Abstract
Fourier transform infrared spectroscopy (FTIRS) is a diagnostic technique historically used in the microbiological field for the characterization of bacterial strains in relation to the specific composition of their lipid, protein, and polysaccharide components. For each bacterial strain, it is possible to obtain a unique absorption spectrum that represents the fingerprint obtained based on the components of the outer cell membrane. In this study, FTIRS was applied for the first time as an experimental diagnostic tool for the discrimination of two pathogenic species belonging to the Bacillus cereus group, Bacillus anthracis and Bacillus cereus sensu stricto; these are two closely related species that are not so easy to differentiate using classical microbiological methods, representing an innovative technology in the field of animal health.
1. Introduction
Bacillus cereus sensu lato is a group of Gram-positive, rod-shaped and spore-forming aerobic bacteria that have close phylogenetic relationships and are therefore genetically very similar. Currently, this group includes several species, but despite being extremely similar and related, only some have an important impact on human and animal health. Bacillus anthracis is the causative agent of anthrax, a serious infectious disease that primarily involves herbivorous animals since they are most frequently exposed to the pathogen in the environment [1].
In fact, B. anthracis survives for decades in a spore-forming form on soil contaminated by the abandoned or buried carcasses of antecedently dead animals. Humans generally acquire anthrax via contact with infected animals or from occupational or nutritional exposure to contaminated animal products such as meat or skin [2]. In the diagnostic field, the identification of B. anthracis is based on phenotypic and genotypic characteristics. The strains of this species are non-hemolytic on Columbia blood agar, susceptible to penicillin and lysed by the gamma phage [2,3]. The virulence of B. anthracis is determined by two virulence plasmids, pXO1 and pXO2, that can be targeted by specific PCRs. The plasmid pXO1 encodes for three proteins: protective antigen (PA), lethal factor (LF) and edema factor (EF). Protective antigen binds to cellular receptors and mediates the entry of the other two into the cytoplasm of the host cell. Lethal factor is a protease that cleaves and inactivates all the protein kinases in the cytoplasm, leading to cell apoptosis and its response to different forms of cellular stress. Edema factor is a calmodulin (CaM)-dependent adenylate cyclase that causes the loss of chloride ions and water from the cell, resulting in extracellular edema [4]. Plasmid pXO2 harbors the genes that encode for the production of a polyglutamate capsule, which allows the pathogen to evade the host immune response by protecting itself from phagocytosis [4].
Anthrax in animals has an extremely rapid course with a fatal outcome that is characterized by sudden death due to acute or hyperacute septicemia and blood leakage from natural openings [5]. In humans, it is predominantly an occupational disease that develops following direct contact with infected animals or their products. Human anthrax can manifest itself in four clinical forms depending on the route via which the pathogen penetrates: cutaneous (the most frequent and non-fatal), gastrointestinal, inhalation and injectional (found in heroin addicts using drugs contaminated with Bacillus anthracis spores) [5].
Bacillus cereus sensu stricto (s.s.) is an opportunistic pathogen able of causing a foodborne disease due to its ability to form endospores that are resistant to high cooking temperatures and its capacity to produce toxins in a wide variety of foods. The symptoms of gastrointestinal infections caused by B. cereus include diarrhea and vomiting, generally acute and mild. B. cereus can also lead to some severe non-gastrointestinal infections, such as endophthalmitis, bacteremia, septicemia, meningitis, and pneumonia [6,7,8].
B. anthracis and Bacillus cereus s.s. have a peculiar biological feature that allows them to alternate between a vegetative phase and a long metabolic dormancy phase as a spore, during which they do not replicate over long periods. Due to this slow evolution, these microorganisms are genetically and phenotypically highly homogeneous. Therefore, in order to characterize them, it is necessary to resort to increasingly sophisticated biomolecular methods.
In recent decades, the improvements obtained in whole-genome sequencing (WGS) technologies and the development of increasingly sophisticated bioinformatics tools have revolutionized the investigation of inter- and intra-species diversity, also for the Bacillus cereus group. Although extremely effective, these techniques require long times, highly qualified personnel and have high costs. In recent years, the differentiation of these two species has also been performed by modern and faster approaches such as mass spectrometry MALDI-TOF, with very promising results [9].
Fourier transform infrared (FTIR) spectroscopy is a technique that, starting from the 1980s, has been used to study and characterize various types of microorganisms (bacteria, yeast, fungi, microalgae, viruses) [10] based on strain-specific absorbance patterns in the infrared spectrum [11]. Through this method, it is possible to characterize microorganisms in relation to the specific composition of their lipid, protein and polysaccharide components [12]. Indeed, for each bacterial strain, it is possible to obtain a unique absorption spectrum that represents the fingerprint obtained on the basis of the biomolecular components of the cell. Notably, the IR Biotyper® (IRBT) system (Bruker Daltonics, Bremen, Germany) based on FTIRS technology was launched in 2017 as a promising system in the field of microbial strain typing. Multiple successful applications have been reported [13] in the field of food, veterinary and water microbiology [14,15,16], as well as in hospital hygiene [17,18,19,20,21] and probiotic production [22,23].
In this study, we evaluated the power of IRBT to discriminate B. anthracis and Bacillus cereus s.s., the pathogenic bacteria belonging to the Bacillus cereus group, in typing different strains of B. anthracis previously identified through the classical methods of molecular epidemiology in order to detect any differences in their biomolecular (carbohydrates, proteins, lipids) composition; this could be useful for the identification.
2. Materials and Methods
2.1. Bacterial Isolates
In this study, a total of n = 52 strains of B. cereus s. s. isolated from food and n = 104 strains of B. anthracis collected at the Anthrax Reference Institute of Italy were tested. Regarding the B. anthracis strains, 3 vaccine strains (Carbosap, Sterne 34F2 and Pasteur type I), 77 bacterial strains isolated during anthrax outbreaks that occurred in Italy from 1989 to 2020, and 25 strains isolated from environmental samples from Albania (n.4), Bangladesh (n.11), Nepal (n. 3), Portugal (n.5) and Zambia (n.2) were included.
2.2. IR Biotyper
For IRBT analysis, isolates were grown on Tryptone Soy Agar (Liofilchem, Roseto degli Abruzzi, Italy) and incubated overnight at 37 °C. A 10 μL loopful of bacterial culture was collected and resuspended in 100 μL of distilled sterile water and incubated at 98 °C for 30 min (to inactivate vegetative and spore-forming forms). Subsequently, 50 μL was taken and added to 50 μL of 70% (vol/vol) ethanol in the Eppendorf tubes provided in the IR Biotyper kit (Bruker Daltonics, Bremen, Germany), which contain metal cylinders; this was vortexed to obtain a homogeneous suspension. Then, 15 μL of the bacterial suspension was spotted onto the IRBT silicon sample plate in three replicates and dried at room temperature. For each sample, three biological replicates (independent bacterial cultures on different days) were analyzed. For each run, quality control was performed with the Infrared Test Standards (IRTS 1 and 2) provided in the IR Biotyper kit (Bruker Daltonics, Bremen, Germany).
Spectra were acquired in transmission mode in the spectral range of 4000–500 cm−1 (mid-IR) using an IR Biotyper spectrometer (Bruker Optics-Daltonics, Bremen, Germany) and OPUS software v7.5 (Bruker Daltonics, Bremen, Germany). The IR Biotyper software (V 4.0) (Bruker Daltonics, Bremen, Germany) was used to process and analyze the acquired spectra. After acquisition, the spectra were vector-normalized using the Savitzky–-Golay algorithm, and the second derivative over 9 datapoints was calculated. An exploratory data analysis was performed using PCA (principal components analysis) and LDA (linear discriminant analysis). Preliminary checks were carried out to determine which wavenumbers provide the greatest discriminatory power in the differentiation of B. anthracis from B. cereus s.s., as well as to discriminate the B. anthracis vaccine strains from the pathogenic field ones. As LDA is a supervised method, it requires the assignment of a group identifier to define the classes to be differentiated and should be checked for overfitting. An LDA model was built using 50% of the strains (randomly selected) for its training, assigning the species as a group identifier. The robustness of the model was checked using the remaining strains, which were not assigned a group identifier. For the vaccine strains, as they are single isolates, the spectra were split between a training and testing set.
Furthermore, machine learning was evaluated to develop and test a classifier for the delineation of the five classes included in this study (B. anthracis field strains, B. anthracis Carbosap, B. anthracis Sterne 34F2, B. anthracis Pasteur type I, and Bacillus cereus s.s.). Artificial neural network (ANN) and support vector machine (SVM) algorithms were investigated. The training and testing sets were the same as those described above for the LDA model.
3. Results
The region of the IR spectrum that proved to be the best for the discrimination of B. anthracis/B. cereus isolates was found to be the region 1300–700 cm−1, corresponding to the absorption wavenumbers of polysaccharides and fingerprint (Figures S1 and S2).
An exploratory data analysis performed with PCA/LDA showed that B. anthracis and the Bacillus cereus s.s. form two well-separated clusters (Figure 1). It should be noted that information about the species is not part of the supervised model (the group identifier is the isolate).
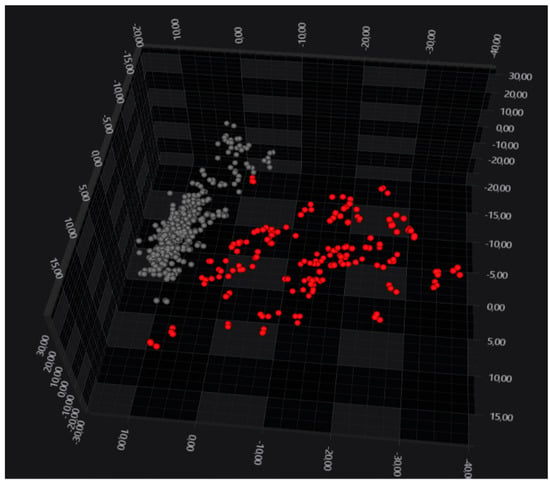
Figure 1.
LDA 3D scatterplot showing the clear separation of B. anthracis isolates (in grey) from B. cereus s.s. (in red). Each sphere represents a spectrum. LDA was performed using 30 principal components, corresponding to 94.1% of variance, and it assigned the isolate as a group identifier.
The LDA model proved to be very robust and showed a very good separation between B. anthracis and B. cereus s.s. The test spectra (represented by crosses) were correctly clustered in the group to which they belong (Figure 2).
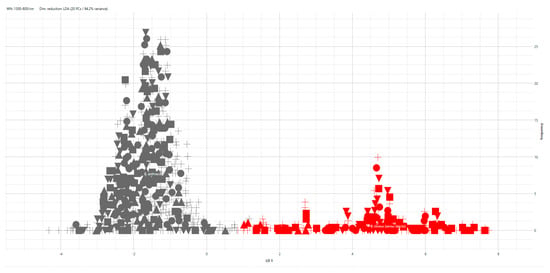
Figure 2.
LDA model using the species as a group identifier. Grey symbols represent the B. anthracis spectra, while red symbols represent the B. cereus s.s. spectra. The filled symbols represent the spectra of the training isolates (50% of the dataset). Crosses represent the test spectra, which were not used to build the model.
Further, the B. anthracis vaccine strains were found to be distinguishable from the field strains (Figure 3).
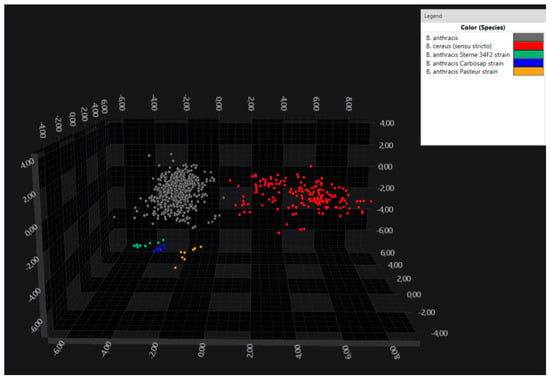
Figure 3.
LDA 3D scatterplot showing the separation of the B. anthracis vaccine strains (Sterne 34F2 in green, Carbosap in blue and Pasteur in yellow) from the field strains (in grey), as well as from B. cereus s. s. (in red). LDA was performed using 30 PCs, variance and by assigning the isolate as a group identifier. Each sphere represents one spectrum.
The LDA model also proved to be very robust regarding the separation between the B. anthracis vaccine strains, B. anthracis field strains and B. cereus s.s. (Figure 4).
Figure 4.
LDA model using the species as a group identifier for the B. anthracis field strains (grey symbols) and B. cereus s.s. (red symbols), and the isolate for the B. anthracis vaccine strains (yellow, blue and green symbols). The filled symbols represent the spectra of the training isolates (50% of the dataset). Crosses represent the test spectra, which were not used to build the model. The ellipses correspond to the 95 CI.
Both machine learning algorithms that were investigated (ANN and linear SVM) showed a very good performance, with SVM being slightly superior (100% vs. 99%)—Figure 5.
Figure 5.
Confusion matrix showing the accuracy of the SVM classifier with the testing set. All spectra were correctly classified with the exception of one spectrum of a field B. anthracis strain misclassified as B. cereus s.s. The class recall (corresponding to sensitivity) and class precision (corresponding to specificity) are 99% for B. anthracis (field strains) and 100% for the B. anthracis vaccine strains and B. cereus s.s.
4. Discussion
Developing extremely sensitive, rapid, and effective protocols for typing microorganisms and reconstructing the epidemiology of an outbreak could be important for reducing the time and cost of analysis that traditional methods require.
The efficacy of FTIRS in identifying bacteria in comparison with DNA-based techniques has been largely demonstrated [21,24]. Although WGS remains the most powerful approach used to characterize strains accurately [25,26], it requires time and highly qualified personnel. In contrast, FTIRS was developed as an easy, fast, and cost-effective method able to type different microorganisms belonging to the same species, with the main objective being to carry out the outbreak investigation in real time.
To the best of our knowledge, the present study was the first to evaluate the ability of IRBT to typify B. anthracis and Bacillus cereus s.s., in order to provide relevant feedback on the usefulness of this new diagnostic tool. From the preliminary results of this study, IRBT showed the ability to successfully differentiate B. anthracis from Bacillus cereus s.s. Furthermore, the three vaccine strains are clearly separated from each other, as well as from the field pathogenic strains of B. anthracis in the multidimensional spectral space. This is presumably related to the fact that the changes in the molecular structures exhibited by the vaccine strains compared to the pathogenic strains are detectable via FTIR.
The application of an artificial intelligence algorithm classifier for the discrimination of B. cereus, B. anthracis field strains and B. anthracis vaccine strains to be created; this could be implemented in routine analysis for a quick and prompt identification of unknown strains.
The next goal will be to explore the potential application of IRBT in the clustering analysis of pathogenic strains of B. anthracis to verify the overlap with the results obtained with MLVA and WGS. Furthermore, the possible application of this method in the discrimination of B. anthracis from other closely related species belonging to the Bacillus cereus group will be studied.
In conclusion, the advantages of IRBT include its ease of use, its fast response time with a relatively high discriminating power, and its low operating costs in comparison to molecular typing methods. For these reasons, IRBT could represent a good solution for the surveillance and typing of microorganisms, including B. anthracis, in addition to the most used genetic methods such as WGS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12010183/s1, Figure S1. Second derivatives of infrared spectra of all isolates in the wavenumber range from 4000 to 500 cm−1; Figure S2. Second derivatives of infrared spectra in the wavenumber range 1300–700 cm−1.
Author Contributions
Conceptualization, V.M., V.R. and L.P.; methodology, V.M., V.R., L.S., D.F., M.C. (Marta Caruso), R.F., L.M.D., F.T., V.V. and D.C.; software, V.M. and M.C. (Miriam Cordovana); validation, V.M. and M.C. (Miriam Cordovana); data curation, V.M., M.C. (Miriam Cordovana) and D.G.; writing—original draft preparation, V.M. and M.C. (Miriam Cordovana); writing—review and editing, D.G.; supervision, D.G.; project administration, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
Miriam Cordovana is an employee of the company Bruker Daltonic GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Turnbull, P.C. Introduction: Anthrax history, disease and ecology. Curr. Top. Microbiol. Immunol. 2002, 271, 1–19. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Anthrax in Humans and Animals, 4th ed.; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Pilo, P.; Frey, J. Pathogenicity, population genetics and dissemination of Bacillus anthracis. Infect. Genet. Evol. 2018, 64, 115–125. [Google Scholar] [CrossRef]
- Moayeri, M.; Leppla, S.H.; Vrentas, C.; Pomerantsev, A.P.; Liu, S. Anthrax Pathogenesis. Annu. Rev. Microbiol. 2015, 69, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Sweeney, D.A.; Cui, X.; Li, Y.; Eichacker, P.Q. An overview of anthrax infection including the recently identified form of disease in injection drug users. Intensive Care Med. 2012, 38, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Drobniewski, F.A. Bacillus cereus and related species. Clin. Microbiol. Rev. 1993, 6, 324–338. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- Schoeni, J.L.; Wong, A.C.L. Bacillus cereus food poisoning and its toxins. J. Food Prot. 2005, 68, 636–648. [Google Scholar] [CrossRef]
- Manzulli, V.; Rondinone, V.; Buchicchio, A.; Serrecchia, L.; Cipolletta, D.; Fasanella, A.; Parisi, A.; Difato, L.; Iatarola, M.; Aceti, A.; et al. Discrimination of Bacillus cereus Group Members by MALDI-TOF Mass Spectrometry. Microorganisms 2021, 9, 1202. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Tugarova, A.V. Specificities of the Fourier Transform Infrared Spectroscopic Methodology and Interpretation of Spectroscopic Data in Microbiological Analyses. J. Anal. Chem. 2023, 78, 1320–1332. [Google Scholar] [CrossRef]
- Novais, Â.; Freitas, A.R.; Rodrigues, C.; Peixe, L. Fourier transform infrared spectroscopy: Unlocking fundamentals and prospects for bacterial strain typing. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 38, 427–448. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, S.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Uribe, G.; Salipante, S.J.; Curtis, L.; Lieberman, J.A.; Kurosawa, K.; Cookson, B.T.; Hoogestraat, D.; Stewart, M.K.; Olmstead, T.; Bourassa, L. Evaluation of Fourier transform-infrared spectroscopy (FT-IR) as a control measure for nosocomial outbreak investigations. J. Clin. Microbiol. 2023, 61, e0034723. [Google Scholar] [CrossRef] [PubMed]
- Tiquia-Arashiro, S.; Li, X.; Pokhrel, K.; Kassem, A.; Abbas, L.; Coutinho, O.; Kasperek, D.; Najaf, H.; Opara, S. Applications of Fourier Transform-Infrared spectroscopy in microbial cell biology and environmental microbiology: Advances, challenges, and future perspectives. Front. Microbiol. 2023, 14, 1304081. [Google Scholar] [CrossRef]
- Cordovana, M.; Mauder, N.; Join-Lambert, O.; Gravey, F.; LeHello, S.; Auzou, M.; Pitti, M.; Zoppi, S.; Buhl, M.; Steinmann, J.; et al. Machine learning-based typing of Salmonella enterica O-serogroups by the Fourier-Transform Infrared (FTIR) Spectroscopy-based IR Biotyper system. J. Microbiol. Methods 2022, 201, 106564. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Romano, A.; Giacometti, F.; Indio, V.; Pitti, M.; Decastelli, L.; Devalle, P.L.; Gorrasi, I.S.R.; Miaglia, S.; Serraino, A. Investigation of a Staphylococcus aureus sequence type 72 food poisoning outbreak associated with food-handler contamination in Italy. Zoonoses Public Health 2023, 70, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Kim, Y.A.; Lee, S.J.; Jung, W.W.; Kim, H.S.; Kim, S.S.; Kim, H.; Yong, D.; Lee, K. Performance Comparison Between Fourier-Transform Infrared Spectroscopy-based IR Biotyper and Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for Strain Diversity. Ann. Lab. Med. 2023, 43, 174–179. [Google Scholar] [CrossRef]
- Azrad, M.; Matok, L.A.; Leshem, T.; Peretz, A. Comparison of FT-IR with whole-genome sequencing for identification of maternal-to-neonate transmission of antibiotic-resistant bacteria. J. Microbiol. Methods. 2022, 202, 106603. [Google Scholar] [CrossRef]
- Contreras, D.A.; Morgan, M.A. Surveillance diagnostic algorithm using real-time PCR assay and strain typing method development to assist with the control of C. auris amid COVID-19 pandemic. Front. Cell Infect. Microbiol. 2022, 12, 887754. [Google Scholar] [CrossRef]
- Kon, H.; Temkin, E.; Elmalih, P.; Keren-Paz, A.; Ben-David, D.; Najjar-Debbiny, R.; Gottesman, T.; Carmeli, Y. Analysis of four carbapenem-resistant Acinetobacter baumannii outbreaks using Fourier-transform infrared spectroscopy. Infect. Control Hosp. Epidemiol. 2023, 44, 991–993. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, H.; Lu, J.; Sun, Q.; Liu, C.; Zeng, Y.; Zhang, R. Evaluation of the IR Biotyper for Klebsiella pneumoniae typing and its potentials in hospital hygiene management. Microb. Biotechnol. 2021, 14, 1343–1352. [Google Scholar] [CrossRef]
- Deidda, F.; Cordovana, M.; BozziCionci, N.; Graziano, T.; Di Gioia, D.; Pane, M. In-process real-time probiotic phenotypic strain identity tracking: The use of Fourier transform infrared spectroscopy. Front. Microbiol. 2022, 13, 1052420. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, L.; Wang, X.; Li, J.; Tang, B. Evaluation of IR Biotyper for Lactiplantibacillus plantarum Typing and Its Application Potential in Probiotic Preliminary Screening. Front. Microbiol. 2022, 13, 823120. [Google Scholar] [CrossRef] [PubMed]
- Martak, D.; Valot, B.; Sauget, M.; Cholley, P.; Thouverez, M.; Bertrand, X.; Hocquet, D. Fourier-transform infrared spectroscopy can quickly type Gram negative bacilli responsible for hospital outbreaks. Front. Microbiol. 2019, 10, 1440. [Google Scholar] [CrossRef]
- Buron-Moles, G.; Chailyan, A.; Dolejs, I.; Forster, J.; Mikš, M.H. Uncovering carbohydrate metabolism through a genotype-phenotype association study of 56 lactic acid bacteria genomes. Appl. Microbiol. Biotechnol. 2019, 103, 3135–3152. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, S.; Park, Y.S. Molecular typing tools for identifying and characterizing lactic acid bacteria: A review. Food Sci. Biotechnol. 2020, 29, 1301–1318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).