Abstract
Methicillin resistance, mediated by the mecA gene in staphylococci and mammaliicocci, has caused tremendous setbacks in the use of antibiotics in human and veterinary medicine due to its high potential of presenting the multidrug resistance (MDR) phenotype. Three other mec analogs exist, of which the mecC has evolutionary been associated with methicillin-resistant Staphylococcus aureus (MRSA) in wild animals, thus loosely referred to as the wild MRSA. In this study, we present an epidemiological review and genomic analysis of non-aureus staphylococci and mammaliicocci that carry the mecC-mediated methicillin resistance trait and determine whether this trait has any relevant link with the One Health niches. All previous studies (2007 till 2023) that described the mecC gene in non-aureus staphylococci and mammaliicocci were obtained from bibliometric databases, reviewed, and systematically analyzed to obtain the antimicrobial resistance (AMR) and virulence determinants, mobilome, and other genetic contents. Moreover, core genome single-nucleotide polymorphism analysis was used to assess the relatedness of these strains. Of the 533 articles analyzed, only 16 studies (on livestock, environmental samples, milk bulk tanks, and wild animals) were eligible for inclusion, of which 17 genomes from 6 studies were used for various in silico genetic analyses. Findings from this systematic review show that all mecC-carrying non-aureus staphylococci were resistant to only beta-lactam antibiotics and associated with the classical SCCmec XI of S. aureus LGA251. Similarly, two studies on wild animals reported mecC-carrying Mammaliicoccus stepanovicii associated with SCCmec XI. Nevertheless, most of the mecC-carrying Mammaliicoccus species presented an MDR phenotype (including linezolid) and carried the SCCmec-mecC hybrid associated with mecA. The phylogenetic analysis of the 17 genomes revealed close relatedness (<20 SNPs) and potential transmission of M. sciuri and M. lentus strains in livestock farms in Algeria, Tunisia, and Brazil. Furthermore, closely related M. sciuri strains from Austria, Brazil, and Tunisia (<40 SNPs) were identified. This systematic review enhances our comprehension of the epidemiology and genetic organization of mecC within the non-aureus staphylococci and mammaliicocci. It could be hypothesized that the mecC-carrying non-aureus staphylococci are evolutionarily related to the wild MRSA-mecC. The potential implications of clonal development of a lineage of mecA/mecC carrying strains across multiple dairy farms in a vast geographical region with the dissemination of MDR phenotype is envisaged. It was observed that most mecC-carrying non-aureus staphylococci and mammaliicocci were reported in mastitis cases. Therefore, veterinarians and veterinary microbiology laboratories must remain vigilant regarding the potential existence of mecA/mecC strains originating from mastitis as a potential niche for this resistance trait.
1. Introduction
The genera Staphylococcus and Mammaliicoccus are predominantly nasal and skin commensals in humans and most animal species [1,2,3]. However, they could be translocated to other parts of the human and animal body to cause clinical infections through the expressions of virulence genes [4]. The antimicrobial resistance (AMR) and virulence potential of staphylococci have long been elucidated in detail in S. aureus. However, non-aureus staphylococci and Mammaliicoccus species have recently been shown to carry critical AMR genes and virulence factors that have been hitherto exclusively reported in S. aureus [5,6,7]. In these regards, it is important to remark on the detection of linezolid resistance genes (cfr, optrA, and poxtA) and virulence determinants such as tst, luk-F/S-PV, eta, seb, sec, and sel in some non-aureus staphylococci and mammaliicocci [5,6,7,8,9]. AMR is a major global health challenge that needs a holistic “One Health” approach, for which Staphylococcus and certain Mammaliicoccus species serve as suitable bacteria models. This is because some species and lineages could “spill over” across multiple hosts, carry emergent resistance mechanisms or transfer critically important AMR zoonotically or anthropogenically [10]. Recently, studies have shown enormous interrelations of the wildlife–livestock interface in the transmission and maintenance of bacterial pathogens and AMR of public health concerns such as those caused by staphylococci and, by extension, mammaliicocci [11,12,13].
The presence of methicillin resistance and resistance to nearly all beta-lactams in staphylococci were historically linked to the acquisition of the mecA gene, which encodes the alternative penicillin-binding protein PBP2a [14]. However, the frequent association of methicillin resistance in staphylococci, mammaliicocci, and macrococci has now been attributed to the presence of other mec-type genes (mecB, mecC, and mecD) (Table 1). These genes also encode for penicillin-binding proteins (PBPs) that exhibit low affinity for beta-lactams [15].

Table 1.
Beta-lactam resistance as well as mobile genetic elements carrying these genes in staphylococci, mammaliicocci, and macrococci.
The mecA-mediated methicillin-resistant S. aureus (MRSA) exhibits a high prevalence on a global scale in human and multiple animal hosts, especially in pigs and dairy animals [10,26,27]. In 2007, an additional mec gene, known as mecC, was discovered to be associated with resistance to beta-lactam antibiotics during an epidemiological investigation of bovine mastitis [16,28]. The mecC gene, previously known as mecALGA251, is a mecA variant that shares 69% nucleotide identity and was initially reported in an S. aureus strain from a bovine sample [29]. Similar to mecA, mecC was discovered to be present inside the mobile genetic element (MGE) referred to as the staphylococcal cassette chromosome mec (SCCmec), which is inserted at the 3′ end of the orfX locus [29].
The SCCmec harboring mecC exhibited notable distinctions from previously identified types and was officially classified as SCCmec type XI [30,31]. In addition to its presence in cattle, mecC has been documented in MRSA strains from people throughout various European countries, as well as in a wide range of wild animal species as reviewed by Abdullahi et al. [27] and Lozano et al. [32]. Furthermore, mecC-carrying MRSA strains have also been demonstrated in river water and livestock such as sheep and goats in Spain and Tunisia [33].
The mecC allotype was subsequently discovered in Mammaliicoccus (previously Staphylococcus) sciuri, located downstream of the newly identified SCCmec type VII [34]. This hybrid SCCmec-mecC element consists of mecA and mecC regions organized within a class E mec complex (mecI-mecR, mecC-blaZ) [34]. It has been demonstrated a strong correlation between M. sciuri and the origin and assembly of the SCCmec element, especially for SCCmec type III [35]. Consequently, several previous investigations have shown multiple lines of evidence indicating that the mecA1 gene originated in M. sciuri encoding the PBPD [36]. Furthermore, Rolo et al. [35] provided evidence that M. sciuri species serve as an innate host and abundant reservoir for ccr, which is the most likely source of these recombinases for the formation of SCCmec [15]. Nevertheless, there are limited data regarding the origin and molecular epidemiology and the clinical importance of mecC-carrying mammaliicoccal strains. So far, M. sciuri has been found in environmental and animal samples [8,37,38] and has been associated with occasional infections in both animals and humans [39,40,41,42]. Previous research has demonstrated that mammaliicocci strains bearing mecA/mecC homologs exhibit the ability to excise both the hybrid SCCmec-mecC and SCCmec type XI from the chromosome [34]. Furthermore, these elements can be subsequently transmitted to more pathogenic staphylococci [43]. In this study, we present an epidemiological review and molecular analysis of non-aureus staphylococci and mammaliicocci that carry the mecC-mediated methicillin resistance trait and determine whether this trait has any relevant link with the One Health niches.
2. Methodology
2.1. Literature Search
A comprehensive literature review was conducted on the PubMed database using the following search terms: “methicillin”, “mecC CoNS”, “mecC methicillin”, “mecC-methicllin resistance”, “mecC mammaliicocci”, “mecC mastitis”, “mecC livestock”, “mecC dairy”, “mecC environment”, “mecC wild animal”, “mecC S. sciuri”, “mecC non-aureus”, “mecC human”, “mecC dog”, and “mecC cat”. Additional search engines such as Google Scholar, ScienceDirect, Scopus, and Web of Science were used to obtain all potentially eligible studies. The inclusion criteria encompassed articles that were published throughout the time frame of October 2007 to October 2023. Of the 533 hits, a total of 341 articles were removed, as they did not address non-aureus staphylococci, as indicated in Supplementary Figure S1. An additional 176 articles were omitted from the study, as they solely concentrated on the mecA-mediated methicillin resistance, inadequate methodology, or review papers. An evaluation was conducted on 16 studies that specifically examined mecC-carrying non-aureus staphylococci and mammaliicocci (Table 2). From these 16 articles, only 6 met the criteria for detailed genomic analyses, as indicated in Supplementary Figure S1 and Table 3.
2.2. Description of the mecC-Carrying Non-aureus Staphylococci and Mammaliicocci Strains and the Methodology Used in the Eligible Studies
The strains included in this analysis and obtained from the eligible studies (Table 2), encompassed a diverse range of subjects, including livestock suffering from mastitis, as well as specimens obtained from farms and wild animals. Non-aureus staphylococci and mammaliicocci from the eligible studies were obtained from several sources, including milk, teat, manure, soil, and skin samples. Following the collection of samples in these studies, they were subjected to cultivation; subsequently, their DNA was extracted for various gene amplifications, and whole-genome sequencing (in some studies). The disc diffusion method was commonly utilized in most studies to assess resistance to oxacillin and/or cefoxitin in antibiotic susceptibility tests. The genomic sequences were utilized to identify the mechanisms for methicillin resistance and other AMRs. Additionally, the genomes of the strains obtained from GenBank were used to determine the sequence types (STs), virulome, plasmids, SCCmec types, and other MGEs (Table 2).
2.3. Phylogenetic and In Silico Genomic Analysis
To determine the relatedness of the non-aureus staphylococci and mammaliicocci strains from the eligible studies, a web-based CSI phylogeny database (https://cge.food.dtu.dk/services/CSIPhylogeny/) (accessed on 10 September 2023) was used to obtain the SNPs by mapping the publicly available genomes of the 17 strains obtained from GenBank to a reference S. aureus LGA251 (accession number FR821779) with the default parameter, except for that the minimum distance between SNPs, which was disabled. The graphical data were added to the phylogenies using iTOL v.6.6 [44]. The sequence types (STs) were determined using MLST v.2.16 [45]. Virulence factors, plasmid replicons, and antimicrobial resistance genes were identified using PlasmidFinder, and Resfinder from the Center for Genomic Epidemiology. Moreover, other databases such as VFDB (http://www.mgc.ac.cn/VFs/main.htm (accessed on 12 September 2023) and CARD (https://card.mcmaster.ca/analyze/rgi) (accessed on 12 September 2023) were used to search for additional virulence and AMR genes. The genetic environment of the mecC gene from 10 non-aureus staphylococci and mammaliicocci strains (one per species per study) was illustrated in comparison with the S. aureus LGA251 strain (accession number FR821779). Computations and graphical designs were performed using EasyFig (https://mjsull.github.io/Easyfig/) (accessed on 28 October 2023) and Inkscape software version 1.3.2. (https://inkscape.org/) (accessed on 28 October 2023).

Table 2.
AMR, virulence genes, genetic lineages, and mobile genetic elements in S. aureus LGA251, and in mecC-carrying non-aureus staphylococci and mammaliicocci.
Table 2.
AMR, virulence genes, genetic lineages, and mobile genetic elements in S. aureus LGA251, and in mecC-carrying non-aureus staphylococci and mammaliicocci.
| Authors | Country | Source of the Strains | Bacterial Species (Number) | AMR Phenotype | Molecular Assays | AMR Genes | Plasmid Reps (Associated AMR) | Genetic Lineage | SCCmec Type | Other MGEs |
|---|---|---|---|---|---|---|---|---|---|---|
| García-Álvarez et al. [16] | UK | Bulk milk | S. aureus (1) | PEN, OXA | WGS | blaZ, mecC | ND | ST425 | XI | None |
| Harrison et al. [22] | UK | Bovine milk | S. xylosus (1) | PEN, OXA | WGS | blaZ, mecC | NT | NT | XI | Tn554-like |
| MacFadyen et al. [46] | UK | Bulk milk tank | S. xylosus (1) | PEN, OXA | WGS | blaZ, mecC | NT | NT | XI | ACME |
| Paterson et al. [40] | UK | Bovine milk tank | M. sciuri (11) | PEN, OXA, CLI, TET, STR | WGS | blaZ, mecC, salA, tet(K), str | NT | NA | SCCmec-mecC hybrid | None |
| Harrison et al. [34] | UK | Bovine | M. sciuri (2) | PEN, OXA, FOX, CHL, CLI, TET, STR, FUS | WGS | blaZ, mecA, mecA1, mecC, fexA, ermC, tet(K), str | NT | NA | SCCmec-mecC hybrid | None |
| Dhaouad et al. [39] | Tunisia | Calves, cow, horses, rabbit | M. sciuri (6) | PEN, FOX, CHL, ERY, CLI, GEN, TOB, STR, TET, FUS | WGS | blaZ, mecA, mecA1, mecC, fexA, erm45, ermB, salA, aac6′-aph2″, ant4, str, dfrK, tet(K), tet(L), fusB/C | rep22 (ant4′, dfrK, tet(L)), repUS76 (ermB) | ST38 | SCCmec-mecC hybrid | Tn558 (fexA) |
| de Moura et al. [47] | Brazil | Bovine | M. sciuri (2) | PEN, FOX, CLI, TET, STR | WGS | blaZ, mecA, mecA1, mecC, salA, str, tet(K) | rep7a (str) | ST71 | SCCmec-mecC hybrid | None |
| Aslantaş [48] | Turkey | Broilers | M. sciuri (7) | PEN, FOX, ERY, CLI, TET, GEN, SXT | PCR | blaZ, mecA, mecC, ermA, lnuA, tet(K), tetM, aac6-aph2 | NT | NT | III (by PCR) | NT |
| Belhout et al. [49] | Algeria | Camels | M. lentus (5) | PEN, FOX, STR, ERY, CLI, TET | WGS | blaZ, mecA, mecC, str, ermB, mphC, tet(K) | rep7a (tet(K), str) | ND | SCCmec-mecC hybrid | None |
| Srednik et al. [50] | Argentina | Bovine | S. saprophyticus (1) | PE, OXA, FOX | PCR | blaZ, mecC | NT | NT | NT | NT |
| Małyszko et al. [23] | Poland | Shrew (small mammal) | S. saprophyticus (1) | PEN, OXA | PCR | blaZ, mecC | NT | NT | NT | NT |
| Loncaric et al. [51] | Austria | Eurasian lynx | M. stepanovicii (1) | PEN, OXA | PCR | blaZ, mecC | NT | NT | NT | NT |
| Semmler et al. [52] | Germany | Wild vole | M. stepanovicii (1) | PEN, OXA | WGS | blaZ, mecC | NT | ND | XI | None |
| Lancoric et al. [53] | Austria | Wild and domestic animals | M. stepanovicii, S. caprae, S. warneri, S. xylosus, and M. sciuri | a. M. sciuri (PEN, OXA, FOX, GEN, TET, ERY, CLI, CHL, SXT) b. M. stepanovicii, S. caprae, S. xylosus, S. warneri (PEN, FOX) | PCR, WGS | a. blaZ, mecA, mecA1, mecC ant4′, tet(M), ermB, cfr, fexA in M. sciuri b. blaZ, mecC in others | ND | M. sciuri (ST22) | a. SCCmec-mecC hybrid in M. sciuri b. XI in others | None |
| Pantůček et al. [54] | The Czech Republic | Stone fragments/sandy soil | S. edaphicus sp. nov. (1) | PEN, OXA | WGS | blaZ, mecC | NT | ND | XI | None |
| Dhaouad et al. [38] | Tunisia | Bovine mastitis and manure | M. sciuri | PEN, OXA, FOX, TET | PCR | mecA, mecC, blaZ, tet(K) | NT | NT | Non-typeable | NT |
| Abdullahi et al. [37] | Spain | Nestling of white stork | M. lentus | PEN, FOX, CLI, TET | PCR | blaZ, mecA, mecC, mphC, tet(K) | NT | NT | blaZ-SCCmec XI | NT |
Abbreviations: PCR: polymerase chain reaction; WGS: whole-genome sequencing; NT: not tested; NA: not applicable; ST: sequence type: CLI: clindamycin; CHL: chloramphenicol; CIP: ciprofloxacin; ERY: erythromycin; FOX: cefoxitin; FUS: fusidic acid; GEN: gentamicin; OXA: oxacillin; PEN: penicillin; TET: tetracycline; TOB: tobramycin; STR: streptomycin; SXT: sulfamethoxazole–trimethoprim.

Table 3.
Species and sources of genomes used for the phylogenomic analyses in this review.
Table 3.
Species and sources of genomes used for the phylogenomic analyses in this review.
| Authors | Country | Strain | GenBank Accession Number |
|---|---|---|---|
| García-Álvarez et al. [16] | UK | S. aureusLGA251 | FR821779 |
| Dhaouad et al. [39] | Tunisia | M. sciuri | SRR20693405 SRR20693403 SRR20693382 SRR20693383 SRR20693384 |
| Paterson et al. [40] | UK | M. sciuri | ERR3350388 |
| Lancoric et al. [53] | Austria | S. xylosus S. warneri M. scuiri | SRR8494495 SRR8494496 SRR8494497 |
| Pantůček et al. [54] | The Czech Republic | S. edaphicus | GCA 002614725 |
| de Moura et al. [47] | Brazil | M. sciuri | GCA 030250115.1 GCA 030250065.1 |
| Belhout et al. [49] | Algeria | M. lentus | GCA 030013965.1 GCA 030012945.1 GCA 030012925.1 GCA 030012985.1 |
3. Findings and Discussion
3.1. SCCmec and Its Classification System in Methicillin-Resistance Trait
SCCmec typing was developed during the 2000s and has since been utilized as a valuable tool in studying the molecular epidemiology of methicillin-resistant staphylococci and investigating the evolution of various Staphylococcus species [31]. Molecular cloning and conventional sequencing techniques have been employed to confirm the existence and arrangement of a newly identified SCCmec type. In practical applications, PCR-based approaches have been widely utilized for the identification of SCCmec, offering convenience and efficiency over an extended period [31]. Moreover, the utilization of whole-genome sequencing has been extensively employed, leading to the recent identification of diverse SCCmec and analogous structures across other species [31,55]. Upon the discovery that the mecA gene was widely distributed across several staphylococcal species, a hypothesis emerged suggesting that mecA might be harbored on a MGE capable of horizontal transmission between staphylococcal species [56]. For the mecC gene, no study has elucidated the potential for its transfer within species of the Staphylococcus and Mammaliicoccus genera through SCCmec elements.
As of now, fourteen distinct types of SCCmec have been documented. These types are further categorized into broad groups [31]. The size of the SCCmec elements varies from 21 to 82 thousand nucleotides [57]. The typical configuration of SCCmec cassettes encompasses five distinct sections. The categorization of SCCmec into distinct types is determined by the specific ccr chromosomal recombinase gene complex, namely ccrA, ccrB, and ccrC [57]. The classification of the mec gene complex also represents a significant factor in the division of SCCmec. Several distinct classes can be identified, including A, B, B2, C1, C2, D, and E. The various classes exhibit variations in the extent of mecI-mecR gene deletion, as well as the relative positioning and distance from the entire or truncated IS431, IS1182, and IS1272 [57]. The categorization of SCCmec subtypes is determined by the subclasses of the mec gene complex and the composition of the J1, J2, and J3 regions [31]. The mec gene complex is composed of mecA or mecC, their regulatory genes, and the accompanying insertion sequences [31]. Currently, five classes of the mec gene complex have been described [31].
3.2. The Mammaliicoccus Genus, a Recent Offshoot from Staphylococcus
The taxonomic characterization of Mammaliicoccus is derived from the existing data presented by Madhaiyan et al. [2]. The cellular composition consists of Gram-positive, nonmotile, non-spore-forming cocci, which are observed in singular form, as well as in pairs and irregular clusters. These organisms demonstrate the ability to develop under aerobic conditions, as well as under facultative anaerobic conditions. The tested samples exhibited good catalase activity, along with varying levels of oxidase activity. According to Madhaiyan et al. [2], the DNA G+C content (mol%) varies between 31.6 and 35.7, while the genome size spans from 2.44 to 2.81 Mbp. The aforementioned description pertains to M. sciuri comb. nov., which serves as the designated type species. The differentiation of the genus from Staphylococcus was achieved by the utilization of various analytical techniques, including the examination of 16S rRNA gene sequences, the construction of phylogenetic trees using whole-genome data, and the assessment of overall genome-related indices. These former Staphylococcus species include M. fleurettii, M. lentus, M. sciuri, M. stepanovicii, and M. vitulinus [2].
3.3. Ecology of mecC Gene in Non-aureus Staphylococci and Mammaliicoccus
The detection of the hybrid SCCmec-mecC in few cases in methicillin-resistant M. sciuri obtained in two different studies from bovine milk [34,40] indicates that the prevalence of this genetic feature in M. sciuri may be more extensive than previously known. Notwithstanding, the mecC gene has been detected in several non-aureus staphylococci and mammaliicocci in Europe, Africa, America, and Turkey (Figure 1); these include M. lentus, S. xylosus, M. stepanovicii, S. caprae, and S. warneri. Remarkably, most of these mecC-carrying strains were identified from dairy animals. Of the 15 studies that reported the detection of the mecC gene in non-aureus staphylococci and Mammaliicoccus, the most frequently identified species were M. scuiri and S. xylosus. The detection of mecC carrying-M. sciuri in both manure and milk samples suggests that contamination may have occurred due to the mammary secretions of cows suffering from mastitis [38,58]. Ecologically, mecC-carrying S. xylosus has been detected in fermented food products such as sausage [59,60] and cheese [61], thus indicating a potential pathway for the transfer of mecC and other resistance genes from the environment or animal product (such as bovine milk) contaminated with bacteria carrying these AMR genes [22,62].
As most mecC-carrying non-aureus staphylococci and Mammaliicoccus are associated with livestock, especially dairy animals, these strains could exert negative impacts on livestock’s health, production, and public health as in the case of bovine mastitis that causes a decline in quality and quantity of milk and milk product [63,64,65]. Moreover, contaminated milk may cause gastroenteritis in humans when they consume dairy products contaminated with mecC-carrying non-aureus staphylococci or Mammaliicoccus that elaborate virulence factors such as the icaABCD biofilm genes [66]. It has been shown that biofilm production could exponentially facilitate the persistence of AMR in bacteria [66]. Thus, biofilms during infections and contamination of dairy products can cause public health concerns from veterinary, food safety, and medical standpoints.
Tracing the origin of mecC-carrying non-aureus staphylococci and mammaliicocci in dairy animals could be difficult, but it could be hypothesized that this methicillin resistance trait might have been acquired from wild animals’ secretions containing the mecC gene, as these hosts are the major and natural reservoirs of mecC-mediated MRSA [26]. Interestingly, two of the three studies on wild animals reported mecC-carrying M. stepanovicii in SCCmec XI. However, the other was a mecA/mecC-carrying M. lentus from a nestling stork whose parent foraged in landfills that could have been contaminated by livestock pasture and feces [37]. In this regard, genomic-based surveillance has become necessary to understand the potential transmission of mecC gene from MRSA to non-aureus staphylococci and mammaliicocci in the same micro-niches or ecosystems.
The predominance of M. sciuri and S. xylosus may be better understood by considering their ability to adapt to various ecological environments and among them the teat canal of dairy animals [67]. The organism’s capacity to inhabit both living and nonliving surfaces is likely ascribed to its capability to form a biofilm and the existence of genes linked to ecological adaptation [66]. These bacteria have widely been recognized as nonpathogenic commensal, with a limited number of documented cases associating them with diseases. In contrast, it is important to highlight that S. saprophyticus, which exhibits the most closely related evolutionary lineage to S. xylosus, possesses considerable significance as an opportunistic pathogen [68]. Specifically, S. saprophyticus contracted from contaminated food has long been implicated in urinary tract infections in young teenagers [68,69]. Moreover, M. lentus and M. sciuri are considered etiological agents of exudative epidermitis with zoonotic potentials [70]. Much more recently, whole-genome data of non-aureus staphylococci species have led to the identification and characterization of numerous putative virulence factors [71,72,73].
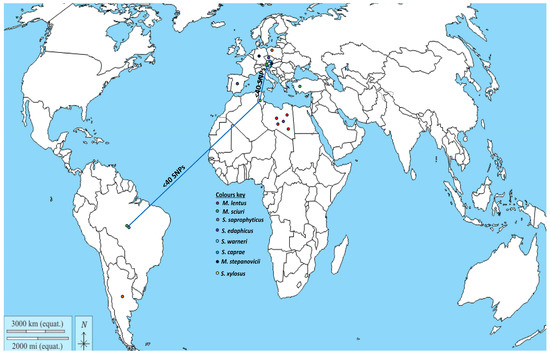
Figure 1.
Geographical distribution of non-aureus staphylococci and Mammaliicoccus species carrying the mecC gene (data obtained from References [22,23,34,37,38,39,40,46,47,48,49,50,51,52,53,54]). NB. The blue connecting line shows countries with genetically related M. sciuri strains.
The finding of a beta-lactam-resistant S. edaphicus strain from an antarctic environment sample showed that the mecC gene located between a pseudo-staphylococcus cassette chromosome mec (ψSCCmecP5085) and other SCCs implies the integration and exchange of foreign DNA [54]. It has been shown that MecC protein exhibits enhanced stability and activity at lower temperatures in comparison to the MecA protein [74]. This phenomenon may provide an evolutionary advantage in mitigating the prevalence of beta-lactam producers in arctic habitats.
3.4. Genetic Environment of the mecC in Staphylococcus and Mammaliicoccus Species
From our in silico analysis of the environment of mecC gene of all Mammaliicoccus species, it appears that this gene is encoded within a hybrid SCCmec element comprising mecA encoding SCCmec type VII [40,47,49]. This is very different from all other mecC-carrying non-aureus staphylococci, which were all in SCCmec type XI (Figure 2). Specifically, the analysis of 10 mecC-carrying non-aureus staphylococci and Mammaliicoccus species showed that all except S. xylosus, M. stepanovicii, S. warneri, S. caprae, and S. edaphicus carried a hybrid SCCmec-mecC (Figure 2). The SCCmec-mecC hybrid consists of a class E mec complex (mecI-mecR1-mecC1-blaZ) located immediately downstream of a SCCmec type VII element (Figure 2). Most of the cassettes comprise mecA/mecI/mecR2 and cadD/cadA/cadC (Figure 2). The mecC gene of the S. xylosus, M. stepanovicii, S. warneri, S. caprae, and S. edaphicus strains was very similar to SCCmec type XI, a classical type that was first found in S. aureus LGA251 (accession number FR821779). Perhaps, this could be because only the mecC gene was related to the methicillin resistance in these strains. Due to the high similarity (>98%) in the environment of the mecC of these strains with that of the reference S. aureus LGA251, it could be hypothesized that this gene might have been transferred to the non-aureus staphylococci through SCCmec XI by horizontal gene transfer (HGT), especially as both mecC-MRSA and mecC-carrying non-aureus staphylococci were reported in the study of Loncaric et al. [53].
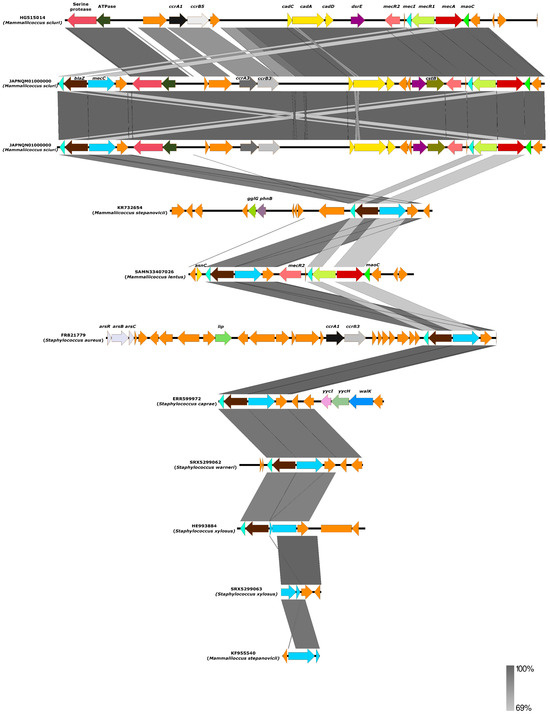
Figure 2.
The environment of the mecC gene of ten non-aureus staphylococci and Mammaliicoccus species compared with previously described S. aureus LGA251 (accession number FR821779). The percentage of identity and scale bar legends are presented on the right side of the image.
It is important to mention that using genome sequences on curated web pipelines could provide an unspecific and incorrect SCCmec type (in most cases SCCmec type III), which could be due to recombination events between the SCCmec type III (intrinsic for most M. lentus and M. sciuri) of the mecA gene and SCCmec type XI of the mecC to produce the SCCmec-mecC hybrid. In this regard, there is a need for caution in using PCR-based assays to detect SCCmec types in mecC-carrying mammaliicocci. Particularly, the intrinsic SCCmec type III or blaZ-SCCmec XI fragment in mammaliicocci could appear PCR-positive. This could be the case of the findings of Abdullahi et al. [37] and Aslantaş [48]. Thus, in silico and computational analyses of mecA/mecC genes from whole-genome sequences of mammaliicocci are necessary to deduce their correct SCCmec type.
3.5. Comparison of AMR Rates in mecC-Carrying S. aureus and Non-aureus Staphylococci and Mammaliicocci
Contrary to the notion that most mecC-carrying MRSA present low-level AMR and rarely present an MDR phenotype, most of the mecC-carrying mammaliicocci presented an MDR phenotype, and AMR genes of clinical relevance. This suggests that the acquisition of other non-beta-lactam resistance genes in these strains is likely to occur with notable frequency. Specifically, many mecC-carrying M. sciuri strains exhibited the highest frequencies of resistance to erythromycin, clindamycin, tetracycline, chloramphenicol, and trimethoprim–sulfamethoxazole (Table 2).
It is important to mention that the majority of mammaliicocci strains exhibit resistance to several clinically relevant AMRs located in plasmids and transposons, especially tet(L), ant4′, ermB, str, fexA, and dfrK genes. Moreover, the presence of M. sciuri strains from a sheep and a goat carrying the cfr gene further highlights the potential of mecC-carrying M. sciuri to carry and transmit critical AMR. It is noteworthy to remark that the cfr gene, responsible for encoding a methyltransferase enzyme that alters the A2503 location of the 23S ribosomal RNA, was initially identified in a calf-derived strain of M. sciuri in the year 2000 [75]. The cfr gene provides resistance to multiple classes of antibiotics, including lincosamides, streptogramin A, phenicols, linezolid, and pleuromutilins [75], especially in staphylococci [7].
It has been observed that fexA gene that encodes for chloramphenicol resistance could co-select the cfr gene and other linezolid resistance genes in staphylococci and mammaliicocci, especially in livestock [5,7,10,76]. This shows that the persistent use of florfenicol (a derivative of chloramphenicol) in livestock farms could have encouraged the re-emergence of cfr-mediated linezolid resistance in many Gram-positive bacteria [7]. Tetracycline and erythromycin are frequently employed in veterinary medicine and their usage may potentially account for the elevated rates of resistance. Contrary to these observations, all the mecC-carrying non-aureus staphylococci did not present an MDR phenotype, a feature that is closely similar to the mecC-carrying-MRSA. This further supports the hypothesis that mecC-carrying non-aureus staphylococci could have similar evolutionary origins of SCCmec type XI and low-level resistance to non-beta-lactams.
3.6. Phylogenomic Relatedness of mecC-Carrying Non-aureus Staphylococci and Mammaliiococci
Mapping of the assembled genomes of the 17 mecC-carrying non-aureus staphylococci and mammaliicocci with the reference S. aureus LGA251 indicated three distinct clusters (Figure 3). Of these, two contained two S. xylosus strains from the UK (cluster 1), four M. lentus strains from Tunisia (cluster 2), and eight M. sciuri strains from Austria, Tunisia, and Brazil (cluster 3). The remaining strains (M. sciuri-ERR3350388, S. warneri, and S. ediphicus) existed as standalone on the tree (with wide SNP difference from other strains) (Supplementary Table S1, Figure 3).
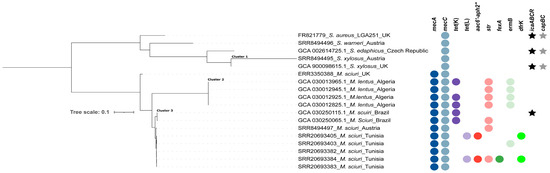
Figure 3.
Phylogenomic tree based on core genome SNP analysis of 17 non-aureus staphylococci and mammaliicocci from six countries. The presence of AMR genes is indicated by filled circles, while the icaABCR operon and capBC genes are indicated by filled stars.
Analysis of a midpoint-rooted phylogenomic tree of the three clusters confirmed the close relatedness (<20 SNPs) and potential transmission of mammaliicoccal strains in livestock farms, as in the case of M. lentus in Algerian camels and M. sciuri from different types of livestock in Tunisia and Brazil (Supplementary Table S1, Figure 3). Moreover, phylogenetic analysis further showed the genetic proximity (<40 SNPs) of M. sciuri strains from Austria, Brazil, and Tunisia (Figure 3). These findings highlight the intercontinental circulation of related M. sciuri strains between various livestock species, as confirmed by the phylogenetic analysis (Figure 3). However, further studies are important to elucidate the pathway of transmission of the genetically related strains to fully understand the factors that facilitated their presence in these countries.
4. Conclusions
This systematic review enhances our comprehension of the epidemiology and genetic organization of mecC within the non-aureus staphylococci and mammaliicocci. From our in silico analyses of the mecC gene, distinct variation in the SCCmec elements of non-aureus staphylococci from other (carrying SCCmec-mecC) hybrids tends to be genus-specific. Furthermore, utilizing core genome phylogenetic analysis, it was determined that the mecA/mecC cassette has been acquired by non-aureus staphylococci and mammaliicocci on separate occasions. The potential implications of clonal development of a lineage of mecA/mecC carrying strains across multiple dairy farms in a vast geographical region with the dissemination of the MDR phenotype is envisaged.
It was observed that most mecC-carrying non-aureus staphylococci and mammaliicocci were detected in mastitis cases. Therefore, veterinarians and veterinary microbiology laboratories must remain vigilant regarding the potential existence of mecA/mecC strains originating from mastitis as a potential niche for this resistance trait.
In summary, enhancing genome-based surveillance of mecC-carrying non-aureus staphylococci and mammaliicocci is vital to ascertaining their origins and impact on human and animal health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12010066/s1, Figure S1: Identification and selection flowchart of articles on the mecC-carrying non-aureus staphylococci and mammaliicocci; Table S1: SNPs matrix of 17 genomes of mecC-carrying non-aureus staphylococci and mammaliicocci.
Author Contributions
Conceptualization: I.N.A. and C.T.; methodology: I.N.A. and J.L.-F.; software analysis: I.N.A., J.L.-F., I.T. and Y.U.; validation: C.T., I.N.A., J.L.-F., I.T., C.G.-A., A.A., Y.U., R.C.R., M.Z. and C.L.; formal analysis: I.N.A., C.T., J.L.-F., C.G.-A., A.A., I.T., Y.U., M.Z. and C.L.; data curation: I.N.A., J.L.-F., I.T., R.C.R. and Y.U.; writing—original draft preparation, I.N.A.; writing—review and editing: C.T., I.N.A., M.Z., R.C.R., I.N.A., C.G.-A., A.A., J.L.-F., I.T., Y.U. and C.L.; supervision: C.T. and C.L.; project administration: C.T.; funding acquisition: C.T., M.Z. and I.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project PID2019-106158RB-I00 of the MCIN/AEI/10.13039/501100011033 of Spain. Also, it received funding from the European Union’s H2020 research and innovation program under the Marie Sklodowska-Curie grant agreement N° 801586.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Szczuka, E.; Wesołowska, M.; Krawiec, A.; Kosicki, J.Z. Staphylococcal species composition in the skin microbiota of domestic pigeons (Columba livia domestica). PLoS ONE 2023, 18, e0287261. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Wirth, J.S.; Saravanan, V.S. Phylogenomic analyses of the Staphylococcaceae family suggest the reclassification of five species within the genus Staphylococcus as heterotypic synonyms, the promotion of five subspecies to novel species, the taxonomic reassignment of five Staphylococcus species to Mammaliicoccus gen. nov., and the formal assignment of Nosocomiicoccus to the family Staphylococcaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 5926–5936. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Brégeon, F.; Mège, J.-L.; Rolain, J.-M.; Blin, O. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef] [PubMed]
- Raineri, E.J.M.; Altulea, D.; van Dijl, J.M. Staphylococcal trafficking and infection—From ‘nose to gut’ and back. FEMS Microbiol. Rev. 2022, 46, fuab041. [Google Scholar] [CrossRef] [PubMed]
- Gostev, V.; Leyn, S.; Kruglov, A.; Likholetova, D.; Kalinogorskaya, O.; Baykina, M.; Dmitrieva, N.; Grigorievskaya, Z.; Priputnevich, T.; Lyubasovskaya, L.; et al. Global Expansion of Linezolid-Resistant Coagulase-Negative Staphylococci. Front. Microbiol. 2021, 12, 661798. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Lozano, C.; Simón, C.; Zarazaga, M.; Torres, C. Within-Host Diversity of Coagulase-Negative Staphylococci Resistome from Healthy Pigs and Pig Farmers, with the Detection of cfr-Carrying Strains and MDR-S. borealis. Antibiotics 2023, 12, 1505. [Google Scholar] [CrossRef]
- Brenciani, A.; Morroni, G.; Schwarz, S.; Giovanetti, E. Oxazolidinones: Mechanisms of resistance and mobile genetic elements involved. J. Antimicrob. Chemother. 2022, 77, 2596–2621. [Google Scholar] [CrossRef]
- Ruiz-Ripa, L.; Gómez, P.; Alonso, C.A.; Camacho, M.C.; Ramiro, Y.; de la Puente, J.; Fernández-Fernández, R.; Quevedo, M.; Blanco, J.M.; Báguena, G.; et al. Frequency and Characterization of Antimicrobial Resistance and Virulence Genes of Coagulase-Negative Staphylococci from Wild Birds in Spain. Detection of tst-Carrying S. sciuri Isolates. Microorganisms 2020, 8, 1317. [Google Scholar] [CrossRef]
- Al-Haqan, A.; Boswihi, S.S.; Pathan, S.; Udo, E.E. Antimicrobial resistance and virulence determinants in coagulase-negative staphylococci isolated mainly from preterm neonates. PLoS ONE 2022, 15, e0236713. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Lozano, C.; Saidenberg, A.B.S.; Latorre-Fernández, J.; Zarazaga, M.; Torres, C. Comparative review of the nasal carriage and genetic characteristics of Staphylococcus aureus in healthy livestock: Insight into zoonotic and anthroponotic clones. Infect. Genet. Evol. 2023, 109, 105408. [Google Scholar] [CrossRef]
- A One Health Priority Research Agenda for Antimicrobial Resistance. Geneva: World Health Organization, Food and Agriculture Organization of the United Nations, United Nations Environment Programme and World Organisation for Animal Health. 2023. Available online: https://www.who.int/publications-detail-redirect/9789240075924 (accessed on 20 October 2023).
- Jori, F.; Hernandez-Jover, M.; Magouras, I.; Dürr, S.; Brookes, V.J. Wildlife–livestock interactions in animal production systems: What are the biosecurity and health implications? Anim. Front. Rev. Mag. Anim. Agric. 2021, 11, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Fan, P.; Liu, T.; Yang, A.; Boughton, R.K.; Pepin, K.M.; Miller, R.S.; Jeong, K.C. Transmission of antibiotic resistance at the wildlife-livestock interface. Commun. Biol. 2022, 5, 585. [Google Scholar] [CrossRef] [PubMed]
- Ambade, S.S.; Gupta, V.K.; Bhole, R.P.; Khedekar, P.B.; Chikhale, R.V. A Review on Five and Six-Membered Heterocyclic Compounds Targeting the Penicillin-Binding Protein 2 (PBP2A) of Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2023, 28, 7008. [Google Scholar] [CrossRef] [PubMed]
- Miragaia, M. Factors Contributing to the Evolution of mecA-Mediated β-lactam Resistance in Staphylococci: Update and New Insights From Whole Genome Sequencing (WGS). Front. Microbiol. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, L.; Holden, M.T.; Lindsay, H.; Webb, C.R.; Brown, D.F.; Curran, M.D.; Walpole, E.; Brooks, K.; Pickard, D.J.; Teale, C.; et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. Lancet Infect. Dis. 2011, 11, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.E.S.; Wireman, J.; Hostetler, J.; Forberger, H.; Borman, J.; Gill, J.; Sanchez, S.; Mankin, A.; LaMarre, J.; Lindsay, J.A.; et al. Major families of multiresistant plasmids from geographically and epidemiologically diverse staphylococci. G3 Genes|Genomes|Genet. 2011, 1, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Andreis, S.N.; Perreten, V.; Schwendener, S. Novel β-Lactamase blaARL in Staphylococcus arlettae. mSphere 2017, 2, e00117-17. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, L.; Lu, Y.; Zhao, X.; Sun, Y.; Tang, X.; Xiao, J.; Wang, C.; Tong, C.; Zhao, L.; et al. Inducible Resistance to β-Lactams in Oxacillin-Susceptible mecA1-Positive Staphylococcus sciuri Isolated from Retail Pork. Front. Microbiol. 2021, 12, 721426. [Google Scholar] [CrossRef]
- Becker, K.; van Alen, S.; Idelevich, E.A.; Schleimer, N.; Seggewiß, J.; Mellmann, A.; Kaspar, U.; Peters, G. Plasmid-Encoded Transferable mecB-Mediated Methicillin Resistance in Staphylococcus aureus. Emerg. Infect. Dis. 2018, 24, 242–248. [Google Scholar] [CrossRef]
- Schwendener, S.; Cotting, K.; Perreten, V. Novel methicillin resistance gene mecD in clinical Macrococcus caseolyticus strains from bovine and canine sources. Sci. Rep. 2017, 7, 43797. [Google Scholar] [CrossRef]
- Harrison, E.M.; Paterson, G.K.; Holden, M.T.G.; Morgan, F.J.E.; Larsen, A.R.; Petersen, A.; Leroy, S.; De Vliegher, S.; Perreten, V.; Fox, L.K.; et al. A Staphylococcus xylosus isolate with a new mecC allotype. Antimicrob. Agents Chemother. 2013, 57, 1524–1528. [Google Scholar] [CrossRef]
- Yszko, I.M.; Schwarz, S.; Hauschild, T. Detection of a new mecC allotype, mecC2, in methicillin-resistant Staphylococcus saprophyticus. J. Antimicrob. Chemother. 2014, 69, 2003–2005. [Google Scholar] [CrossRef]
- Greninger, A.L.; Chatterjee, S.S.; Chan, L.C.; Hamilton, S.M.; Chambers, H.F.; Chiu, C.Y. Whole-Genome Sequencing of Methicillin-Resistant Staphylococcus aureus Resistant to Fifth-Generation Cephalosporins Reveals Potential Non-mecA Mechanisms of Resistance. PLoS ONE 2016, 11, e0149541. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, E.-J.; Kim, D.; Kim, J.W.; Lee, K.-J.; Kim, H.S.; Kim, Y.R.; Shin, J.H.; Shin, J.H.; Shin, K.S.; et al. Ceftaroline Resistance by Clone-Specific Polymorphism in Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2018, 62, e00485-18. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Fernández-Fernández, R.; Juárez-Fernández, G.; Martínez-Álvarez, S.; Eguizábal, P.; Zarazaga, M.; Lozano, C.; Torres, C. Wild Animals Are Reservoirs and Sentinels of Staphylococcus aureus and MRSA Clones: A Problem with “One Health” Concern. Antibiotics 2021, 10, 1556. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Lozano, C.; Ruiz-Ripa, L.; Fernández-Fernández, R.; Zarazaga, M.; Torres, C. Ecology and Genetic Lineages of Nasal Staphylococcus aureus and MRSA Carriage in Healthy Persons with or without Animal-Related Occupational Risks of Colonization: A Review of Global Reports. Pathogens 2021, 10, 1000. [Google Scholar] [CrossRef]
- Shore, A.C.; Deasy, E.C.; Slickers, P.; Brennan, G.; O’Connell, B.; Monecke, S.; Ehricht, R.; Coleman, D.C. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 3765–3773. [Google Scholar] [CrossRef]
- Paterson, G.K.; Larsen, A.R.; Robb, A.; Edwards, G.E.; Pennycott, T.W.; Foster, G.; Mot, D.; Hermans, K.; Baert, K.; Peacock, S.J.; et al. The newly described mecA homologue, mecALGA251, is present in methicillin-resistant Staphylococcus aureus isolates from a diverse range of host species. J. Antimicrob. Chemother. 2012, 67, 2809–2813. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Uehara, Y. Current Status of Staphylococcal Cassette Chromosome mec (SCCmec). Antibiotics 2022, 11, 86. [Google Scholar] [CrossRef]
- Lozano, C.; Fernández-Fernández, R.; Ruiz-Ripa, L.; Gómez, P.; Zarazaga, M.; Torres, C. Human mecC-Carrying MRSA: Clinical Implications and Risk Factors. Microorganisms 2022, 8, 1615. [Google Scholar] [CrossRef]
- Gómez, P.; Ruiz-Ripa, L.; Fernández-Fernández, R.; Gharsa, H.; Ben Slama, K.; Höfle, U.; Zarazaga, M.; Holmes, M.A.; Torres, C. Genomic Analysis of Staphylococcus aureus of the Lineage CC130, Including mecC-Carrying MRSA and MSSA Isolates Recovered of Animal, Human, and Environmental Origins. Front. Microbiol. 2021, 12, 655994. [Google Scholar] [CrossRef]
- Harrison, E.M.; Paterson, G.K.; Holden, M.T.G.; Ba, X.; Rolo, J.; Morgan, F.J.E.; Pichon, B.; Kearns, A.; Zadoks, R.N.; Peacock, S.J.; et al. A novel hybrid SCCmec-mecC region in Staphylococcus sciuri. J. Antimicrob. Chemother. 2014, 69, 911–918. [Google Scholar] [CrossRef]
- Rolo, J.; de Lencastre, H.; Miragaia, M. High frequency and diversity of cassette chromosome recombinases (ccr) in methicillin-susceptible Staphylococcus sciuri. J. Antimicrob. Chemother. 2014, 69, 1461–1469. [Google Scholar] [CrossRef]
- Rolo, J.; Worning, P.; Nielsen, J.B.; Bowden, R.; Bouchami, O.; Damborg, P.; Guardabassi, L.; Perreten, V.; Tomasz, A.; Westh, H.; et al. Evolutionary Origin of the Staphylococcal Cassette Chromosome mec (SCC mec). Antimicrob. Agents Chemother. 2017, 61, e02302-16. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Lozano, C.; Höfle, Ú.; Cardona-Cabrera, T.; Zarazaga, M.; Torres, C. Antimicrobial resistome of coagulase-negative staphylococci from nasotracheal cavities of nestlings of Ciconia ciconia in Southern Spain: Detection of mecC-SCCmec type-XI-carrying S. lentus. Comp. Immunol. Microbiol. Infect. Dis. 2023, 99, 102012. [Google Scholar] [CrossRef]
- Dhaouadi, S.; Soufi, L.; Campanile, F.; Dhaouadi, F.; Sociale, M.; Lazzaro, L.; Cherif, A.; Stefani, S.; Elandoulsi, R.B. Prevalence of meticillin-resistant and -susceptible coagulase-negative staphylococci with the first detection of the mecC gene among cows, humans and manure in Tunisia. Int. J. Antimicrob. Agents 2022, 55, 105826. [Google Scholar] [CrossRef]
- Dhaouadi, S.; Bouchami, O.; Soufi, L.; Dhaouadi, F.; Chaari, S.; Bouglita, W.; Cherif, A.; de Lencastre, H.; Elandoulsi, R.B.; Miragaia, M. Frequent dissemination and carriage of an SCCmec-mecC hybrid in methicillin-resistant Mammaliicoccus sciuri in farm animals from Tunisia. J. Glob. Antimicrob. Resist. 2022, 31, 228–235. [Google Scholar] [CrossRef]
- Paterson, G.K. Genomic epidemiology of methicillin-resistant Staphylococcus sciuri carrying a SCCmec-mecC hybrid element. Infect. Genet. Evol. 2020, 79, 104148. [Google Scholar] [CrossRef]
- Coimbra, D.G.; Almeida, A.G.C.S.; Jùnior, J.B.O.; Silva, L.A.F.D.; Pimentel, B.J.; Gitaí, D.; Moreira, L.S.; Silva-Filho, E.A.; de Andrade, T. Wound infection by multiresistant Staphylococcus sciuri identified by molecular methods. New Microbiol. 2011, 34, 425–427. [Google Scholar]
- Nemeghaire, S.; Argudín, M.A.; Feßler, A.T.; Hauschild, T.; Schwarz, S.; Butaye, P. The ecological importance of the Staphylococcus sciuri species group as a reservoir for resistance and virulence genes. Vet. Microbiol. 2014, 171, 342–356. [Google Scholar] [CrossRef]
- Grazul, M.; Balcerczak, E.; Sienkiewicz, M. Analysis of the Presence of the Virulence and Regulation Genes from Staphylococcus aureus (S. aureus) in Coagulase Negative Staphylococci and the Influence of the Staphylococcal Cross-Talk on Their Functions. Int. J. Environ. Res. Public Health 2023, 20, 5155. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- MacFadyen, A.C.; Harrison, E.M.; Ellington, M.J.; Parkhill, J.; Holmes, M.A.; Paterson, G.K. A highly conserved mecC-encoding SCCmectype XI in a bovine isolate of methicillin-resistant Staphylococcus xylosus. J. Antimicrob. Chemother. 2018, 73, 3516–3518. [Google Scholar] [CrossRef]
- de Moura, G.S.; de Carvalho, E.; Sanchez, E.M.R.; Sellera, F.P.; Marques, M.F.; Heinemann, M.B.; De Vliegher, S.; Souza, F.N.; Mota, R.A. Emergence of livestock-associated Mammaliicoccus sciuri ST71 co-harbouring mecA and mecC genes in Brazil. Vet. Microbiol. 2023, 283, 109792. [Google Scholar] [CrossRef]
- Aslantas, O. High Occurence of Methicillin Resistant Staphylococcus sciuri (MRSS) and First Detection of mecC from Broiler Flocks in Turkey. Isr. J. Vet. Med. 2020, 75, 185–192. [Google Scholar]
- Belhout, C.; Boyen, F.; Vereecke, N.; Theuns, S.; Taibi, N.; Stegger, M.; de la Fé-Rodríguez, P.Y.; Bouayad, L.; Elgroud, R.; Butaye, P. Prevalence and Molecular Characterization of Methicillin-Resistant Staphylococci (MRS) and Mammaliicocci (MRM) in Dromedary Camels from Algeria: First Detection of SCCmec-mecC Hybrid in Methicillin-Resistant Mammaliicoccus lentus. Antibiotics 2023, 12, 674. [Google Scholar] [CrossRef]
- Srednik, M.E.; Archambault, M.; Jacques, M.; Gentilini, E.R. Detection of a mecC-positive Staphylococcus saprophyticus from bovine mastitis in Argentina. J. Glob. Antimicrob. Resist. 2017, 10, 261–263. [Google Scholar] [CrossRef]
- Loncaric, I.; Kübber-Heiss, A.; Posautz, A.; Stalder, G.L.; Hoffmann, D.; Rosengarten, R.; Walzer, C. Characterization of methicillin-resistant Staphylococcus spp. carrying the mecC gene, isolated from wildlife. J. Antimicrob. Chemother. 2013, 68, 2222–2225. [Google Scholar] [CrossRef]
- Semmler, T.; Harrison, E.M.; Lübke-Becker, A.; Ulrich, R.G.; Wieler, L.H.; Guenther, S.; Stamm, I.; Hanssen, A.-M.; Holmes, M.A.; Vincze, S.; et al. A Look into the Melting Pot: The mecC-Harboring Region Is a Recombination Hot Spot in Staphylococcus stepanovicii. PLoS ONE 2016, 11, e0147150. [Google Scholar] [CrossRef] [PubMed]
- Loncaric, I.; Kübber-Heiss, A.; Posautz, A.; Ruppitsch, W.; Lepuschitz, S.; Schauer, B.; Feßler, A.T.; Krametter-Frötscher, R.; Harrison, E.M.; Holmes, M.A.; et al. Characterization of mecC gene-carrying coagulase-negative Staphylococcus spp. isolated from various animals. Vet. Microbiol. 2019, 230, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Pantůček, R.; Sedláček, I.; Indráková, A.; Vrbovská, V.; Mašlaňová, I.; Kovařovic, V.; Švec, P.; Králová, S.; Krištofová, L.; Kekláková, J.; et al. Staphylococcus edaphicus sp. nov., Isolated in Antarctica, Harbors the mecC Gene and Genomic Islands with a Suspected Role in Adaptation to Extreme Environments. Appl. Environ. Microbiol. 2018, 84, e01746-17. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Hasman, H.; Larsen, J.; Stegger, M.; Johannesen, T.B.; Allesøe, R.L.; Lemvigh, C.K.; Aarestrup, F.M.; Lund, O.; Larsen, A.R. SCC mec Finder, a Web-Based Tool for Typing of Staphylococcal Cassette Chromosome mec in Staphylococcus aureus Using Whole-Genome Sequence Data. mSphere 2018, 3, e00612-17. [Google Scholar] [CrossRef] [PubMed]
- Wielders, C.L.C.; Fluit, A.C.; Brisse, S.; Verhoef, J.; Schmitz, F.J. mecA gene is widely disseminated in Staphylococcus aureus population. J. Clin. Microbiol. 2002, 40, 3970–3975. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef] [PubMed]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Leroy, S.; Christieans, S.; Talon, R. Tetracycline Gene Transfer in Staphylococcus xylosus in situ during Sausage Fermentation. Front. Microbiol. 2019, 10, 392. [Google Scholar] [CrossRef]
- Leroy, S.; Vermassen, A.; Ras, G.; Talon, R. Insight into the Genome of Staphylococcus xylosus, a Ubiquitous Species Well Adapted to Meat Products. Microorganisms 2017, 5, 52. [Google Scholar] [CrossRef]
- Leroy, S.; Even, S.; Micheau, P.; De La Foye, A.; Laroute, V.; Le Loir, Y.; Talon, R. Transcriptomic Analysis of Staphylococcus xylosus in Solid Dairy Matrix Reveals an Aerobic Lifestyle Adapted to Rind. Microorganisms 2020, 8, 1807. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Transmission of antimicrobial resistance (AMR) during animal transport. EFSA J. 2022, 20, e07586. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, R.; Gopal, D.R.; Dhandapani, R.; Subbarayalu, R.; Elangovan, M.P.; Prabhu, B.; Veerappan, V.; Nandheeswaran, A.; Paramasivam, S.; Muthupandian, S. Is AMR in Dairy Products a Threat to Human Health? An Updated Review on the Origin, Prevention, Treatment, and Economic Impacts of Subclinical Mastitis. Infect. Drug Resist. 2023, 16, 155–178. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Yatoo, M.I.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2022, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- França, A.; Gaio, V.; Lopes, N.; Melo, L.D.R. Virulence Factors in Coagulase-Negative Staphylococci. Pathogens 2021, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Traversari, J.; Borne, B.H.P.v.D.; Dolder, C.; Thomann, A.; Perreten, V.; Bodmer, M. Non-aureus Staphylococci Species in the Teat Canal and Milk in Four Commercial Swiss Dairy Herds. Front. Vet. Sci. 2019, 6, 186. [Google Scholar] [CrossRef]
- Lawal, O.U.; Fraqueza, M.J.; Bouchami, O.; Worning, P.; Bartels, M.D.; Gonçalves, M.L.; Paixão, P.; Gonçalves, E.; Toscano, C.; Empel, J.; et al. Foodborne Origin and Local and Global Spread of Staphylococcus saprophyticus Causing Human Urinary Tract Infections. Emerg. Infect. Dis. 2021, 27, 880–893. [Google Scholar] [CrossRef]
- Lawal, O.U.; Fraqueza, M.J.; Worning, P.; Bouchami, O.; Bartels, M.D.; Goncalves, L.; Paixão, P.; Goncalves, E.; Toscano, C.; Empel, J.; et al. Staphylococcus saprophyticus Causing Infections in Humans Is Associated with High Resistance to Heavy Metals. Antimicrob. Agents Chemother. 2021, 65, e0268520. [Google Scholar] [CrossRef]
- Kalai, S.; Roychoudhury, P.; Dutta, T.; Subudhi, P.; Chakraborty, S.; Barman, N.; Sen, A. Multidrug resistant staphylococci isolated from pigs with exudative epidermitis in North eastern Region of India. Lett. Appl. Microbiol. 2021, 72, 535–541. [Google Scholar] [CrossRef]
- Lin, S.; Sun, B.; Shi, X.; Xu, Y.; Gu, Y.; Gu, X.; Ma, X.; Wan, T.; Xu, J.; Su, J.; et al. Comparative Genomic and Pan-Genomic Characterization of Staphylococcus epidermidis from Different Sources Unveils the Molecular Basis and Potential Biomarkers of Pathogenic Strains. Front. Microbiol. 2021, 12, 770191. [Google Scholar] [CrossRef]
- Ayala, D.I.; Grum, D.S.; Evans, N.P.; Russo, K.N.; Kimminau, E.A.; Trible, B.R.; Lahoti, M.M.; Novak, C.L.; Karnezos, T.P. Identification and characterization of the causative agents of Focal Ulcerative Dermatitis in commercial laying hens. Front. Vet. Sci. 2023, 10, 1110573. [Google Scholar] [CrossRef] [PubMed]
- Argemi, X.; Hansmann, Y.; Prola, K.; Prévost, G. Coagulase-Negative Staphylococci Pathogenomics. Int. J. Mol. Sci. 2019, 20, 1215. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Milheiriço, C.; Gardete, S.; Holmes, M.A.; Holden, M.T.G.; de Lencastre, H.; Tomasz, A. Properties of a novel PBP2A protein homolog from Staphylococcus aureus strain LGA251 and its contribution to the β-lactam-resistant phenotype. J. Biol. Chem. 2012, 287, 36854–36863. [Google Scholar] [CrossRef] [PubMed]
- Long, K.S.; Poehlsgaard, J.; Kehrenberg, C.; Schwarz, S.; Vester, B. The Cfr rRNA methyltransferase confers resistance to Phenicols, Lincosamides, Oxazolidinones, Pleuromutilins, and Streptogramin A antibiotics. Antimicrob. Agents Chemother. 2006, 50, 2500–2505. [Google Scholar] [CrossRef]
- Schouls, L.M.; Veldman, K.; Brouwer, M.S.M.; Dierikx, C.; Witteveen, S.; van Santen-Verheuvel, M.; Hendrickx, A.P.A.; Landman, F.; Hengeveld, P.; Wullings, B.; et al. cfr and fexA genes in methicillin-resistant Staphylococcus aureus from humans and livestock in the Netherlands. Commun. Med. 2020, 2, 135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).