Limosilactobacillus reuteri DSM 17938 Produce Bioactive Components during Formulation in Sucrose
Abstract
1. Introduction
2. Materials and Methods
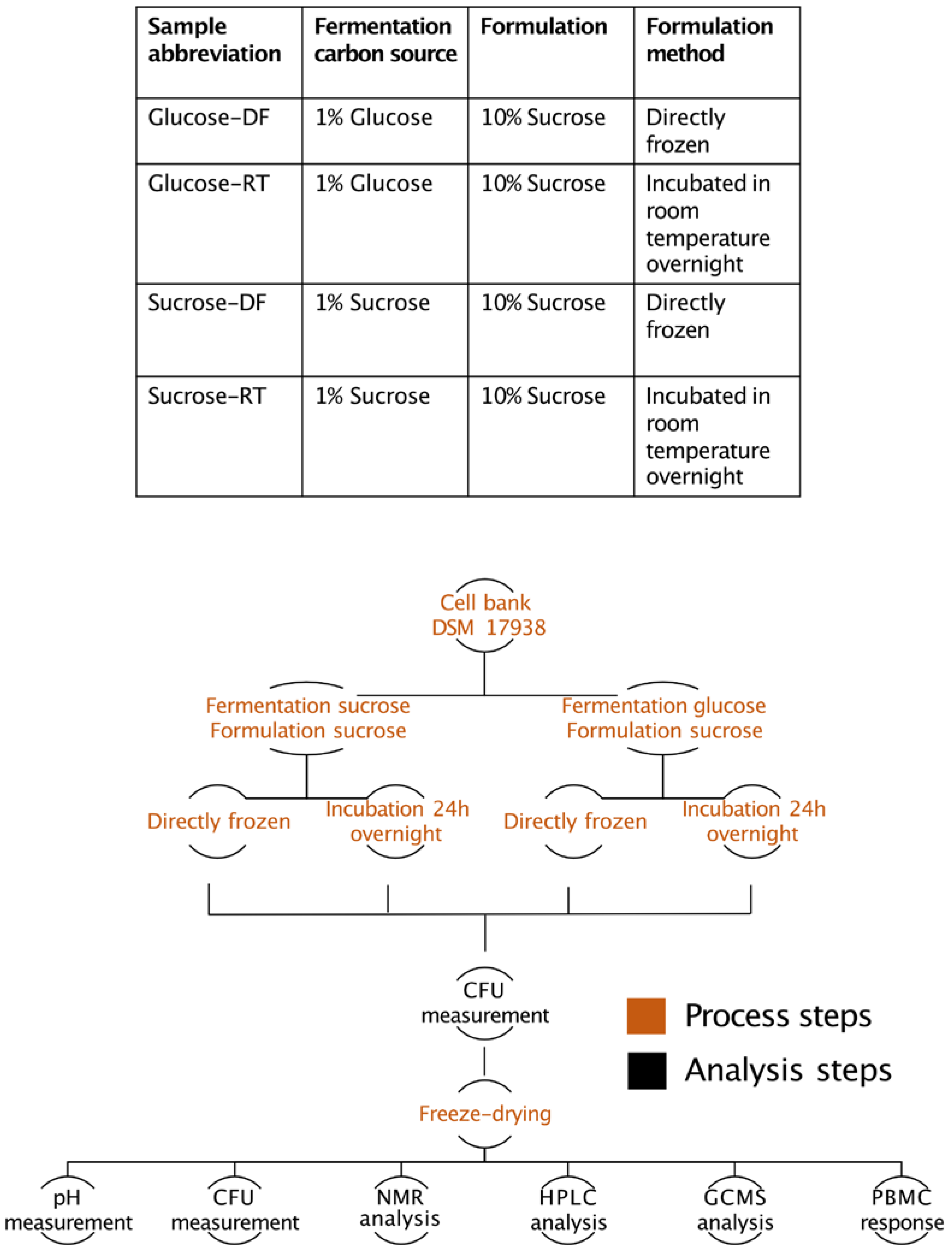
2.1. Fermentation, Lyoconversion, and Freeze-Drying
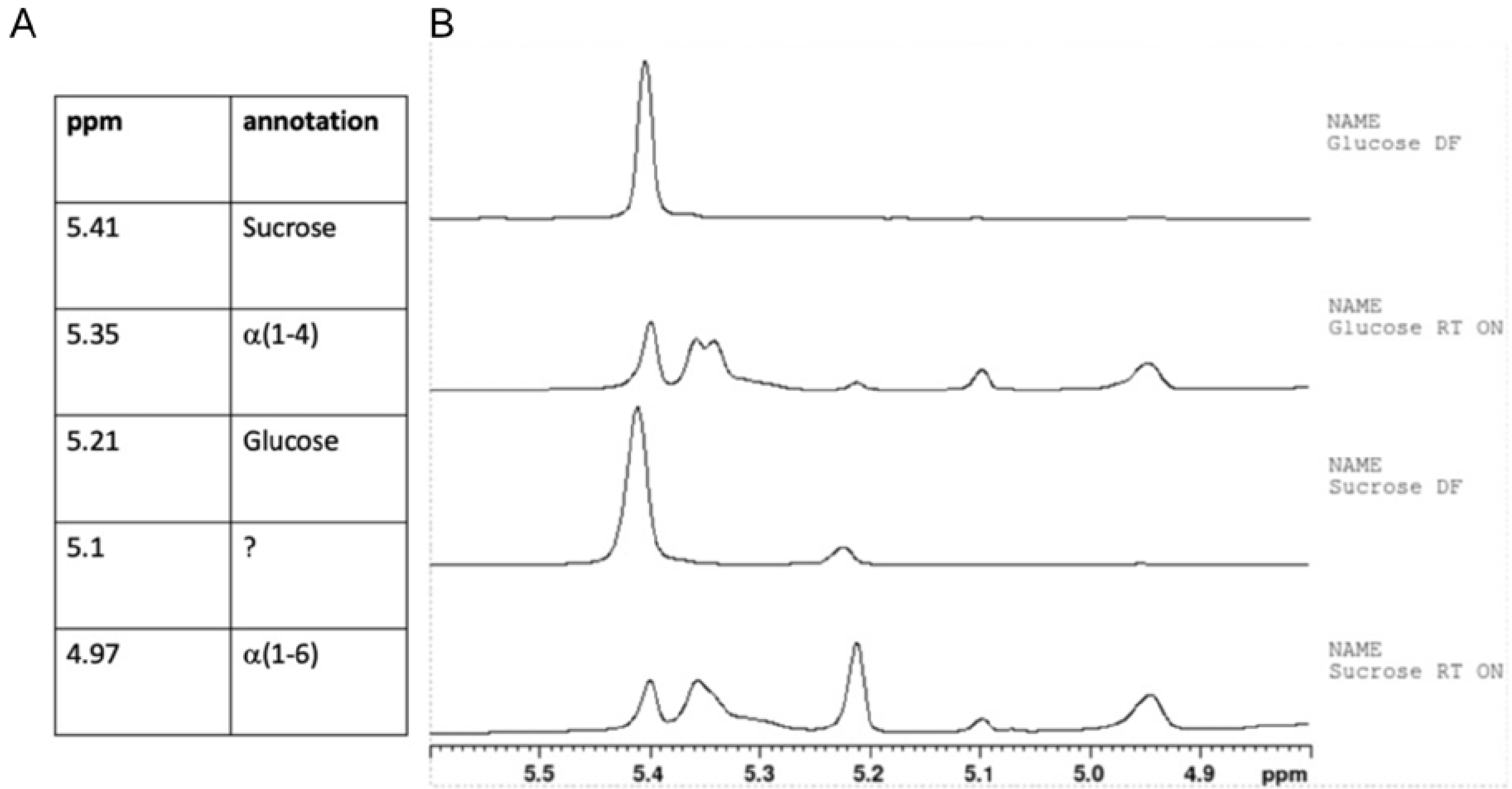
2.2. NMR Spectroscopy
2.3. Extraction of Exopolysaccharides
2.4. Cytokine Secretion in Peripheral Blood Mononuclear Cells
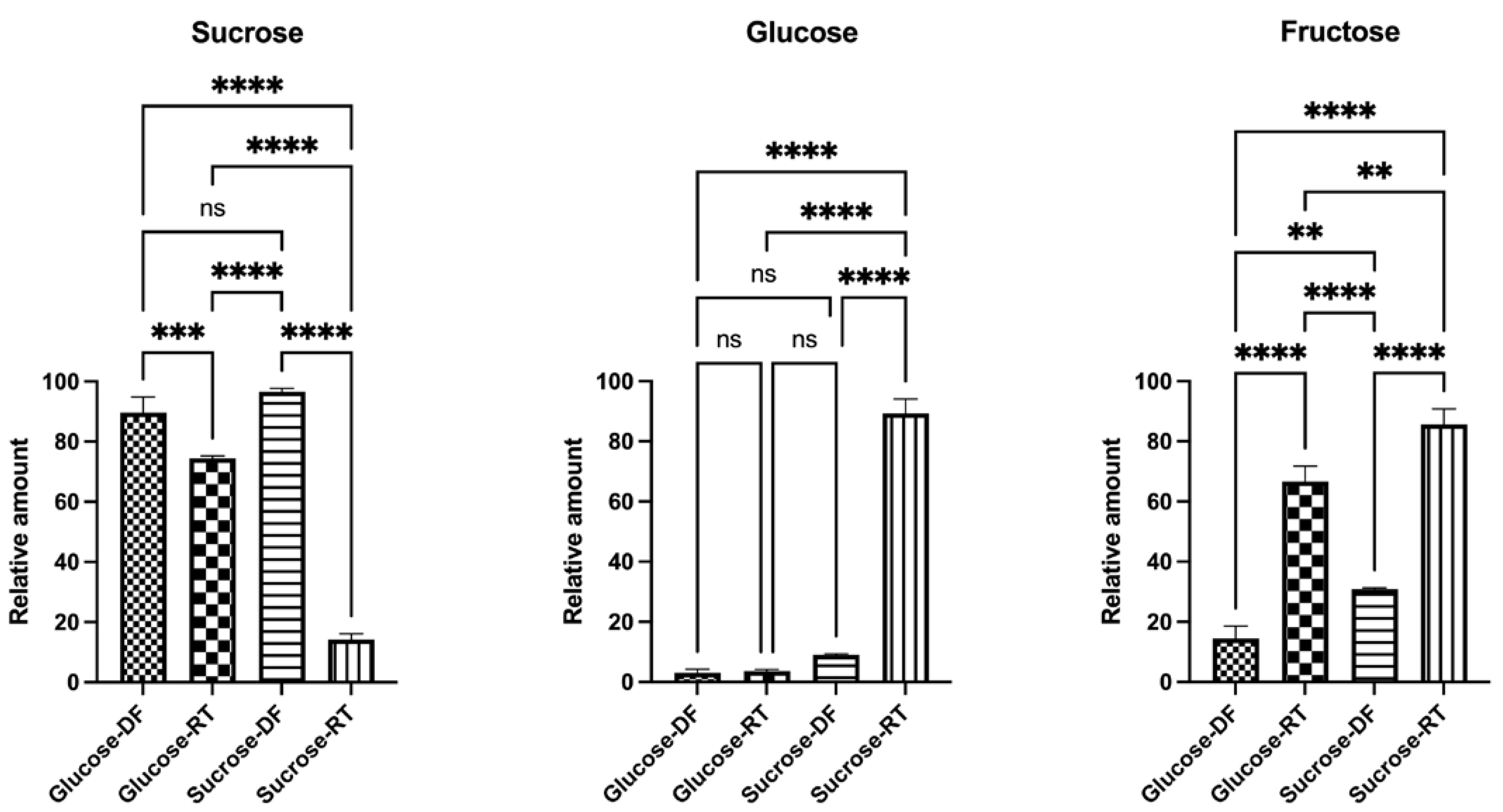
2.5. High-Performance Liquid Chromatography (HPLC)
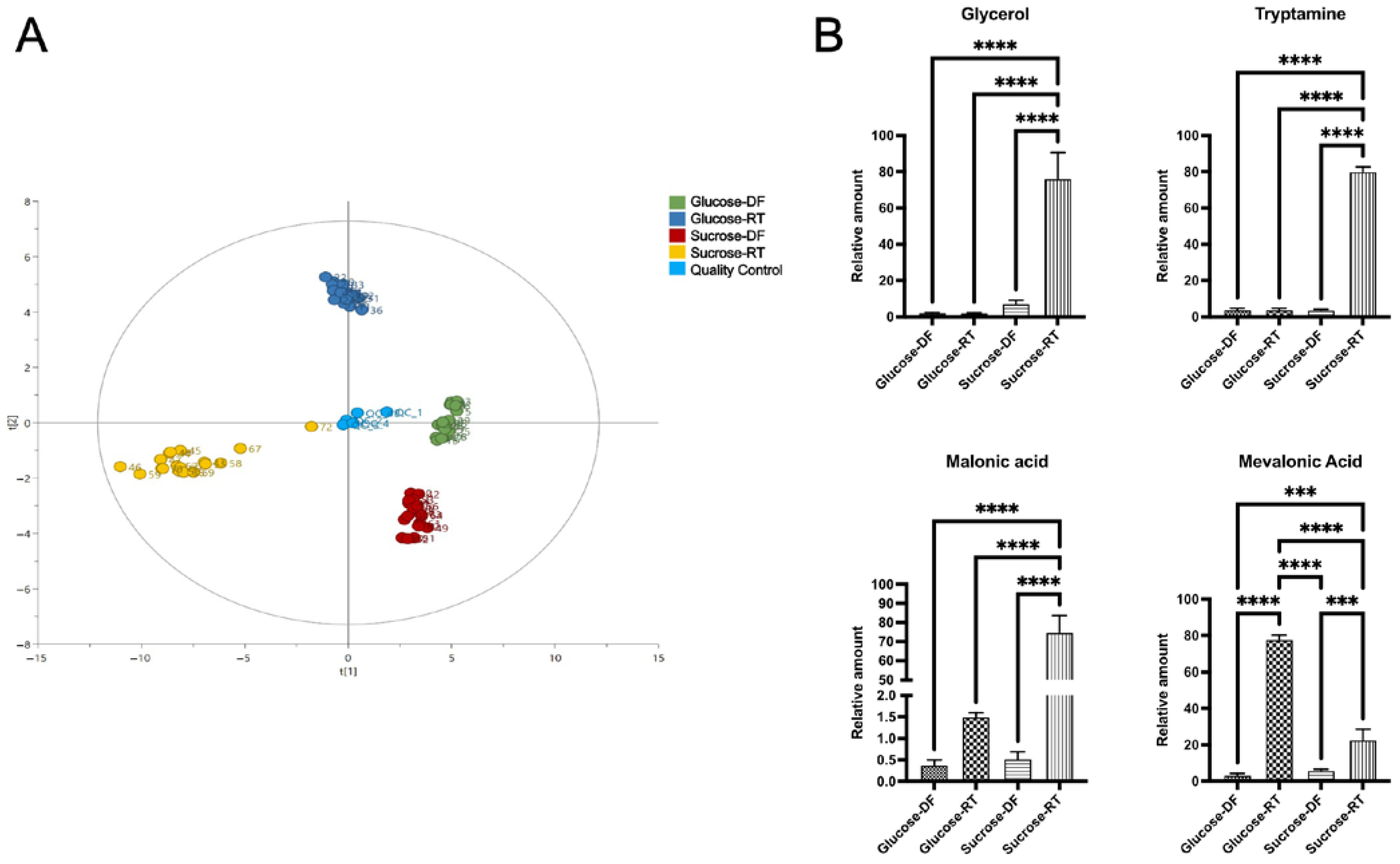
2.6. Gas Chromatography–Mass Spectrophotometry
2.7. Statistical Analysis
3. Results
3.1. L. reuteri DSM 17938 Produce an α-Glucan during Formulation
3.2. The Sugar Substrate Impacts Outcome of Lyoconversion
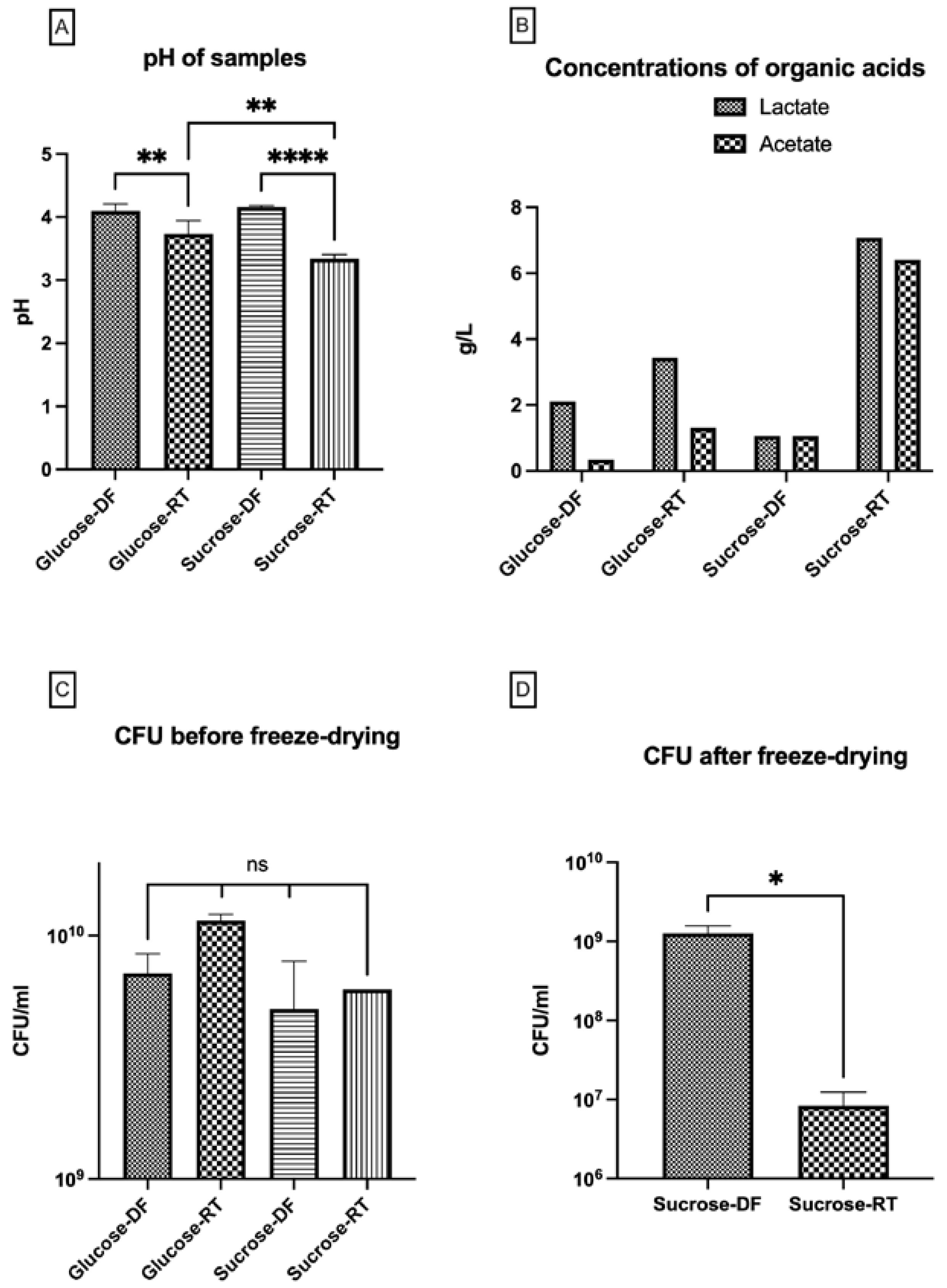
3.3. Lyoconversion Decreases pH, Increases Organic Acid Production, and Reduces Freeze-Drying Survival
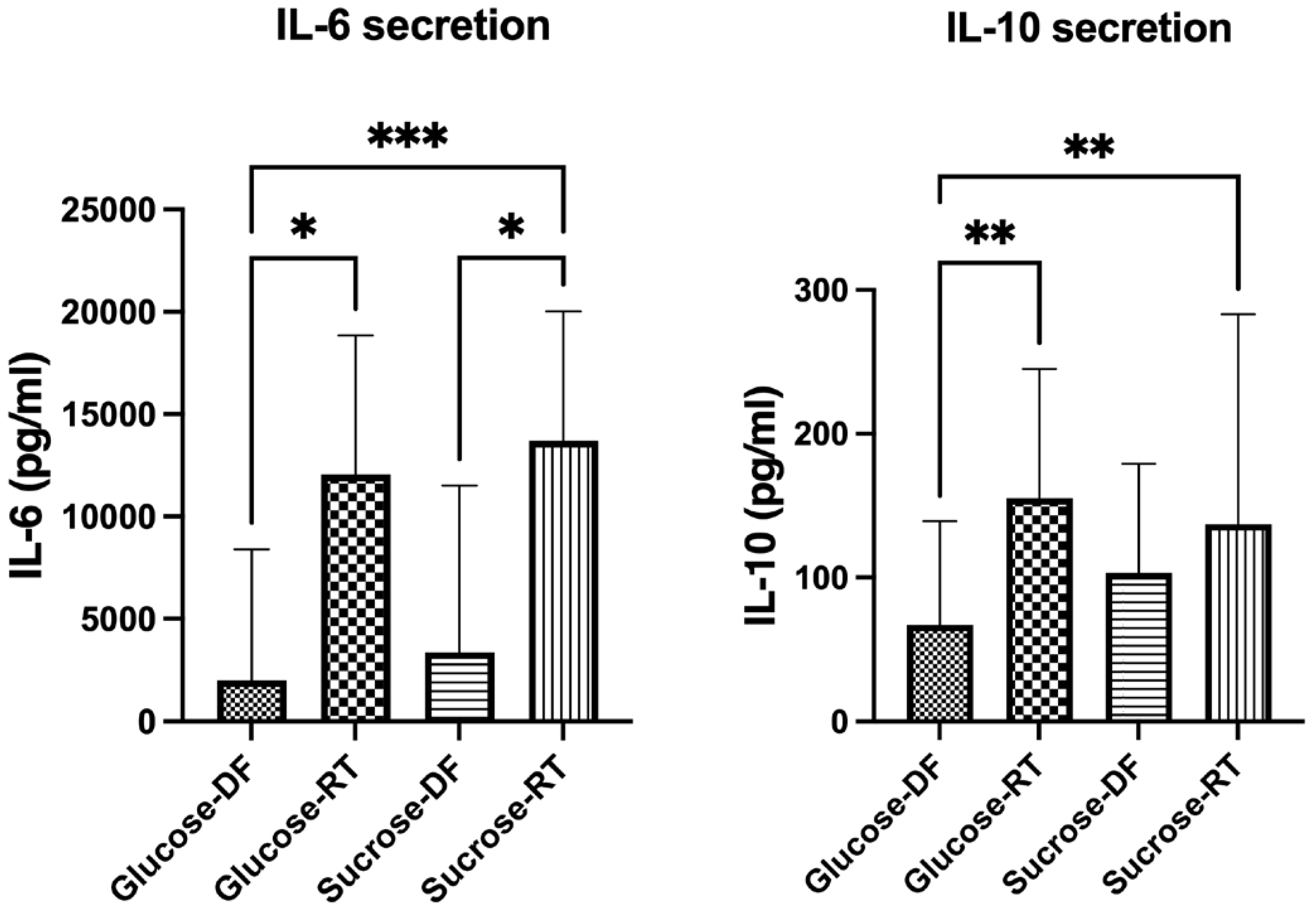
3.4. Lyoconverted Samples Increase Cytokine Secretion from PBMC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, M.; Szajewska, H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: A review of the current evidence. Eur. J. Pediatr. 2014, 173, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Forsgård, R.A.; Rode, J.; Lobenius-Palmér, K.; Kamm, A.; Patil, S.; Tacken, M.G.J.; Lentjes, M.A.H.; Axelsson, J.; Grompone, G.; Montgomery, S.; et al. Limosilactobacillus reuteri DSM 17938 supplementation and SARS-CoV-2 specific antibody response in healthy adults: A randomized, triple-blinded, placebo-controlled trial. Gut Microbes 2023, 15, 2229938. [Google Scholar] [CrossRef]
- Hunter, C.; Dimaguila, M.A.V.T.; Gal, P.; Wimmer, J.E.; Ransom, J.L.; Carlos, R.Q.; Smith, M.; Davanzo, C.C. Effect of routine probiotic, Lactobacillus reuteri DSM 17938, use on rates of necrotizing enterocolitis in neonates with birthweight < 1000 grams: A sequential analysis. BMC Pediatr. 2012, 12, 142–146. [Google Scholar]
- Pang, Y.; Ermann Lundberg, L.; Mata Forsberg, M.; Ahl, D.; Bysell, H.; Pallin, A.; Sverremark-Ekström, E.; Karlsson, R.; Jonsson, H.; Roos, S. Extracellular membrane vesicles from Limosilactobacillus reuteri strengthen the intestinal epithelial integrity, modulate cytokine responses and antagonize activation of TRPV1. Front. Microbiol. 2022, 13, 1032202. [Google Scholar] [CrossRef]
- van Hijum, S.A.F.T.; Szalowska, E.; van der Maarel, M.J.E.C.; Dijkhuizen, L. Biochemical and molecular characterization of a levansucrase from Lactobacillus reuteri. Microbiology 2004, 150, 621–630. [Google Scholar] [CrossRef]
- Kralj, S.; Stripling, E.; Sanders, P.; van Geel-Schutten, G.H.; Dijkhuizen, L. Highly hydrolytic reuteransucrase from probiotic Lactobacillus reuteri strain ATCC 55730. Appl. Environ. Microbiol. 2005, 71, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.R.K.; Mias, G.I. Streptococcus pneumoniae’s Virulence and Host Immunity: Aging, Diagnostics, and Prevention. Front. Immunol. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Kšonžeková, P.; Bystrický, P.; Vlčková, S.; Pätoprstý, V.; Pulzová, L.; Mudroňová, D.; Kubašková, T.; Csank, T.; Tkáčiková, L. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef]
- İspirli, H.; Şimşek, Ö.; Skory, C.; Sağdıç, O.; Dertli, E. Characterization of a 4,6-α-glucanotransferase from Lactobacillus reuteri E81 and production of malto-oligosaccharides with immune-modulatory roles. Int. J. Biol. Macromol. 2019, 124, 1213–1219. [Google Scholar] [CrossRef]
- Gerwig, G.J. Structural Analysis of Exopolysaccharides from Lactic Acid Bacteria. In Lactic Acid Bacteria; Methods and Protocols; Humana Press: New York, NY, USA, 2019; Volume 1887, pp. 67–84. [Google Scholar]
- van Leeuwen, S.S.; Kralj, S.; van Geel-Schutten, I.H.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P. Structural analysis of the α-d-glucan (EPS180) produced by the Lactobacillus reuteri strain 180 glucansucrase GTF180 enzyme. Carbohydr. Res. 2008, 343, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Arsköld, E.; Svensson, M.; Grage, H.; Roos, S.; Rådström, P.; van Niel, E.W.J. Environmental influences on exopolysaccharide formation in Lactobacillus reuteri ATCC 55730. Int. J. Food Microbiol. 2007, 116, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Rosander, A.; Connolly, E.; Roos, S. Removal of Antibiotic Resistance Gene-Carrying Plasmids from Lactobacillus reuteri ATCC 55730 and Characterization of the Resulting Daughter Strain, L. reuteri DSM 17938. Appl. Environ. Microbiol. 2008, 74, 6032–6040. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.C.; Axelsson, L.; Lindgren, S.E.; Dobrogosz, W.J. In Vitro Studies on Reuterin Synthesis by Lactobacillus reuteri. Microb. Ecol. Health Dis. 2009, 2, 137–144. [Google Scholar]
- Jiye, A.; Trygg, J.; Gullberg, J.; Johansson, A.I.; Chem, P.J.A. 2005 Extraction and GC/MS analysis of the human blood plasma metabolome. Anal. Chem. 2005, 77, 8086–8094. [Google Scholar]
- Schauer, N.; Steinhauser, D.; Strelkov, S.; Schomburg, D.; Allison, G.; Mortitz, T.; Lundgren, K.; Roessner-Tunali, U.; Forbes, M.G.; Willmitzer, L.; et al. GC–MS libraries for the rapid identification of metabolites in complex biological samples. FEBS Lett. 2005, 579, 1332–1337. [Google Scholar] [CrossRef]
- Gidley, M.J. Quantification of the structural features of starch polysaccharides by n.m.r. spectroscopy. Carbohydr. Res. 1985, 139, 85–93. [Google Scholar] [CrossRef]
- Van Geel-Schutten, G.H.; Faber, E.J.; Smit, E.; Bonting, K.; Smith, M.R.; Brink, B.T.; Kamerling, J.P.; Vliegenthart, J.F.; Dijkhuizen, L. Biochemical and structural characterization of the glucan and fructan exopolysaccharides synthesized by the lactobacillus reuteri wild-type strain and by mutant strains. Appl. Environ. Microbiol. 1999, 65, 3008–3014. [Google Scholar] [CrossRef]
- Cheng, H.N.; Neiss, T.G. Solution NMR Spectroscopy of Food Polysaccharides. Polym. Rev. 2012, 52, 81–114. [Google Scholar] [CrossRef]
- Meng, X.; Dobruchowska, J.M.; Pijning, T.; Gerwig, G.J.; Dijkhuizen, L. Synthesis of New Hyperbranched α-Glucans from Sucrose by Lactobacillus reuteri 180 Glucansucrase Mutants. J. Agric. Food Chem. 2016, 64, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Dertli, E.; Colquhoun, I.J.; Côté, G.L.; Le Gall, G.; Narbad, A. Structural analysis of the α-d-glucan produced by the sourdough isolate Lactobacillus brevis E25. Food Chem. 2018, 242, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Arsköld, E.; Lohmeier-Vogel, E.; Cao, R.; Roos, S.; Rådström, P.; van Niel, E.W.J. Phosphoketolase pathway dominates in Lactobacillus reuteri ATCC 55730 containing dual pathways for glycolysis. J. Bacteriol. 2008, 190, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ciszek-Lenda, M.; Nowak, B.; Sróttek, M.; Gamian, A.; Marcinkiewicz, J. Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37: Effects on the production of inflammatory mediators by mouse macrophages. Int. J. Exp. Pathol. 2011, 92, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Rabe, H.; Lundell, A.-C.; Sjöberg, F.; Ljung, A.; Strömbeck, A.; Gio-Batta, M.; Maglio, C.; Nordström, I.; Andersson, K.; Nookaew, I.; et al. Neonatal gut colonization by Bifidobacterium is associated with higher childhood cytokine responses. Gut Microbes 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. CRI 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Siaterlis, A.; Deepika, G.; Charalampopoulos, D. Effect of culture medium and cryoprotectants on the growth and survival of probiotic lactobacilli during freeze drying. Lett. Appl. Microbiol. 2009, 48, 295–301. [Google Scholar] [CrossRef]
- Monchois, V.; Willemot, R.M.; Monsan, P. Glucansucrases: Mechanism of action and structure-function relationships. FEMS Microbiol. Rev. 1999, 23, 131–151. [Google Scholar] [CrossRef]
- Nishimura, J. Probiotic characteristics and carbohydrate metabolism of Lactobacillus reuteri. Milk Sci. 2020, 69, 71–82. [Google Scholar]
- Kurtmann, L.; Skibsted, L.H.; Carlsen, C.U. Browning of freeze-dried probiotic bacteria cultures in relation to loss of viability during storage. J. Agric. Food Chem. 2009, 57, 6736–6741. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Nguyen, P.-T.; Nguyen, T.-T.-U.; Nguyen, T.-B.-N.; Bui, N.-B.; Nguyen, H.T. Efficacy of the incorporation between self-encapsulation and cryoprotectants on improving the freeze-dried survival of probiotic bacteria. J. Appl. Microbiol. 2022, 132, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Palmkron, S.B.; Bergenståhl, B.; Hall, S.; Håkansson, S.; Wahlgren, M.; Larsson, E.; Fureby, A.M. The Impact of Annealing Methods on the Encapsulating Structure and Storage-Stability of Freeze-Dried Pellets of Probiotic Bacteria. Pharm. Res. 2024, 41, 1671–1682. [Google Scholar] [CrossRef]
- Tsau, J.L.; Guffanti, A.A.; Montville, T.J. Conversion of Pyruvate to Acetoin Helps To Maintain pH Homeostasis in Lactobacillus plantarum. Appl. Environ. Microbiol. 1992, 58, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-J.; Huang, H.; Ouyang, P.-K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Zografi, G. The relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids. Pharm. Res. 1994, 11, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Waterland, M.; Janssen, P.; Anderson, R.; Singh, H. Importance of intact secondary protein structures of cell envelopes and glass transition temperature of the stabilization matrix on the storage stability of probiotics. Food Res. Int. 2019, 123, 198–207. [Google Scholar] [CrossRef]
- Pallin, A. Improving the functional properties of Lactobacillus reuteri. Acta Univ. Agric. Sueciae 2018, 2018, 41–82. [Google Scholar]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef]
- Nguyen, T.-L.-P.; Saulou-Bérion, C.; Delettre, J.; Béal, C. Culture conditions affect Lactobacillus reuteri DSM 17938 ability to perform glycerol bioconversion into 3-hydroxypropionic acid. J. Biosci. Bioeng. 2021, 131, 501–508. [Google Scholar] [CrossRef]
- Begley, M.; Bron, P.A.; Heuston, S.; Casey, P.G.; Englert, N.; Wiesner, J.; Jomaa, H.; Gahan, C.G.M.; Hill, C. Analysis of the isoprenoid biosynthesis pathways in Listeria monocytogenes reveals a role for the alternative 2-C-methyl-D-erythritol 4-phosphate pathway in murine infection. Infect. Immun. 2008, 76, 5392–5401. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.C.; Stanton, C.; Fitzgerald, G.F.; Daly, C.; Ross, R.P. Anhydrobiotics: The challenges of drying probiotic cultures. Food Chem. 2008, 106, 1406–1416. [Google Scholar] [CrossRef]
- Rao, N.S.; Ermann Lundberg, L.; Tomasson, J.; Tullberg, C.; Brink, D.P.; Palmkron, S.B.; van Niel, E.W.J.; Håkansson, S.; Carlquist, M. Non-inhibitory levels of oxygen during cultivation increase freeze-drying stress tolerance in Limosilactobacillus reuteri DSM 17938. Front. Microbiol. 2023, 14, 1152389. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Larsson, C.U.; Sawicki, R.; van Niel, E.W.J.; Roos, S.; Håkansson, S. Impact of the fermentation parameters pH and temperature on stress resilience of Lactobacillus reuteri DSM 17938. AMB Expr. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Filho, M.L.M.; Busanello, M.; Garcia, S. Optimization of the fermentation parameters for the growth of Lactobacillus in soymilk with okara flour. LWT 2016, 74, 456–464. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermann Lundberg, L.; Mata Forsberg, M.; Lemanczyk, J.; Sverremark-Ekström, E.; Sandström, C.; Roos, S.; Håkansson, S. Limosilactobacillus reuteri DSM 17938 Produce Bioactive Components during Formulation in Sucrose. Microorganisms 2024, 12, 2058. https://doi.org/10.3390/microorganisms12102058
Ermann Lundberg L, Mata Forsberg M, Lemanczyk J, Sverremark-Ekström E, Sandström C, Roos S, Håkansson S. Limosilactobacillus reuteri DSM 17938 Produce Bioactive Components during Formulation in Sucrose. Microorganisms. 2024; 12(10):2058. https://doi.org/10.3390/microorganisms12102058
Chicago/Turabian StyleErmann Lundberg, Ludwig, Manuel Mata Forsberg, James Lemanczyk, Eva Sverremark-Ekström, Corine Sandström, Stefan Roos, and Sebastian Håkansson. 2024. "Limosilactobacillus reuteri DSM 17938 Produce Bioactive Components during Formulation in Sucrose" Microorganisms 12, no. 10: 2058. https://doi.org/10.3390/microorganisms12102058
APA StyleErmann Lundberg, L., Mata Forsberg, M., Lemanczyk, J., Sverremark-Ekström, E., Sandström, C., Roos, S., & Håkansson, S. (2024). Limosilactobacillus reuteri DSM 17938 Produce Bioactive Components during Formulation in Sucrose. Microorganisms, 12(10), 2058. https://doi.org/10.3390/microorganisms12102058







