. Crit. Rev. Food Sci. Nutr. 2023, 64, 8155–8183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).