In-Vitro Antimicrobial Activities of Grape Seed, Green Tea, and Rosemary Phenolic Extracts Against Liver Abscess Causing Bacterial Pathogens in Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Extracts
2.2. Extraction of Phytophenols
2.3. Measurement of Total Phenolic Contents
2.4. Bacterial Strains
2.5. Preparation of Bacterial Inoculums
2.6. Broth Macro-Dilution Method
2.7. Broth Micro-Dilution Method
2.8. Statistical Analysis
3. Results
3.1. Total Phenolic Content
3.2. Broth Macro-Dilution Method
3.2.1. Grape Seed Phenolic Extract
3.2.2. Green Tea Phenolic Extract
3.2.3. Rosemary Phenolic Extract
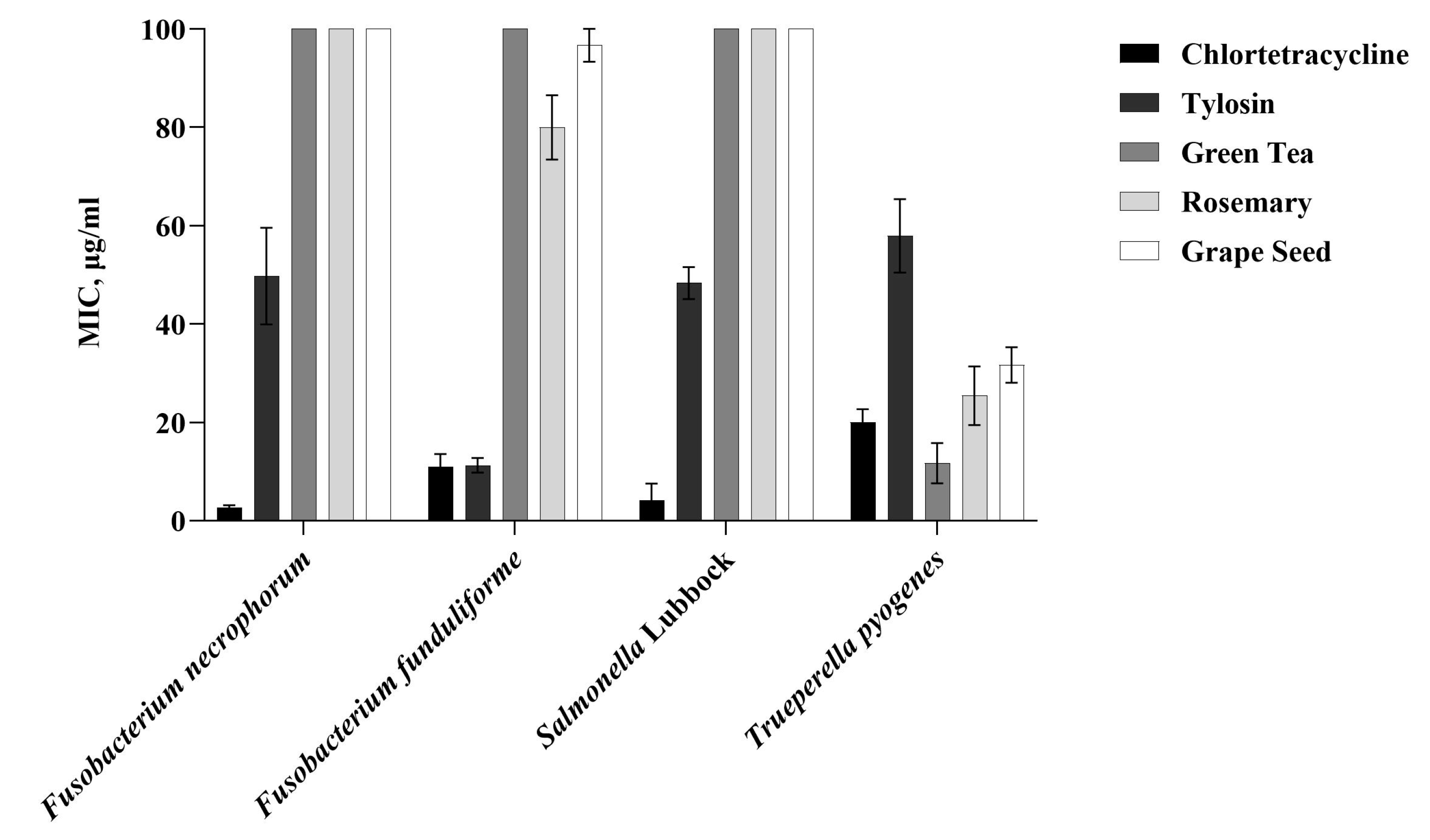
3.3. Broth Micro-Dilution Method
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amachawadi, R.G.; Nagaraja, T.G. Liver abscesses in cattle: A review of incidence in Holsteins and of bacteriology and vaccine approaches to control in feedlot cattle. J. Anim. Sci. 2016, 94, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.F.; Holland, B.P. Liver Abnormalities in Cattle. Effect of Liver Abscessation on Growth and Productivity of Cattle. Vet. Clin. Food Anim. 2022, 38, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.D.; Hubbert, M.E. Review: Control of liver abscesses in feedlot cattle. Prof. Anim. Sci. 2015, 31, 101–108. [Google Scholar] [CrossRef]
- Amachawadi, R.G.; Nagaraja, T.G. First report of anaerobic isolation of Salmonella enterica from liver abscesses of feedlot cattle. J. Clin. Microbiol. 2015, 53, 3100–3101. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Annual Report 2022. Advancing Public Health Through Therapeutic Individualization. Available online: https://www.fda.gov/media/155771/download (accessed on 15 July 2024).
- Davedow, T.; Narvaez-Bravo, C.; Zaheer, R.; Sanderson, H.; Rodas-Gonzalez, A.; Klima, C.; Booker, C.W.; Hannon, S.J.; Bras, A.L.; Gow, S.; et al. Investigation of a Reduction in Tylosin on the Prevalence of Liver Abscesses and Antimicrobial Resistance in Enterococci in Feedlot Cattle. Front. Vet. Sci. 2020, 7, 90. [Google Scholar] [CrossRef]
- Brown, T.R.; Lawrence, T.E. Association of liver abnormalities with carcass grading performance and value. J. Anim. Sci. 2010, 88, 4037–4043. [Google Scholar] [CrossRef]
- Galyean, M.; Hales, K. Non-Antimicrobial Methods to Control Liver Abscesses. Vet. Clin. Food Anim. 2022, 38, 395–404. [Google Scholar] [CrossRef]
- Pereira, I.C.; Costa, C.F.; Martins, C.L.; Pereira, M.C.S.; Squizatti, M.M.; Owens, F.N.; Cruz, G.D.; Millen, D.D.; Arrigoni, M.D.B. Voluntary daily fluctuation in dry matter intake is associated to feedlot performance, feeding behavior and rumen morphometrics in beef cattle. Livest. Sci. 2021, 250, 104565. [Google Scholar] [CrossRef]
- He, Z.X.; He, M.L.; Walker, N.D.; McAllister, T.A.; Yang, W.Z. Using a fibrolytic enzyme in barley-based diets containing wheat dried distillers’ grains with soluble: Ruminal fermentation, digestibility, and growth performance of feedlot steers. J. Anim. Sci. 2014, 92, 3978–3987. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- López, N.A.; Salazar, J.A.G.; Santos, E.M.; Campagnol, P.C.B.; Teixeira, A.; Lorenzo, J.M.; Morales, M.E.S.; Domínguez, R. Natural Antimicrobials: A Clean Label Strategy to Improve the Shelf Life and Safety of Reformulated Meat Products. Foods 2022, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape Pomace as a Promising Antimicrobial Alternative in Feed: A Critical Review. Grape Pomace as a Promising Antimicrobial Alternative in Feed: A Critical Review. J. Agric. Food Chem. 2019, 67, 9705–9718. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Negut, I. Coatings Based on Essential Oils for Combating Antibiotic Resistance. Antibiotics 2024, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Sochorova, L.; Prusova, B.; Cebova, M.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Nedomova, S.; Baron, M.; Sochor, J. Health Effects of Grape Seed and Skin Extracts and Their Influence on Biochemical Markers. Molecules 2020, 25, 5311. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2016, 174, 1244–1262. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Akhtar, W.; Shahzad, T.; Malik, A.; Ajmal Shah, M.; Iqbal, S.; Rauf, A.; Zengin, G.; Bouyahya, A.; et al. Rosemary species: A review of phytochemicals, bioactivities and industrial applications. S. Afr. J. Bot. 2022, 151, 3–18. [Google Scholar] [CrossRef]
- Bouammali, B.; Zraibi, L.; Ziani, I.; Merzouki, M.; Bourassi, L.; Fraj, E.; Challioui, A.; Azzaoui, K.; Sabbahi, R.; Hammouti, B.; et al. Rosemary as a Potential Source of Natural Antioxidants and Anticancer Agents: A Molecular Docking Study. Plants 2024, 13, 89. [Google Scholar] [CrossRef]
- Olivas-Méndez, P.; Chávez-Martínez, A.; Santellano-Estrada, E.; Asorey, L.G.; Sánchez-Vega, R.; Rentería-Monterrubio, A.L.; Chávez-Flores, D.; Tirado-Gallegos, J.M.; Méndez-Zamora, G. Antioxidant and Antimicrobial Activity of Rosemary (Rosmarinus officinalis) and Garlic (Allium sativum) Essential Oils and Chipotle Pepper Oleoresin (Capsicum annum) on Beef Hamburgers. Foods 2022, 11, 2018. [Google Scholar] [CrossRef]
- Amachawadi, R.G.; Nagaraja, T.G. Pathogenesis of Liver Abscesses in Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2022, 38, 335–346. [Google Scholar] [CrossRef]
- Callaway, T.R.; Lillehoj, H.; Chuanchuen, R.; Gay, C.G. Alternatives to Antibiotics: A Symposium on the Challenges and Solutions for Animal Health and Production. Antibiotics 2021, 10, 471. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Tian, W.; Li, Y. Phenolic acid composition an antioxidant activity of hard red winter wheat varieties. Food Biochem. 2018, 42, e12682. [Google Scholar] [CrossRef]
- Tian, W.; Chen, G.; Zhang, G.; Wang, D.; Tilley, M.; Li, Y. Rapid determination of total phenolic content of whole wheat flour using near-infrared spectroscopy and chemometrics. Food Chem. 2021, 344, 128633. [Google Scholar] [CrossRef]
- Amachawadi, R.G.; Purvis, T.J.; Lubbers, B.V.; Homm, J.W.; Maxwell, C.L.; Nagaraja, T.G. Bacterial flora of liver abscesses in crossbred beef cattle and Holstein steers fed finishing diets with or without tylosin. J. Anim. Sci. 2017, 95, 3425–3434. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute (CLSI). Performance Standard for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 6th ed.; CLSI Supplement VET01S: Wayne, PA, USA, 2023. [Google Scholar]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The Antimicrobial Resistance Crisis: Causes, Consequences, and Management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Nwobodo, D.C.; Ugwu, M.C.; Anie, C.O.; Al-Ouqaili, M.T.S.; Ikem, J.C.; Chigozie, U.V.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Chavada, J.; Muneshwar, K.N.; Ghulaxe, Y.; Wani, M.; Sarda, P.P.; Huse, S. Antibiotic Resistance: Challenges and Strategies in Combating Infections. Cureus 2023, 15, e46013. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, S. Role of alternatives to antibiotics in mitigating the antimicrobial resistance crisis. Indian J. Med. Res. 2022, 156, 464–477. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Miyakawa, M.E.F.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Rio, D.D.; Mateos, A.R.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly) phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Salem, Y.; Rajha, H.N.; Franjieh, D.; Hoss, I.; Manca, M.L.; Manconi, M.; Castangia, I.; Perra, M.; Maroun, R.G.; Louka, N. Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams. Antioxidants 2022, 11, 1348. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Kara, Z.; Baykan, M.; Dogan, M.; Ege, D. Effectiveness of Grape (Vitis vinifera L.) Seed Extracts on Fungi and Bacteria Management. Selcuk J. Agric. Food 2018, 32, 366–372. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Huang, G.; Wang, J. Ruminal microbiota–host interaction and its effect on nutrient metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef]
- Zokti, J.A.; Baharin, B.S.; Mohammed, A.S.; Abas, F. Green Tea Leaves Extract: Microencapsulation, Physicochemical and Storage Stability Study. Molecules 2016, 21, 940. [Google Scholar] [CrossRef]
- Labbé, D.; Tetu, B.; Trudel, D.; Bazinet, L. Catechin stability of EGC- and EGCG-enriched tea drinks produced by a two-step extraction procedure. Food Chem. 2008, 111, 139–143. [Google Scholar] [CrossRef]
- Bazinet, L.; Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2007, 23, 1361–1394. [Google Scholar] [CrossRef]
- Hameed, I.H.; Ibraheam, I.A.; Kadhim, H.J. Gas chromatography mass spectrum and fouriertransform infrared spectroscopy analysis of methanolic extract of Rosmarinus oficinalis leaves. J. Pharmacogn. Phytother. 2015, 7, 90–106. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef]
- Fung, D.Y.C.; Tayler, S.; Khan, J. Effects of butylated hydroxyanisole (BHA) and buyulated hydroxytoluene (BHT) on growth and aflatoxin production of Aspergillus flavus. Food Saf. 1977, 1, 39–51. [Google Scholar] [CrossRef]
- Bouloumpasi, E.; Hatzikamari, M.; Christaki, S.; Lazaridou, A.; Chatzopou, P.; Biliaderis, C.G.; Irakli, M. Assessment of Antioxidant and Antibacterial Potential of Phenolic Extracts from Post-Distillation Solid Residues of Oregano, Rosemary, Sage, Lemon Balm, and Spearmint. Processes 2024, 12, 140. [Google Scholar] [CrossRef]
- Manilal, A.; Sabu, K.R.; Woldemariam, M.; Aklilu, A.; Biresaw, G.; Yohanes, T.; Seid, M.; Merdekios, B. Antibacterial Activity of Rosmarinus officinalis against Multidrug-Resistant Clinical Isolates and Meat-Borne Pathogens. Evid. Based Complement. Altern. Med. 2021, 14, 17–26. [Google Scholar] [CrossRef]
| Bacteria | Phenolic Extracts | Concentration Tested (mg/mL) | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Replication | Strain | Growth Condition | Strain * Growth Condition (2-Way Interaction) | Time | Strain * Time (2-Way Interaction) | Growth Condition * Time (2-Way Interaction) | Strain * Growth Condition * Time (3-Way Interaction) | |||
| Fusobacterium necrophorum subsp. necrophorum | Grape seed | 1 | - | 0.9928 | 0.0003 | 0.9992 | <0.0001 | 0.8995 | <0.0001 | 0.9909 |
| Fusobacterium necrophorum subsp. funduliforme | Grape seed | 1 | - | 0.9143 | 0.0023 | 0.8008 | <0.0001 | 0.3004 | <0.0001 | 0.5370 |
| Fusobacterium necrophorum subsp. necrophorum | Grape seed | 2 | 0.0201 | 0.9754 | 0.0022 | 0.6221 | <0.0001 | 0.1228 | <0.0001 | 0.6699 |
| Fusobacterium necrophorum subsp. funduliforme | Grape seed | 2 | 0.0176 | 0.6959 | 0.0051 | 0.5194 | <0.0001 | 0.4498 | <0.0001 | 0.4845 |
| Fusobacterium necrophorum subsp. necrophorum | Green tea | 0.1 | - | 0.1063 | <0.0001 | 0.0169 | <0.0001 | 0.0009 | <0.0001 | 0.0001 |
| Fusobacterium necrophorum subsp. funduliforme | Green tea | 0.1 | - | 0.0042 | <0.0001 | 0.0024 | <0.0001 | <.0001 | <0.0001 | <0.0001 |
| Fusobacterium necrophorum subsp. necrophorum | Green tea | 1 | 0.9151 | 0.8256 | 0.0374 | 0.9311 | <0.0001 | 0.0162 | <0.0001 | 0.1001 |
| Fusobacterium necrophorum subsp. funduliforme | Green tea | 1 | 0.5745 | 0.6105 | 0.0309 | 0.7263 | <0.0001 | 0.6430 | <0.0001 | 0.4465 |
| Salmonella enterica serotype Lubbock | Green tea | 1 | - | 0.0089 | <0.0001 | 0.3375 | <0.0001 | 0.4992 | <0.0001 | 0.1494 |
| Salmonella enterica serotype Lubbock | Green tea | 2 | - | 0.2230 | <0.0001 | 0.6609 | <0.0001 | 0.9963 | <0.0001 | 0.9998 |
| Fusobacterium necrophorum subsp. necrophorum | Rosemary | 1 | - | 0.4324 | <0.0001 | 0.5407 | <0.0001 | 0.0997 | <0.0001 | 0.0002 |
| Fusobacterium necrophorum subsp. funduliforme | Rosemary | 1 | - | 0.2968 | <0.0001 | 0.1652 | <0.0001 | 0.0982 | <0.0001 | 0.1070 |
| Salmonella enterica serotype Lubbock | Rosemary | 1 | - | 0.9990 | 0.0001 | 1.0000 | <0.0001 | 1.0000 | <0.0001 | 1.0000 |
| Fusobacterium necrophorum subsp. necrophorum | Rosemary | 2 | - | 0.0143 | <0.0001 | 0.0551 | <0.0001 | 0.3012 | <0.0001 | 0.0092 |
| Fusobacterium necrophorum subsp. funduliforme | Rosemary | 2 | - | 0.0082 | <0.0001 | 0.0014 | <0.0001 | 0.0268 | <0.0001 | 0.0110 |
| Alpha = 0.05 | ||||||||||
| Bacteria | Phenolic Extracts | Concentration (mg/mL) | p-values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Replication | Strain | Growth Condition | Strain * Growth Condition (2-Way Interaction) | Time | Strain * Time (2-Way Interaction) | Growth Condition * Time (2-Way Interaction) | Strain * Growth Condition * Time (3-Way Interaction) | |||
| Fusobacterium necrophorum subsp. necrophorum | Grape seed | 1 | - | 0.3603 | <0.0001 | 0.2315 | 0.4216 | 0.3093 | 0.1574 | 0.4378 |
| Fusobacterium necrophorum subsp. funduliforme | Grape seed | 1 | - | 0.0131 | <0.0001 | 0.0109 | <0.0001 | 0.0002 | <0.0001 | 0.0002 |
| Fusobacterium necrophorum subsp. necrophorum | Green tea | 1 | 0.4278 | 0.8984 | 0.0004 | 0.8158 | 0.7739 | 0.5887 | 0.8503 | 0.9274 |
| Fusobacterium necrophorum subsp. necrophorum | Rosemary | 1 | - | 0.5622 | <0.0001 | 0.3149 | 0.1592 | 0.9817 | 0.2339 | 0.9812 |
| Fusobacterium necrophorum subsp. funduliforme | Rosemary | 1 | - | 0.3531 | <0.0001 | 0.5415 | 0.0021 | 0.1215 | 0.0063 | 0.1021 |
| Alpha = 0.05 | ||||||||||
| Bacteria | Phenolic Extracts | Concentration Tested (mg/mL) | Bacterial Concentration (Log Values) | ||||
|---|---|---|---|---|---|---|---|
| Time | Growth Condition | LSM | SEM | * | |||
| Fusobacterium necrophorum subsp. necrophorum | Grape seed | 1 | 24 h | Bacteria control | 9.62 | 0.21 | A |
| DMSO + Bacteria | 9.72 | 0.21 | A | ||||
| Phenolic extract + Bacteria | 4.78 | 0.15 | B | ||||
| 48 h | Bacteria control | 9.50 | 0.21 | A | |||
| DMSO + Bacteria | 9.57 | 0.21 | A | ||||
| Phenolic extract + Bacteria | 4.90 | 0.15 | B | ||||
| Fusobacterium necrophorum subsp. funduliforme | Grape seed | 1 | 24 h | Bacteria control | 9.91 | 0.13 | A |
| DMSO + Bacteria | 9.11 | 0.13 | B | ||||
| Phenolic extract + Bacteria | 4.61 | 0.09 | C | ||||
| 48 h | Bacteria control | 9.97 | 0.13 | A | |||
| DMSO + Bacteria | 5.82 | 0.13 | B | ||||
| Phenolic extract + Bacteria | 4.99 | 0.09 | C | ||||
| Fusobacterium necrophorum subsp. necrophorum | Green tea | 1 | 24 h | Bacteria control | 8.12 | 0.68 | A |
| DMSO + Bacteria | 9.15 | 0.64 | A | ||||
| Phenolic extract + Bacteria | 1.23 | 0.22 | B | ||||
| 48 h | Bacteria control | 7.22 | 0.68 | A | |||
| DMSO + Bacteria | 9.10 | 0.64 | A | ||||
| Phenolic extract + Bacteria | 1.75 | 0.22 | B | ||||
| Fusobacterium necrophorum subsp. necrophorum | Rosemary | 1 | 24 h | Bacteria control | 10.72 | 0.43 | A |
| DMSO + Bacteria | 7.31 | 0.43 | B | ||||
| Phenolic extract + Bacteria | 3.51 | 0.30 | C | ||||
| 48 h | Bacteria control | 10.87 | 0.43 | A | |||
| DMSO + Bacteria | 7.37 | 0.43 | B | ||||
| Phenolic extract + Bacteria | 4.60 | 0.30 | C | ||||
| Fusobacterium necrophorum subsp. funduliforme | Rosemary | 1 | 24 h | Bacteria control | 10.40 | 0.38 | A |
| DMSO + Bacteria | 8.59 | 0.38 | B | ||||
| Phenolic extract + Bacteria | 2.81 | 0.27 | C | ||||
| 48 h | Bacteria control | 8.75 | 0.38 | A | |||
| DMSO + Bacteria | 8.27 | 0.38 | A | ||||
| Phenolic extract + Bacteria | 2.70 | 0.27 | B | ||||
| Alpha = 0.005 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, H.M.; Amachawadi, R.G.; Kang, Q.; Li, Y.; Nagaraja, T.G. In-Vitro Antimicrobial Activities of Grape Seed, Green Tea, and Rosemary Phenolic Extracts Against Liver Abscess Causing Bacterial Pathogens in Cattle. Microorganisms 2024, 12, 2291. https://doi.org/10.3390/microorganisms12112291
Salih HM, Amachawadi RG, Kang Q, Li Y, Nagaraja TG. In-Vitro Antimicrobial Activities of Grape Seed, Green Tea, and Rosemary Phenolic Extracts Against Liver Abscess Causing Bacterial Pathogens in Cattle. Microorganisms. 2024; 12(11):2291. https://doi.org/10.3390/microorganisms12112291
Chicago/Turabian StyleSalih, Harith M., Raghavendra G. Amachawadi, Qing Kang, Yonghui Li, and Tiruvoor G. Nagaraja. 2024. "In-Vitro Antimicrobial Activities of Grape Seed, Green Tea, and Rosemary Phenolic Extracts Against Liver Abscess Causing Bacterial Pathogens in Cattle" Microorganisms 12, no. 11: 2291. https://doi.org/10.3390/microorganisms12112291
APA StyleSalih, H. M., Amachawadi, R. G., Kang, Q., Li, Y., & Nagaraja, T. G. (2024). In-Vitro Antimicrobial Activities of Grape Seed, Green Tea, and Rosemary Phenolic Extracts Against Liver Abscess Causing Bacterial Pathogens in Cattle. Microorganisms, 12(11), 2291. https://doi.org/10.3390/microorganisms12112291







