Quorum Sensing Regulates the Production of Methanethiol in Vibrio harveyi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Cultivation Conditions
2.2. Quantification of MeSH and DMS Production
2.3. Identification of Potential Proteins Related to MeSH and DMS Metabolism
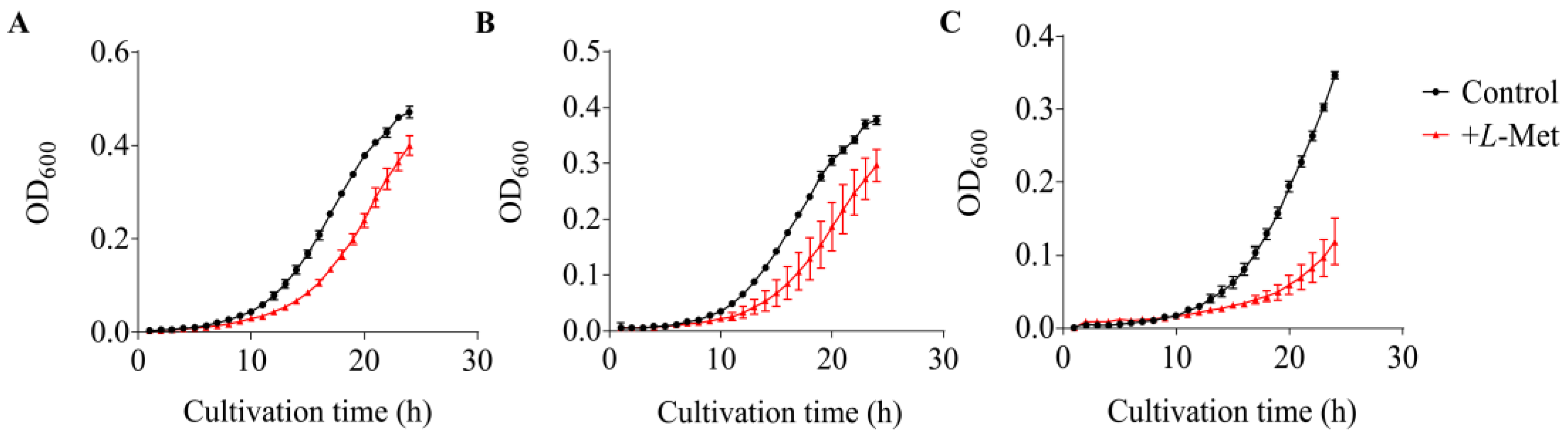
2.4. Growth of V. harveyi and Its Mutants with L-Met
2.5. MeSH Production with Autoinducer and Quorum-Quenching Enzyme
3. Results
3.1. MeSH and DMS Production by Vibrio harveyi BB120
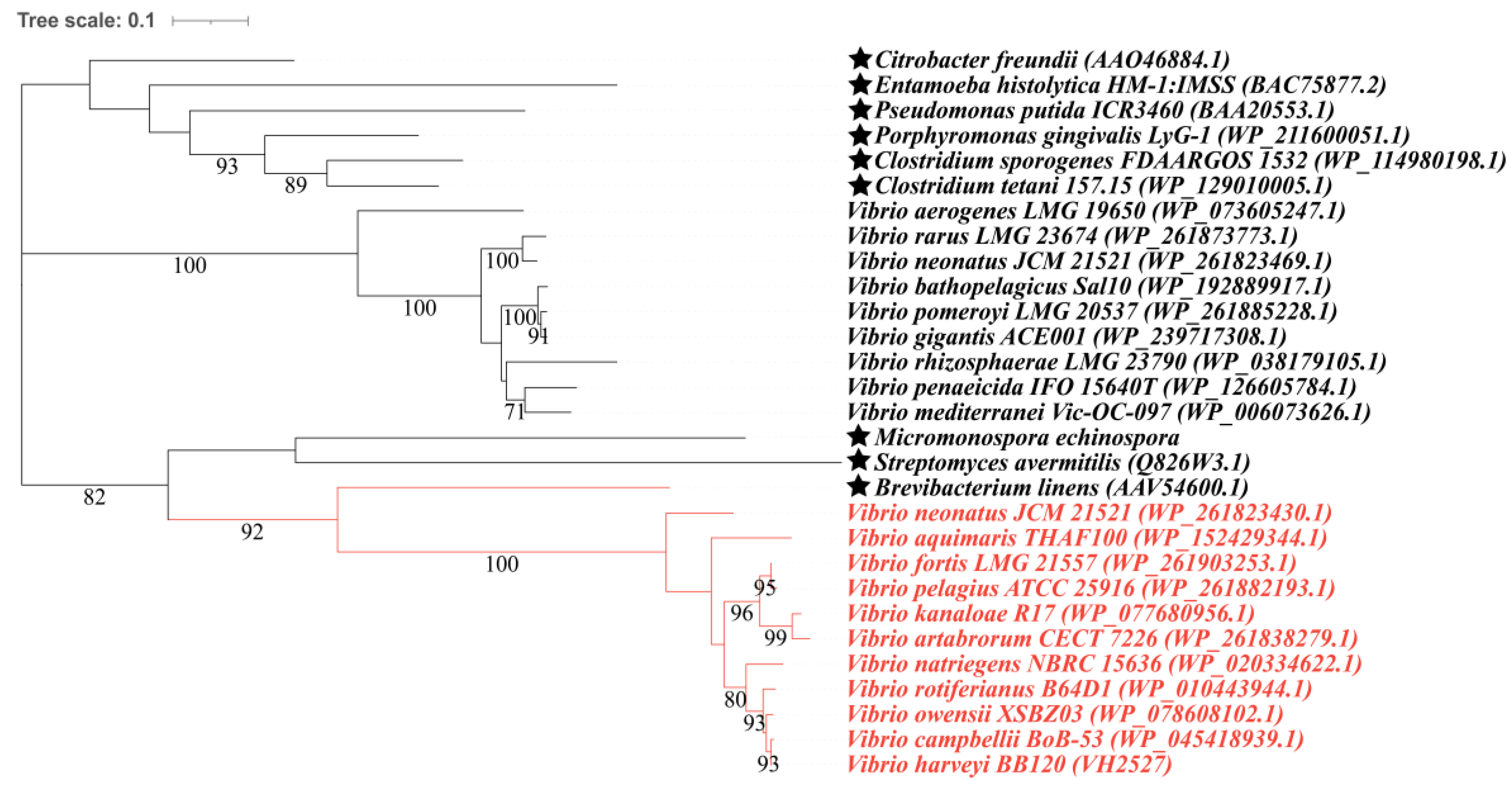
3.2. Candidate Proteins Involved in MeSH and DMS Cycling in Vibrio
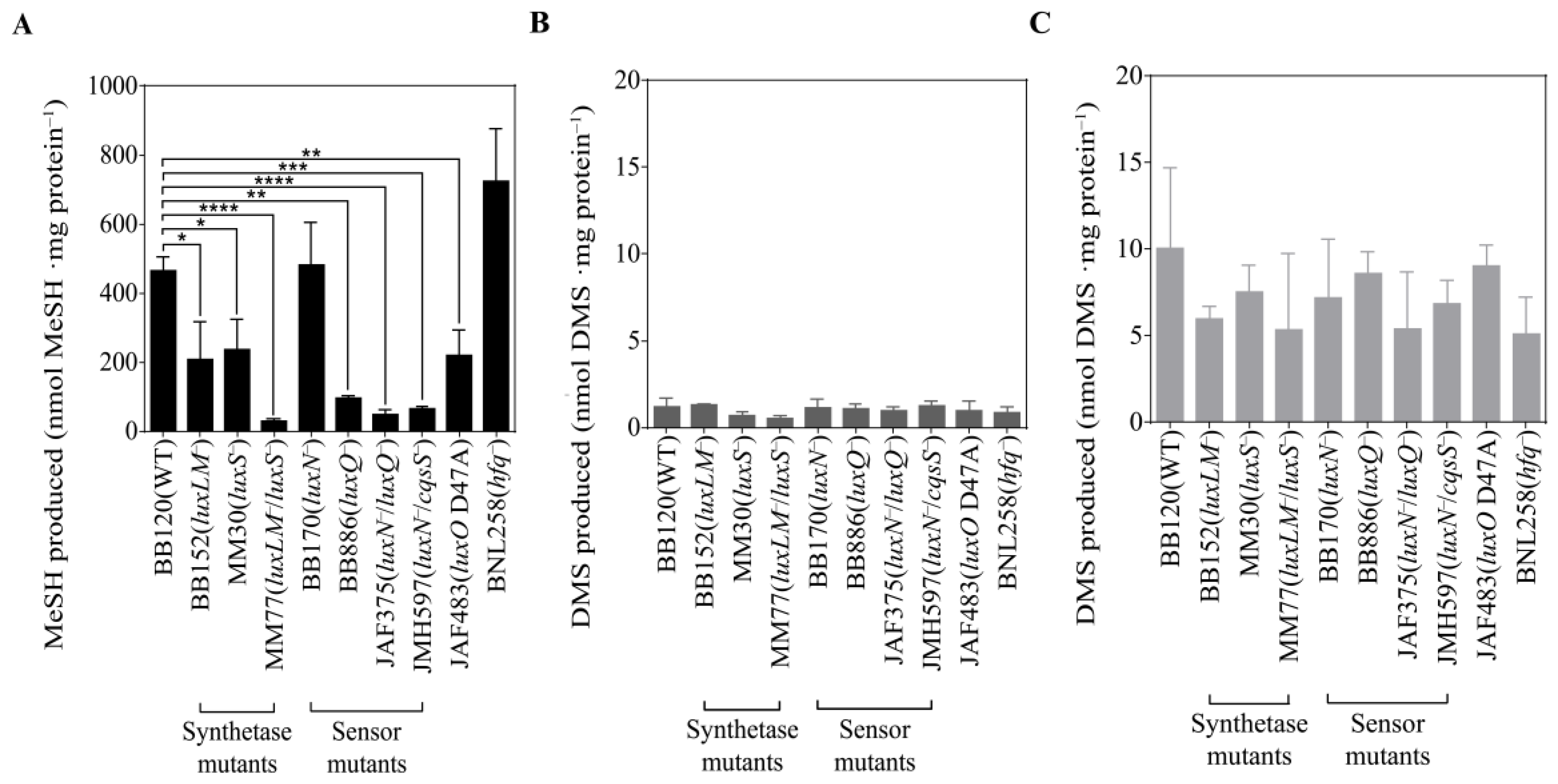
3.3. QS Mutants of V. harveyi BB120 Differ in MeSH Production
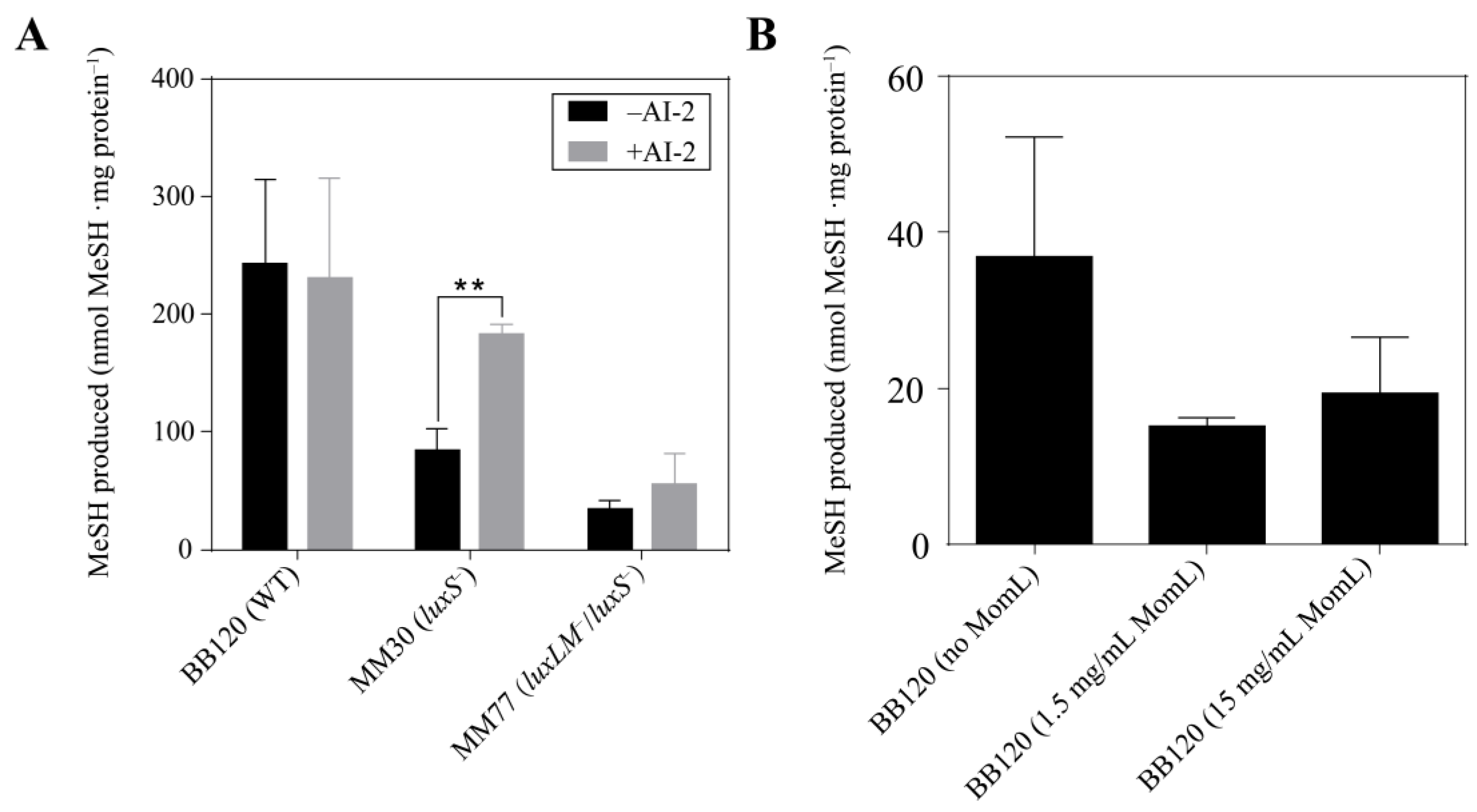
3.4. Signal Molecules Affect MeSH Production in Vibrio harveyi
3.5. Growth Inhibition Caused by L-Met on V. harveyi BB120 and Its QS Mutants
4. Discussion
4.1. Metabolism of Organic Sulfur in Vibrios
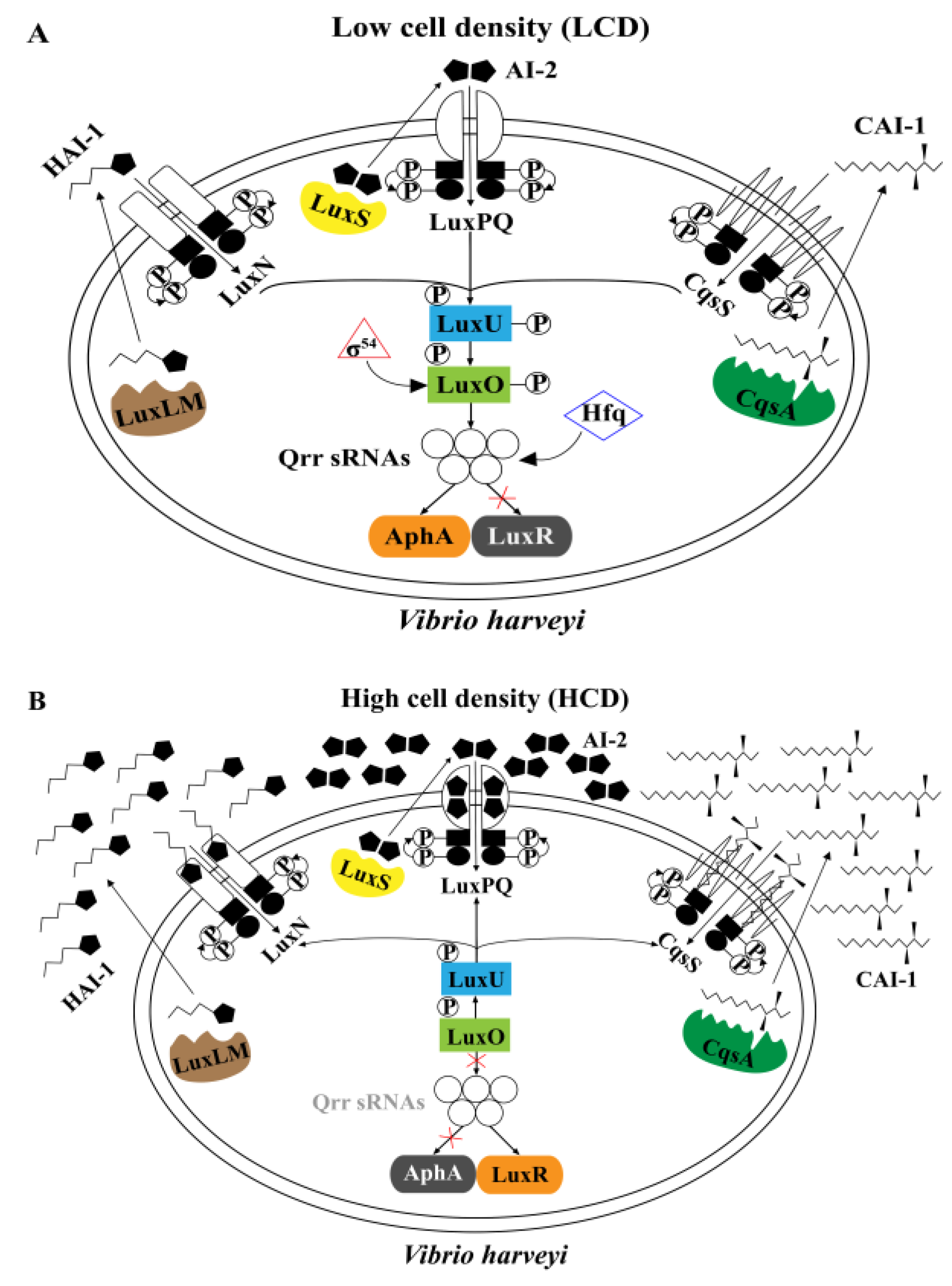
4.2. QS Systems Regulate MeSH Metabolism in Vibrio harveyi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bentley, R.; Chasteen, T.G. Environmental VOSCs––formation and degradation of dimethyl sulfide, methanethiol and related materials. Chemosphere 2004, 55, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Charlson, R.J.; Lovelock, J.E.; Andreae, M.O.; Warren, S.G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326, 655–661. [Google Scholar] [CrossRef]
- Lavoie, M.; Galí, M.; Sévigny, C.; Kieber, D.J.; Sunda, W.G.; Spiese, C.E.; Maps, F.; Levasseur, M. Modelling dimethylsulfide diffusion in the algal external boundary layer: Implications for mutualistic and signalling roles. Environ. Microbiol. 2018, 20, 4157–4169. [Google Scholar] [CrossRef] [PubMed]
- Lana, A.; Bell, T.G.; Simó, R.; Vallina, S.M.; Ballabrera-Poy, J.; Kettle, A.J.; Dachs, J.; Bopp, L.; Saltzman, E.S.; Stefels, J.; et al. An updated climatology of surface dimethlysulfide concentrations and emission fluxes in the global ocean. Glob. Biogeochem. Cycles 2011, 25, GB1004. [Google Scholar] [CrossRef]
- Liu, C.; Gao, P.; Yang, G.; Zhang, H.; Li, P. Study progress on methyl-mercaptan in ocean. Trans. Oceanol. Limnol. 2014, 3, 161–166. [Google Scholar] [CrossRef]
- Kettle, A.J.; Rhee, T.S.; von Hobe, M.; Poulton, A.; Aiken, J.; Andreae, M.O. Assessing the flux of different volatile sulfur gases from the ocean to the atmosphere. J. Geophys. Res. 2001, 106, 193–209. [Google Scholar] [CrossRef]
- Gros, V.; Bonsang, B.; Sarda-Estève, R.; Nikolopoulos, A.; Metfies, K.; Wietz, M.; Peeken, I. Concentrations of dissolved dimethyl sulfide (DMS), methanethiol and other trace gases in context of microbial communities from the temperate Atlantic to the Arctic Ocean. Biogeosciences 2023, 20, 851–867. [Google Scholar] [CrossRef]
- Lee, C.-L.; Brimblecombe, P. Anthropogenic contributions to global carbonyl sulfide, carbon disulfide and organosulfides fluxes. Earth-Sci. Rev. 2016, 160, 1–18. [Google Scholar] [CrossRef]
- Li, C.-Y.; Cao, H.-Y.; Wang, Q.; Carrión, O.; Zhu, X.; Miao, J.; Wang, P.; Chen, X.-L.; Todd, J.D.; Zhang, Y.-Z. Aerobic methylation of hydrogen sulfide to dimethylsulfide in diverse microorganisms and environments. ISME J. 2023, 17, 1184–1193. [Google Scholar] [CrossRef]
- Carrión, O.; Curson, A.R.J.; Kumaresan, D.; Fu, Y.; Lang, A.S.; Mercadé, E.; Todd, J.D. A novel pathway producing dimethylsulphide in bacteria is widespread in soil environments. Nat. Commun. 2015, 6, 6579. [Google Scholar] [CrossRef]
- Kim, A.-H.; Yum, S.S.; Lee, H.; Chang, D.Y.; Shim, S. Polar cooling effect due to increase of phytoplankton and dimethyl-sulfide emission. Atmosphere 2018, 9, 384. [Google Scholar] [CrossRef]
- Johnston, A.W.B.; Todd, J.D.; Sun, L.; Nikolaidou-Katsaridou, M.N.; Curson, A.R.J.; Rogers, R. Molecular diversity of bacterial production of the climate-changing gas, dimethyl sulphide, a molecule that impinges on local and global symbioses. J. Exp. Bot. 2008, 59, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Steinke, M.; Stefels, J.; Stamhuis, E. Dimethyl sulfide triggers search behavior in copepods. Limnol. Oceanogr. 2006, 51, 1925–1930. [Google Scholar] [CrossRef]
- Nevitt, G.A. Sensory ecology on the high seas: The odor world of the procellariiform seabirds. J. Exp. Biol. 2008, 211, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Bullock, H.A.; Luo, H.; Whitman, W.B. Evolution of dimethylsulfoniopropionate metabolism in marine phytoplankton and bacteria. Front. Microbiol. 2017, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- González, J.M.; Simó, R.; Massana, R.; Covert, J.S.; Casamayor, E.O.; Pedrós-Alió, C.; Moran, M.A. Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl. Environ. Microb. 2000, 66, 4237–4246. [Google Scholar] [CrossRef]
- Tanaka, H.; Esaki, N.; Soda, K. Properties of L-methionine γ-lyase from Pseudomonas ovalis. Biochemistry 1977, 16, 100–106. [Google Scholar] [CrossRef]
- Ito, S.; Nakamura, T.; Eguchi, Y. Purification and characterization of methioninase from Pseudomonas putida. J. Biochem. 1976, 79, 1263–1272. [Google Scholar] [CrossRef]
- Dias, B.; Weimer, B. Purification and characterization of L-methionine γ-lyase from Brevibacterium linens BL2. Appl. Environ. Microb. 1998, 64, 3327–3331. [Google Scholar] [CrossRef]
- Mamaeva, D.V.; Morozova, E.A.; Nikulin, A.D.; Revtovich, S.V.; Nikonov, S.V.; Garber, M.B.; Demidkina, T.V. Structure of Citrobacter freundii L-methionine γ-lyase. Acta Crystallogr. F 2005, 61, 546–549. [Google Scholar] [CrossRef]
- Kudou, D.; Yasuda, E.; Hirai, Y.; Tamura, T.; Inagaki, K. Molecular cloning and characterization of L-methionine γ-lyase from Streptomyces avermitilis. J. Biosci. Bioeng. 2015, 120, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Boden, R.; Borodina, E.; Wood, A.P.; Kelly, D.P.; Murrell, J.C.; Schäfer, H. Purification and characterization of dimethylsulfide monooxygenase from Hyphomicrobium sulfonivorans. J. Bacteriol. 2011, 193, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Tripp, H.J.; Kitner, J.B.; Schwalbach, M.S.; Dacey, J.W.H.; Wilhelm, L.J.; Giovannoni, S.J. SAR11 marine bacteria require exogenous reduced sulphur for growth. Nature 2008, 452, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Losick, R. How and why bacteria talk to each other. Cell 1993, 73, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Winzer, K.; Chan, W.C.; Cámara, M. Look who’s talking: Communication and quorum sensing in the bacterial world. Phil. Trans. R. Soc. B 2007, 362, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, N.A.; Barnard, A.M.L.; Slater, H.; Simpson, N.J.L.; Salmond, G.P.C. Quorum-sensing in gram-negative bacteria. FEMS Microbiol. Rev. 2001, 25, 365–404. [Google Scholar] [CrossRef]
- Winzer, K.; Hardie, K.R.; Williams, P. Bacterial cell-to-cell communication: Sorry, can’t talk now—gone to lunch! Curr. Opin. Microbiol. 2002, 5, 216–222. [Google Scholar] [CrossRef]
- Decho, A.W.; Norman, R.S.; Visscher, P.T. Quorum sensing in natural environments: Emerging views from microbial mats. Trends Microbiol. 2010, 18, 73–80. [Google Scholar] [CrossRef]
- Ng, W.-L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zeng, X.; Zheng, J.; Zou, Y.; Qiu, S.; Dai, Y. AHL-mediated quorum sensing to regulate bacterial substance and energy metabolism: A review. Microbiol. Res. 2022, 262, 127102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, S.W.; Li, H. Significantly improved production of Welan gum by Sphingomonas sp. WG through a novel quorum-sensing-interfering dipeptide cyclo(L-Pro-L-Phe). Int. J. Biol. Macromol. 2019, 126, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-Y.; Liu, X.-J.; Fu, C.-A.; Gu, X.-F.; Lin, J.-Q.; Liu, X.-M.; Pang, X.; Lin, J.-Q.; Chen, L.-X. Novel strategy for improvement of the bioleaching efficiency of Acidithiobacillus ferrooxidans based on the AfeI/R quorum sensing system. Minerals 2020, 10, 222. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Fu, C.-A.; Hao, L.; Gu, X.-F.; Wang, R.; Lin, J.-Q.; Liu, X.-M.; Pang, X.; Zhang, C.-J.; Lin, J.-Q.; et al. The substrate-dependent regulatory effects of the AfeI/R system in Acidithiobacillus ferrooxidans reveals the novel regulation strategy of quorum sensing in acidophiles. Environ. Microb. 2020, 23, 757–773. [Google Scholar] [CrossRef]
- Milton, D.L. Quorum sensing in vibrios: Complexity for diversification. Int. J. Med. Microbiol. 2006, 296, 61–71. [Google Scholar] [CrossRef]
- Henke, J.M.; Bassler, B.L. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 2004, 186, 6902–6914. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Henke, J.M.; Bassler, B.L. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 2004, 186, 3794–3805. [Google Scholar] [CrossRef]
- Shao, Y.; Bassler, B.L. Quorum-sensing non-coding small RNAs use unique pairing regions to differentially control mRNA targets. Mol. Microbiol. 2012, 83, 599–611. [Google Scholar] [CrossRef]
- Rutherford, S.T.; van Kessel, J.C.; Shao, Y.; Bassler, B.L. AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Gene. Dev. 2011, 25, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Greenberg, E.P.; Stevens, A.M. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 1997, 179, 4043–4045. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Baumann, L. The marine gram-negative eubacteria: Genera Photobacterium, Beneckea, Alteromonas, Pseudomonas and Alcaligenes. In The Prokaryotes: A Handbook on Habitats, Isolation and Identification of Bacteria, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1981; pp. 1302–1331. [Google Scholar] [CrossRef]
- Curson, A.R.J.; Fowler, E.K.; Dickens, S.; Johnston, A.W.B.; Todd, J.D. Multiple DMSP lyases in the γ-proteobacterium Oceanimonas doudoroffii. Biogeochemistry 2011, 110, 109–119. [Google Scholar] [CrossRef]
- Inoue, H.; Inagaki, K.; Sugimoto, M.; Esaki, N.; Soda, K.; Tanaka, H. Structural analysis of the L-methionine γ-lyase gene from Pseudomonas putida. J. Biochem. 1995, 117, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Amarita, F.; Yvon, M.; Nardi, M.; Chambellon, E.; Delettre, J.; Bonnarme, P. Identification and functional analysis of the gene encoding methionine-γ-lyase in Brevibacterium linens. Appl. Environ. Microb. 2004, 70, 7348–7354. [Google Scholar] [CrossRef]
- Manukhov, I.V.; Mamaeva, D.V.; Rastorguev, S.M.; Faleev, N.G.; Morozova, E.A.; Demidkina, T.V.; Zavilgelsky, G.B. A gene encoding L-methionine γ-lyase is present in Enterobacteriaceae family genomes: Identification and characterization of Citrobacter freundii L-methionine γ-lyase. J. Bacteriol. 2005, 187, 3889–3893. [Google Scholar] [CrossRef]
- Morozova, E.A.; Anufrieva, N.V.; Davydov, D.Z.; Komarova, M.V.; Dyakov, I.N.; Rodionov, A.N.; Pokrovsky, V.S. Plasma methionine depletion and pharmacokinetic properties in mice of methionine γ-lyase from Citrobacter freundii, Clostridium tetani and Clostridium sporogenes. Biomed. Pharmacother. 2017, 88, 978–984. [Google Scholar] [CrossRef]
- Song, H.; Xu, R.; Guo, Z. Identification and characterization of a methionine γ-lyase in the calicheamicin biosynthetic cluster of Micromonospora echinospora. ChemBioChem 2014, 16, 100–109. [Google Scholar] [CrossRef]
- Xue, C.-X.; Lin, H.; Zhu, X.-Y.; Liu, J.; Zhang, Y.; Rowley, G.; Todd, J.D.; Li, M.; Zhang, X.-H. DiTing: A pipeline to infer and compare biogeochemical pathways from metagenomic and metatranscriptomic data. Front. Microbiol. 2021, 12, 698286. [Google Scholar] [CrossRef]
- Curson, A.R.J.; Liu, J.; Bermejo Martínez, A.; Green, R.T.; Chan, Y.; Carrión, O.; Williams, B.T.; Zhang, S.-H.; Yang, G.-P.; Bulman Page, P.C.; et al. Dimethylsulfoniopropionate biosynthesis in marine bacteria and identification of the key gene in this process. Nat. Microbiol. 2017, 2, e17009. [Google Scholar] [CrossRef]
- Williams, B.T.; Cowles, K.; Bermejo Martínez, A.; Curson, A.R.J.; Zheng, Y.; Liu, J.; Newton-Payne, S.; Hind, A.J.; Li, C.-Y.; Rivera, P.P.L.; et al. Bacteria are important dimethylsulfoniopropionate producers in coastal sediments. Nat. Microbiol. 2019, 4, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Curson, A.R.J.; Todd, J.D.; Sullivan, M.J.; Johnston, A.W.B. Catabolism of dimethylsulphoniopropionate: Microorganisms, enzymes and genes. Nat. Rev. Microbiol. 2011, 9, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Tang, K.; Su, Y.; Brackman, G.; Cui, F.; Zhang, Y.; Shi, X.; Coenye, T.; Zhang, X.-H. MomL, a novel marine-derived N-acyl homoserine lactonase from Muricauda olearia. Appl. Environ. Microb. 2014, 81, 774–782. [Google Scholar] [CrossRef]
- Foo, T.C.; Terentis, A.C.; Venkatachalam, K.V. A continuous spectrophotometric assay and nonlinear kinetic analysis of methionine γ-lyase catalysis. Anal. Biochem. 2016, 507, 21–26. [Google Scholar] [CrossRef]
- Reisch, C.R.; Stoudemayer, M.J.; Varaljay, V.A.; Amster, I.J.; Moran, M.A.; Whitman, W.B. Novel pathway for assimilation of dimethylsulphoniopropionate widespread in marine bacteria. Nature 2011, 473, 208–211. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, H.-Y.; Zhang, N.; Teng, Z.-J.; Yu, Y.; Wang, Z.-B.; Peng, W.; Fu, H.-H.; Chen, X.-L.; Zhang, Y.-Z.; et al. Novel insights into dimethylsulfoniopropionate catabolism by cultivable bacteria in the Arctic Kongsfjorden. Appl. Environ. Microb. 2022, 88, e01806-21. [Google Scholar] [CrossRef]
- Bassler, B.L.; Wright, M.; Silverman, M.R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: Sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 1994, 13, 273–286. [Google Scholar] [CrossRef]
- Surette, M.G.; Miller, M.B.; Bassler, B.L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: A new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 1999, 96, 1639–1644. [Google Scholar] [CrossRef]
- Mok, K.C.; Wingreen, N.S.; Bassler, B.L. Vibrio harveyi quorum sensing: A coincidence detector for two autoinducers controls gene expression. EMBO J. 2003, 22, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Wright, M.; Showalter, R.E.; Silverman, M.R. Intercellular signalling in Vibrio harveyi: Sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 1993, 9, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.A.; Bassler, B.L. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 1999, 31, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Lenz, D.H.; Mok, K.C.; Lilley, B.N.; Kulkarni, R.V.; Wingreen, N.S.; Bassler, B.L. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 2004, 118, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.E. Amino acid toxicities and imbalances. In Mammalian Protein Metabolism; Academic Press: New York, NY, USA, 1964; pp. 87–134. [Google Scholar] [CrossRef]
- Harper, A.E.; Benevenga, N.J.; Wohlhueter, R.M. Effects of ingestion of disproportionate amounts of amino acids. Physiol. Rev. 1970, 50, 428–558. [Google Scholar] [CrossRef] [PubMed]
- Lomans, B.P.; van der Drift, C.; Pol, A.; Op den Camp, H.J.M. Microbial cycling of volatile organic sulfur compounds. Cell. Mol. Life Sci. 2002, 59, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, J.V.; Turchyn, A.V.; Redeker, K.R.; Mills, J.V.; Antler, G.; Carrión, O.; Todd, J.D. The production and fate of volatile organosulfur compounds in sulfide and ferruginous sediment. J. Geophys. Res. Biogeosci. 2019, 124, 3390–3402. [Google Scholar] [CrossRef]
- Foley, J.A.; Taylor, K.E.; Ghan, S.J. Planktonic dimethylsulfide and cloud albedo: An estimate of the feedback response. Clim. Chang. 1991, 18, 1–15. [Google Scholar] [CrossRef]
- Lawrence, M.G. An empirical analysis of the strength of the phytoplankton-dimethylsulfide-cloud-climate feedback cycle. J. Geophys. Res. -Atmos. 1993, 98, 20663–20673. [Google Scholar] [CrossRef]
- Simó, R. Production of atmospheric sulfur by oceanic plankton: Biogeochemical, ecological and evolutionary links. Trends Ecol. Evol. 2001, 16, 287–294. [Google Scholar] [CrossRef]
- Yoch, D.C. Dimethylsulfoniopropionate: Its sources, role in the marine food web, and biological degradation to dimethylsulfide. Appl. Environ. Microb. 2002, 68, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Ostrowski, M.; Siboni, N.; Seymour, J.R.; Petrou, K. Uptake of dimethylsulfoniopropionate (DMSP) by natural microbial communities of the Great Barrier Reef (GBR), Australia. Microorganisms 2021, 9, 1891. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Ben-Dor, S.; Feldmesser, E.; Levin, Y.; Tawfik, D.S.; Vardi, A. Identification of the algal dimethyl sulfide-releasing enzyme: A missing link in the marine sulfur cycle. Science 2015, 348, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- del Valle, D.A.; Martínez-García, S.; Sañudo-Wilhelmy, S.A.; Kiene, R.P.; Karl, D.M. Methionine and dimethylsulfoniopropionate as sources of sulfur to the microbial community of the North Pacific Subtropical Gyre. Aquat. Microb. Ecol. 2015, 75, 103–116. [Google Scholar] [CrossRef]
- Simpson, C.A.; Peterson, B.D.; Haas, N.W.; Geyman, L.J.; Lee, A.H.; Podicheti, R.; Pepin, R.; Brown, L.C.; Rusch, D.B.; Manzella, M.P.; et al. The quorum-sensing systems of Vibrio campbellii DS40M4 and BB120 are genetically and functionally distinct. Environ. Microb. 2021, 23, 5412–5432. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.A.; Hawver, L.A.; Ng, W.L. Parallel quorum sensing signaling pathways in Vibrio cholerae. Curr. Genet. 2015, 62, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Henares, B.M.; Xu, Y.; Boon, E.M. A nitric oxide-responsive quorum sensing circuit in Vibrio harveyi regulates flagella production and biofilm formation. Int. J. Mol. Sci. 2013, 14, 16473–16484. [Google Scholar] [CrossRef]
- Natrah, F.M.I.; Ruwandeepika, H.A.D.; Pawar, S.; Karunasagar, I.; Sorgeloos, P.; Bossier, P.; Defoirdt, T. Regulation of virulence factors by quorum sensing in Vibrio harveyi. Vet. Microbiol. 2011, 154, 124–129. [Google Scholar] [CrossRef]
- Pena, R.T.; Blasco, L.; Ambroa, A.; Gonzalez Pedrajo, B.M.; Fernandez-Garcia, L.; Lopez, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship between quorum sensing and secretion systems. Front. Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef]
- Hawver, L.A.; Giulietti, J.M.; Baleja, J.D.; Ng, W.-L. Quorum sensing coordinates cooperative expression of pyruvate metabolism genes to maintain a sustainable environment for population stability. mBio 2016, 7, e01863. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Wang, J.; Todd, J.D.; Zhang, X.-H.; Zhang, Y. Quorum Sensing Regulates the Production of Methanethiol in Vibrio harveyi. Microorganisms 2024, 12, 35. https://doi.org/10.3390/microorganisms12010035
Zhou T, Wang J, Todd JD, Zhang X-H, Zhang Y. Quorum Sensing Regulates the Production of Methanethiol in Vibrio harveyi. Microorganisms. 2024; 12(1):35. https://doi.org/10.3390/microorganisms12010035
Chicago/Turabian StyleZhou, Tiantian, Jinyan Wang, Jonathan D. Todd, Xiao-Hua Zhang, and Yunhui Zhang. 2024. "Quorum Sensing Regulates the Production of Methanethiol in Vibrio harveyi" Microorganisms 12, no. 1: 35. https://doi.org/10.3390/microorganisms12010035
APA StyleZhou, T., Wang, J., Todd, J. D., Zhang, X.-H., & Zhang, Y. (2024). Quorum Sensing Regulates the Production of Methanethiol in Vibrio harveyi. Microorganisms, 12(1), 35. https://doi.org/10.3390/microorganisms12010035





