Application of the Electrical Microbial Growth Analyzer Method for Efficiently Quantifying Viable Bacteria in Ready-to-Eat Sea Cucumber Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of Artificially Contaminated Samples
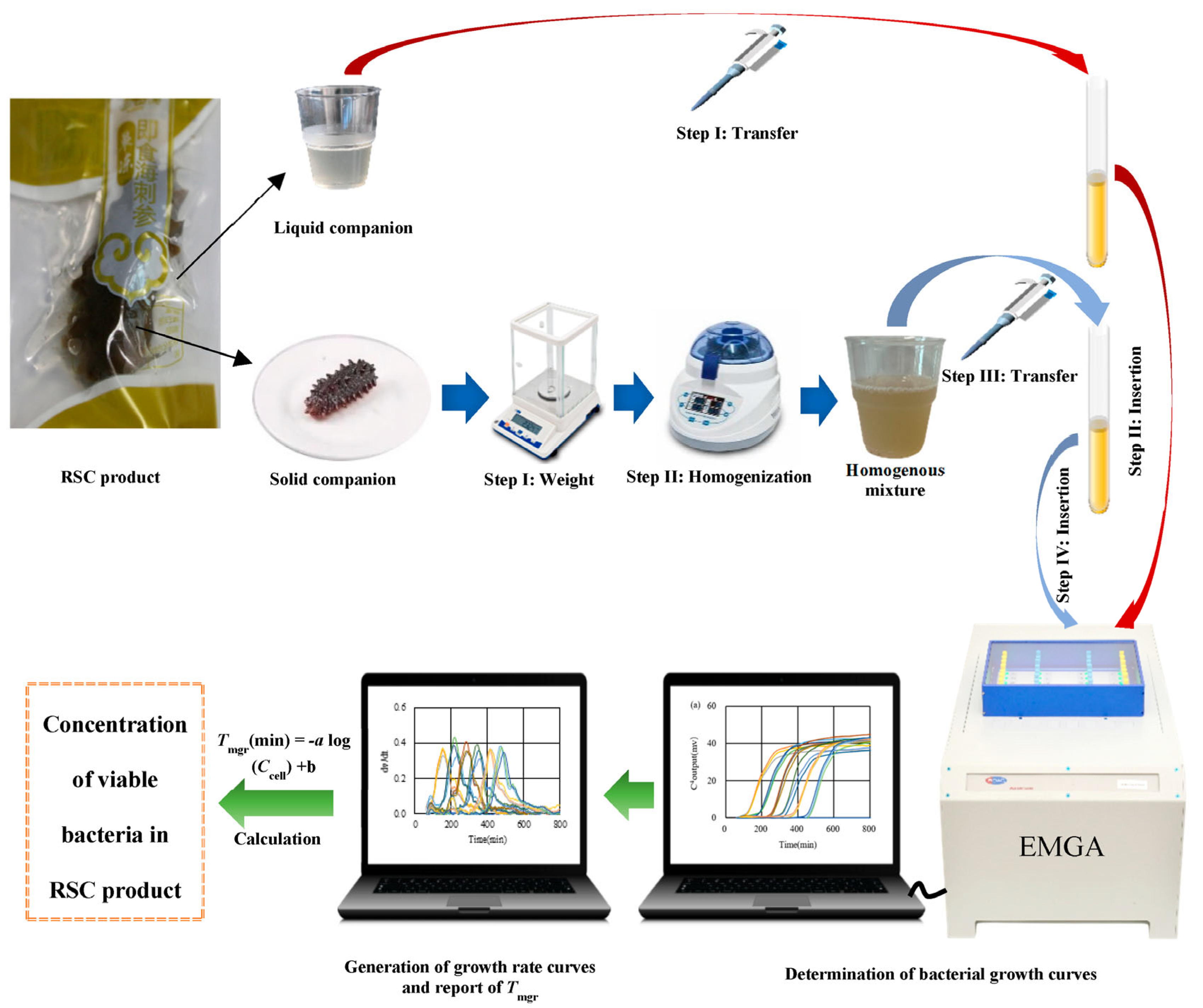
2.3. Quantification of Bacteria in RSC Products with EMGA
2.4. Quantification of Bacteria in RSC Products with Plate Counting
2.5. Quantification of Bacteria in RSC Products with PCR
2.6. Limit of Detection (LOD)
2.7. Recovery Rate
2.8. Statistical Analysis
3. Results and Discussion
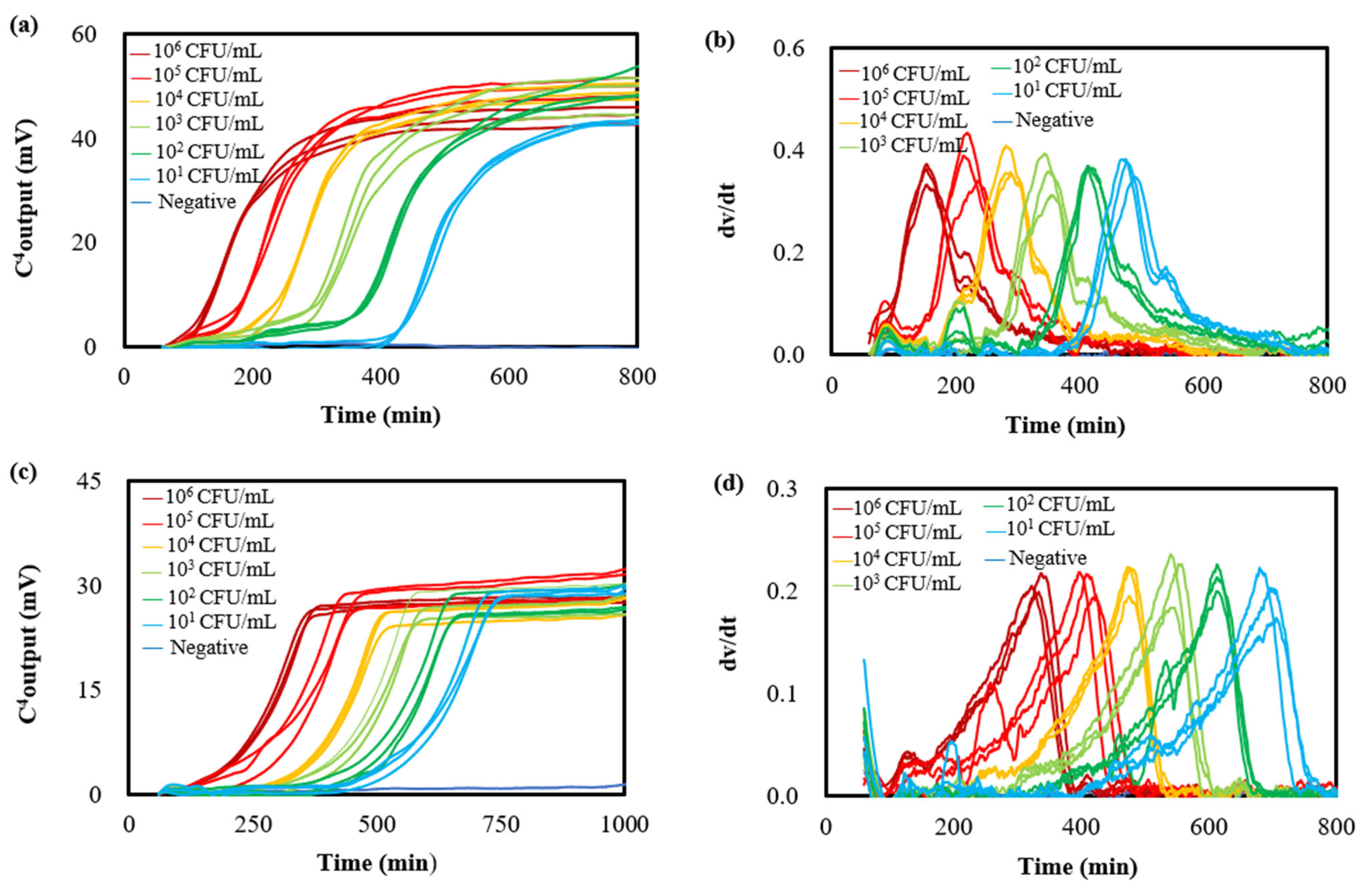
3.1. Bacterial Growth Curves and Growth Rate Curves
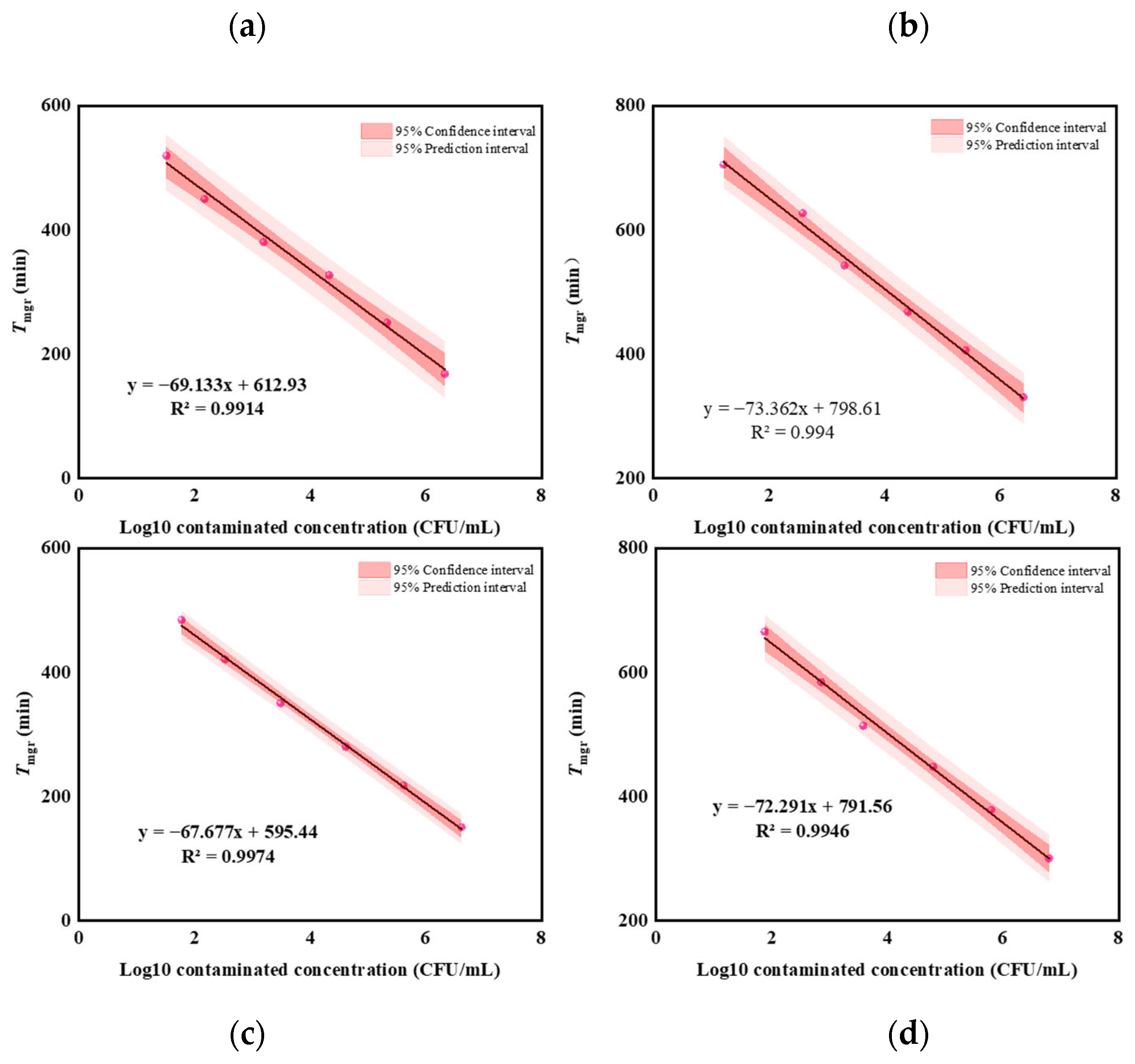
3.2. Linear Range
3.3. Simplicity and Efficiency
3.4. Sensitivity
3.5. Accuracy and Precision
3.6. Cost
3.7. Limitations and Further Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Ding, Y.; Tian, J.; Liu, Z.; Bi, J.; Zhou, D.; Song, L.; Chen, G. Inhibition of ultraviolet-induced sea cucumber (Stichopus japonicus) autolysis by maintaining coelomocyte intracellular calcium homeostasis. Food Chem. 2022, 368, 130768. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, G.; Wang, Q.; Zhao, Y.; Xie, Y.; Xu, S.; Li, R.; Shen, Y.; Gong, S.; Gao, J.; et al. Comparative effects of in-package high pressure steam sterilization and high temperature boiling on the quality changes and shelf life of ready-to-eat sea cucumber. Food Compos. Anal. 2024, 129, 106138. [Google Scholar] [CrossRef]
- Arienzo, A.; Gallo, V.; Tomassetti, F.; Antonini, G. Implication of sodium hypochlorite as a sanitizer in ready-to-eat salad processing and advantages of the use of alternative rapid bacterial detection methods. Foods 2023, 12, 3021. [Google Scholar] [CrossRef]
- Bodie, A.; O’Bryan, C.; Olson, E.; Ricke, S. Natural antimicrobials for Listeria monocytogenes in ready-to-eat meats: Current challenges and future prospects. Microorganisms 2023, 11, 1301. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, X.; Chen, B.; Wang, X.; Liang, Z.; Wang, L. The detection of foodborne pathogenic bacteria in seafood using a multiplex polymerase chain reaction system. Foods 2022, 11, 113909. [Google Scholar] [CrossRef]
- Aladhadh, M. A review of modern methods for the detection of foodborne pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Hammerl, J.A.; Boone, I.; Jansen, W.; Fohler, S.; Klein, G.; Dieckmann, R.; Dahouk, S.A. Overview of validated alternative methods for the detection of foodborne bacterial pathogens. Trends Food Sci. Technol. 2017, 62, 113–118. [Google Scholar] [CrossRef]
- Sekhon, A.S.; Unger, P.; Sharma, S.; Singh, B.; Chen, X.; Ganjyal, G.M.; Michael, M. Hyperspectral imaging of foodborne pathogens at colony and cellular levels for rapid identification in dairy products. Food Sci. Nutr. 2024, 12, 239–254. [Google Scholar] [CrossRef]
- Hiremath, S.D.; Bhosle, A.A.; Nayse, A.; Biswas, S.; Biswas, M.; Bhasikuttan, A.C.; Banerjee, M.; Chatterjee, A. A redox-coupled carbon dots-MnO2 nanosheets based sensory platform for label-free and sensitive detection of E. coli. Sens. Actuators B-Chem. 2021, 339, 129918. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Yang, Q.; Jiang, Y.; Li, Y.; Zhao, J.; Qu, M. Conductometric sensor for viable Escherichia coli and Staphylococcus aureus based on magnetic analyte separation via aptamer. Microchim. Acta 2020, 187, 43. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Dixit, Y.; Reis, M.; Brightwell, G. Hyperspectral imaging and machine learning in food microbiology: Developments and challenges in detection of bacterial, fungal, and viral contaminants. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3717–3745. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.; Zec, H.C.; Chen, L.; Kaushik, A.M.; Mach, K.E.; Liao, J.C.; Wang, T.-H. Simple and precise counting of viable bacteria by resazurin-amplified picoarray detection. Anal. Chem. 2018, 90, 9449–9456. [Google Scholar] [CrossRef] [PubMed]
- Marutescu, L.G. Current and future flow cytometry applications contributing to antimicrobial resistance control. Microorganisms 2023, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, X.; Ma, L.; Shi, Y.; Zhang, D.; Qu, K. Analytical methods for assessing antimicrobial activity of nanomaterials in complex media: Advances, challenges, and perspectives. J. Nanobiotechnol. 2023, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.P.; Franca, A.S.; Irudayaraj, J. Surface-enhanced Raman spectroscopy applied to food safety. Annu. Rev. Food Sci. Technol. 2013, 4, 369–380. [Google Scholar] [CrossRef]
- Belkum, A.; Bachmann, T.T.; Luedke, G.; Lisby, J.G.; Kahlmeter, G.; Mohess, A.; Becker, K.; Hays, J.P.; Woodford, N.; Mitsakakis, K.; et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2019, 17, 51–62. [Google Scholar] [CrossRef]
- An, X.; Zuo, P.; Ye, B.-C. A single cell droplet microfluidic system for quantitative determination of food-borne pathogens. Talanta 2020, 209, 120571. [Google Scholar] [CrossRef]
- Du, H.; Wang, X.; Yang, Q.; Wu, W. Quantum dot: Lightning invisible foodborne pathogens. Trends Food Sci. Technol. 2021, 110, 1–12. [Google Scholar] [CrossRef]
- Castle, L.M.; Schuh, D.A.; Reynolds, E.E.; Furst, A.L. Electrochemical sensors to detect bacterial foodborne pathogens. ACS Sens. 2021, 6, 1717–1730. [Google Scholar] [CrossRef]
- Nnachi, R.C.; Sui, N.; Ke, B.; Luo, Z.; Bhalla, N.; He, D.; Yang, Z. Biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ. Int. 2022, 166, 107357. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, L.; Johnson, N.; Begley, M.; O’Leary, P.; Callanan, M. Comparison of predicted and impedance determined growth of Listeria innocua in complex food matrices. Food Microbiol. 2020, 87, 103381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling strategies for selective in vitro and in vivo sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, Y.; He, G.-X.; Katagori, N.; Chen, H. A comparison of conventional methods for the quantification of bacterial cells after exposure to metal oxide nanoparticles. BMC Microbiol. 2014, 14, 222. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Yang, Q.; Wang, X.; Zhang, Y.; Zhao, J.; Qu, K.; Zhao, C. Online monitoring of bacterial growth with an electrical sensor. Anal. Chem. 2018, 90, 6006–6011. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Q.; Ma, L.; Zhang, D.; Lin, W.; Schlensky, N.; Cheng, H.; Zheng, Y.; Luo, X.; Ding, C.; et al. Automatically showing microbial growth kinetics with a high-performance microbial growth analyzer. Biosens. Bioelectron. 2023, 239, 115626. [Google Scholar] [CrossRef]
- Hauser, P.; Kubáň, P. Capacitively coupled contactless conductivity detection for analytical techniques–developments from 2018 to 2020. J. Chromatogr. A 2020, 1632, 461616. [Google Scholar] [CrossRef]
- Tuma, P. Monitoring of biologically active substances in clinical samples by capillary and microchip electrophoresis with contactless conductivity detection: A review. Anal. Chim. Acta 2022, 1225, 340161. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Q.; Lu, F.; Wang, X.; Liang, R.; Pu, X.; Chen, J.; Zhang, D.; Chen, Z.; Zhang, X. Inhibitory effects of potassium sorbate and ZnO nanoparticles on Escherichia coli and Staphylococcus aureus in milk-basedbeverage. J. Int. Dairy 2024, 206, 106073. [Google Scholar] [CrossRef]
- Zeng, X.; Pang, Z.; Lu, E.; Zhang, G.; Han, X.; Zhang, Z.; Yu, L.; Luo, X. A stable and low-fouling lead ion-selective electrode employing polyaniline-MXene composite for solid contact and polypeptide for antifouling. Sens. Actuators B-Chem. 2024, 418, 136237. [Google Scholar] [CrossRef]
- Settu, K.; Chen, C.-J.; Liu, J.-T.; Chen, C.-L.; Tsai, J.-Z. Impedimetric method for measuring ultra-low E. coli concentrations in human urine. Biosens. Bioelectron. 2015, 66, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Gao, Y.; Li, X.; Yuan, L.; Zhu, G.; Gu, X.; Yang, Z. Nanozyme colourimetry based on temperate bacteriophage for rapid and sensitive detection of Staphylococcus aureus in food matrices. Int. J. Food Microbiol. 2024, 416, 110657. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.J.; Wei, Q.K.; Liao, C.W.; Hung, M.J.; Wang, T.H. Microbiological quality of 18 °C ready-to-eat food products sold in Taiwan. Int. J. Food Microbiol. 2003, 80, 241–250. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, B.; Liu, S.; Zhu, L.; Zhu, F. The MBS microbial rapid detection system for rapid detection of major pathogenic bacteria in feed: Comparison with plate counting method. BMC Microbiol. 2022, 22, 242. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Brück, W.M.; Brück, T.B.; Allahyari, S.; Tamai, I.A. A quantitative prevalence of Escherichia coli O157 in different food samples using real-time qPCR method. Food Sci. Nutr. 2023, 11, 228–235. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Wei, S.; Oh, D.-H. A highly selective enrichment broth combined with real-time PCR for detection of Staphylococcus aureus in food samples. Lwt 2018, 94, 103–110. [Google Scholar] [CrossRef]
- Yang, L.; Banada, P.P.; Liu, Y.; Bhunia, A.K.; Bashir, R. Conductivity and pH dual detection of growth profile of healthy and stressed Listeria monocytogenes. Biotechnol. Bioeng. 2005, 92, 685–694. [Google Scholar] [CrossRef]
- Ghafar-Zadeh, E.; Sawan, M.; Chodavarapu, V.P.; Hosseini-Nia, T. Bacteria growth monitoring through a differential CMOS capacitive sensor. IEEE Trans. Biomed. Circ. Syst. 2010, 4, 232–238. [Google Scholar] [CrossRef]
- Qiu, T.A.; Nguyen, T.H.T.; Hudson-Smithm, N.V.; Clement, P.L.; Forester, D.-C.; Frew, H.; Hang, M.N.; Murphy, C.J.; Hamers, R.J.; Feng, Z.V.; et al. Growth-based bacterial viability assay for interference-free and high-throughput toxicity screening of nanomaterials. Anal. Chem. 2017, 89, 2057–2064. [Google Scholar] [CrossRef]
- Zhuang, L.; Gong, J.; Zhao, Y.; Yang, J.; Liu, G.; Zhao, B.; Song, C.; Zhang, Y.; Shen, Q. Progress in methods for the detection of viable Escherichia coli. Analyst 2024, 149, 1022–1049. [Google Scholar] [CrossRef]
- Gong, L.; Liang, J.; Zhang, Y.; Zhang, M.; Ao, H.; Yang, T. An antifouling electrochemical biosensor using self-signal for Salmonella typhimurium direct detection in food sample. Food Chem. 2024, 452, 139536. [Google Scholar] [CrossRef] [PubMed]
- Anvarian, A.H.P.; Smith, M.P.; Overton, T.W. Use of flow cytometry and total viable count to determine the effects of orange juice composition on the physiology of Escherichia coli. Food Sci. Nutr. 2018, 6, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, W.B.; Dudley, E.G.; Doores, S.; Cutter, C.N. Commercially available rapid methods for detection of selected food-borne pathogens. Crit. Rev. Food Sci. Nutr. 2016, 56, 1519–1531. [Google Scholar] [CrossRef]
- Uria, N.; Moral-Vico, J.; Abramova, N.; Bratov, A.; Munoz, X.F. Fast determination of viable bacterial cells in milk samples using impedimetric sensor and a novel calibration method. Electrochim. Acta 2016, 198, 249–258. [Google Scholar] [CrossRef]
- Zhao, X.; Bhat, A.; O’Connor, C.; Curtin, J.; Singh, B.; Tian, F. Review of detection limits for various techniques for bacterial detection in food samples. Nanomaterials 2024, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Clais, S.; Boulet, G.; Van Kerckhoven, M.; Lanckacker, E.; Delputte, P.; Maes, L.; Cos, P. Comparison of viable plate count, turbidity measurement and real-time PCR for quantification of Porphyromonas gingivalis. Lett. Appl. Microbiol. 2015, 60, 79–84. [Google Scholar] [CrossRef]
- Fukushima, H.; Katsube, K.; Hata, Y.; Kishi, R.; Fujiwara, S. Rapid separation and concentration of food-borne pathogens in food samples prior to quantification by viable-cell counting and real-time PCR. Appl. Environ. Microb. 2007, 73, 92–100. [Google Scholar] [CrossRef]
- Francois, P.; Pittet, D.; Bento, M.; Pepey, B.; Vaudaux, P.; Lew, D.; Schrenzel, J. Rapid detection of methicillin-resistant Staphylococcus aureus directly from sterile or nonsterile clinical samples by a new molecular assay. J. Clin. Microbiol. 2003, 41, 254–260. [Google Scholar] [CrossRef]
- Hospodsky, D.; Yamamoto, N.; Peccia, J. Accuracy, precision, and method detection limits of quantitative PCR for airborne bacteria and fungi. Appl. Environ. Microb. 2010, 76, 7004–7012. [Google Scholar] [CrossRef]
- Si, Y.; Grazon, C.; Clavier, G.; Rieger, J.; Audibert, J.-F.; Sclavi, B.; Meallet-Renault, R. Rapid and accurate detection of Escherichia coli growth by fluorescent pH-sensitive organic nanoparticles for high-throughput screening applications. Biosens. Bioelectron. 2016, 75, 320–327. [Google Scholar] [CrossRef]
- Jo, N.; Kim, B.; Lee, S.-M.; Oh, J.; Park, I.H.; Lim, K.J.; Shin, J.-S.; Yoo, K.-H. Aptamer-functionalized capacitance sensors for real-time monitoring of bacterial growth and antibiotic susceptibility. Biosens. Bioelectron. 2018, 102, 164–170. [Google Scholar] [CrossRef] [PubMed]


| Strains | Concentration CFU/mL | EMGA | Plate Counting | PCR | |||
|---|---|---|---|---|---|---|---|
| Recovery Rate (%) | SD | Recovery Rate (%) | SD | Recovery Rate (%) | SD | ||
| E. coli | 5.0 × 106 | 89.05–94.36 | 2.50 | 74.28–98.63 | 10.60 | 69.66–80.75 | 4.81 |
| 5.0 × 105 | 91.52–112.93 | 5.84 | 78.12–85.71 | 9.10 | 79.15–83.77 | 6.12 | |
| 5.0 × 104 | 101.44–117.79 | 6.77 | 93.44–123.78 | 11.43 | 54.19–81.60 | 11.20 | |
| 5.0 × 103 | 108.85–112.77 | 1.60 | 86.66–114.74 | 6.36 | 70.00–94.31 | 10.04 | |
| 5.0 × 102 | 87.37–96.71 | 3.83 | 85.71–101.40 | 7.32 | 67.51–86.41 | 8.20 | |
| 5.0 × 101 | 76.13–81.38 | 2.22 | 80.20–89.00 | 9.43 | 68.90–81.36 | 11.85 | |
| S. aureus | 5.0 × 106 | 89.52–110.99 | 5.86 | 68.06–87.72 | 12.11 | 63.29–93.09 | 12.71 |
| 5.0 × 105 | 103.04–125.81 | 5.25 | 96.00–132.26 | 15.64 | 88.12–92.33 | 4.82 | |
| 5.0 × 104 | 96.88–123.75 | 3.76 | 86.49–113.51 | 12.40 | 83.06–97.65 | 6.27 | |
| 5.0 × 103 | 107.01–130.54 | 3.92 | 88.24–102.70 | 5.93 | 51.71–86.66 | 14.27 | |
| 5.0 × 102 | 95.20–117.28 | 8.19 | 78.26–108.33 | 13.18 | 60.84–83.31 | 14.58 | |
| 5.0 × 101 | 104.28–133.33 | 9.95 | 50.00–114.29 | 17.56 | Not available | Not available | |
| Strains | Concentration CFU/mL | EMGA | Plate Counting | PCR | |||
|---|---|---|---|---|---|---|---|
| Recovery Rate (%) | SD | Recovery Rate (%) | SD | Recovery Rate (%) | SD | ||
| E. coli | 5.0 × 106 | 100.70–110.82 | 3.54 | 88.70–116.32 | 13.04 | 82.90–98.31 | 6.39 |
| 5.0 × 105 | 97.72–106.88 | 3.92 | 71.53–110.63 | 22.21 | 94.21–98.02 | 4.78 | |
| 5.0 × 104 | 87.15–116.46 | 2.67 | 98.36–112.90 | 6.86 | 65.70–82.00 | 7.13 | |
| 5.0 × 103 | 91.94–102.24 | 2.34 | 82.14–95.24 | 5.75 | 88.91–94.72 | 4.79 | |
| 5.0 × 102 | 82.14–95.24 | 3.75 | 82.40–106.43 | 9.93 | 72.52–85.14 | 3.50 | |
| 5.0 × 101 | 91.80–109.68 | 5.91 | 89.78–108.59 | 24.97 | 63.76–80.98 | 7.14 | |
| S. aureus | 5.0 × 106 | 103.92–110.68 | 2.96 | 71.78–85.00 | 8.67 | 62.42–92.37 | 9.19 |
| 5.0 × 105 | 95.39–109.73 | 3.62 | 90.28–116.32 | 6.25 | 87.81–95.30 | 4.64 | |
| 5.0 × 104 | 94.51–108.70 | 2.84 | 69.57–70.42 | 9.26 | 83.67–97.59 | 5.04 | |
| 5.0 × 103 | 81.43–104.46 | 2.38 | 73.28–71.43 | 5.09 | 89.82–93.56 | 2.72 | |
| 5.0 × 102 | 76.99–89.48 | 4.10 | 75.96–106.99 | 14.21 | 69.63–85.69 | 5.63 | |
| 5.0 × 101 | 74.51–83.06 | 5.96 | 68.85–73.68 | 19.80 | Not aviable | Not aviable | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liang, R.; Pu, X.; Zhang, Y.; Lu, F.; Yang, Q.; Zhu, X.; Kong, Q.; Zhang, X. Application of the Electrical Microbial Growth Analyzer Method for Efficiently Quantifying Viable Bacteria in Ready-to-Eat Sea Cucumber Products. Microorganisms 2024, 12, 2301. https://doi.org/10.3390/microorganisms12112301
Wang X, Liang R, Pu X, Zhang Y, Lu F, Yang Q, Zhu X, Kong Q, Zhang X. Application of the Electrical Microbial Growth Analyzer Method for Efficiently Quantifying Viable Bacteria in Ready-to-Eat Sea Cucumber Products. Microorganisms. 2024; 12(11):2301. https://doi.org/10.3390/microorganisms12112301
Chicago/Turabian StyleWang, Xiaoyang, Ruohan Liang, Xiaodan Pu, Yuanyuan Zhang, Feng Lu, Qianqian Yang, Xueting Zhu, Qing Kong, and Xuzhi Zhang. 2024. "Application of the Electrical Microbial Growth Analyzer Method for Efficiently Quantifying Viable Bacteria in Ready-to-Eat Sea Cucumber Products" Microorganisms 12, no. 11: 2301. https://doi.org/10.3390/microorganisms12112301
APA StyleWang, X., Liang, R., Pu, X., Zhang, Y., Lu, F., Yang, Q., Zhu, X., Kong, Q., & Zhang, X. (2024). Application of the Electrical Microbial Growth Analyzer Method for Efficiently Quantifying Viable Bacteria in Ready-to-Eat Sea Cucumber Products. Microorganisms, 12(11), 2301. https://doi.org/10.3390/microorganisms12112301






