Simultaneous Differential Detection of H5, H7, H9 and Nine NA Subtypes of Avian Influenza Viruses via a GeXP Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of High-Performance, Gene-Specific Primers for a GeXP Multiplex
2.2. Viral DNA/RNA Nucleic Acid Extraction
2.3. Reaction Procedures and Conditions of the GeXP Assay
2.4. Validation of a Single Primer and Preliminary Evaluation of Multiple Primers
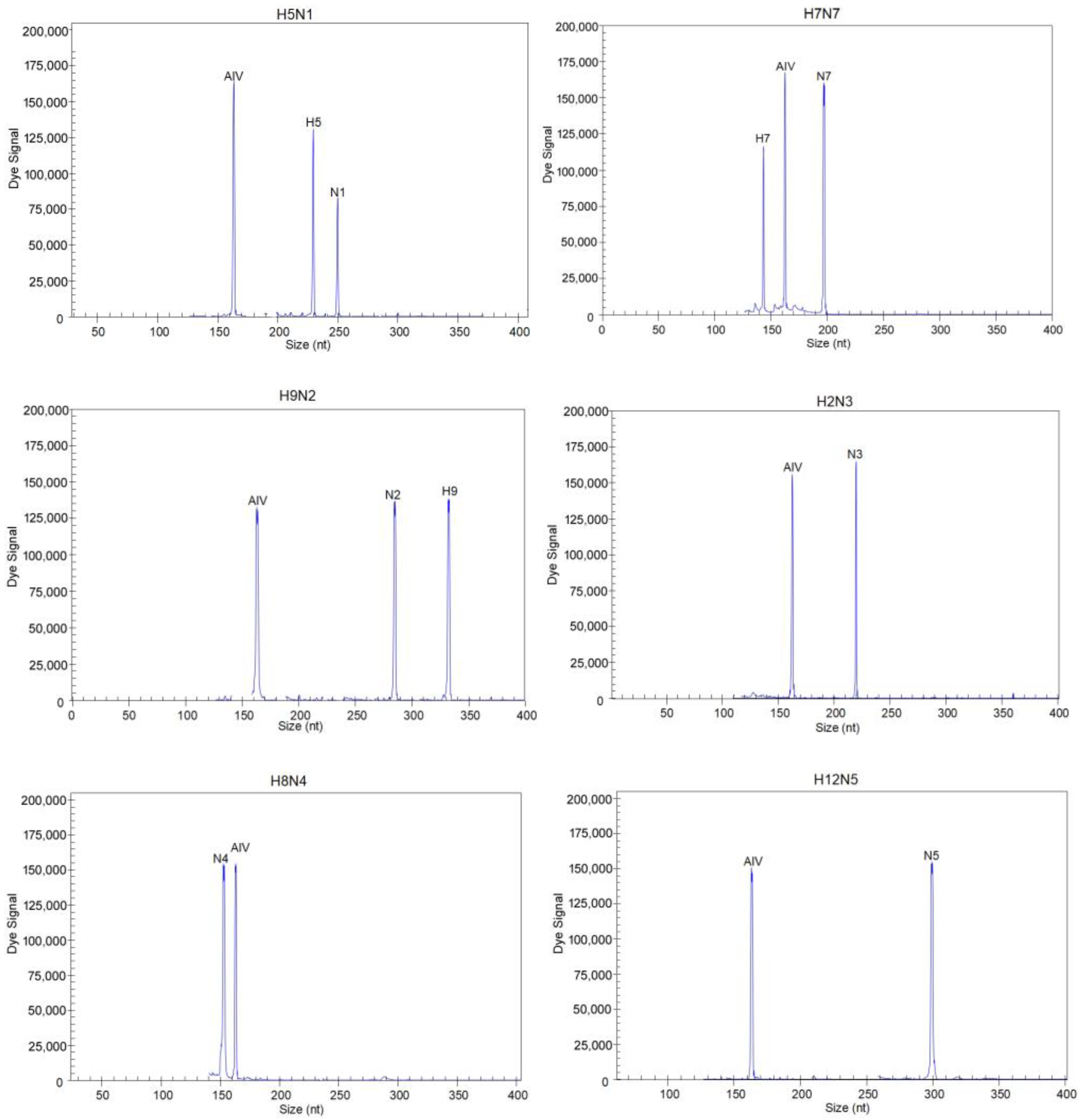
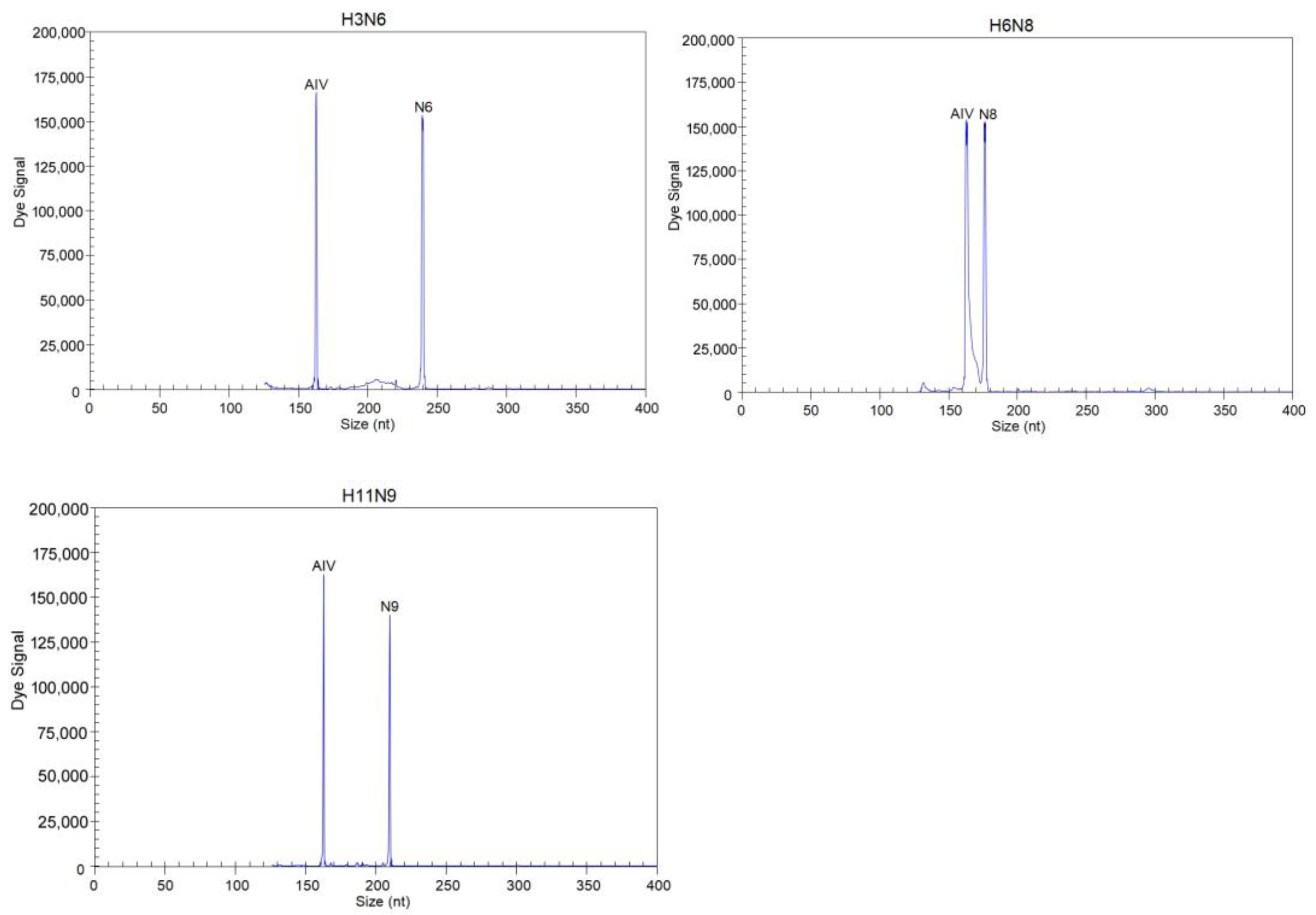
2.5. Evaluation of the Specificity of the GeXP Assay
2.6. Evaluation of the Sensitivity of the GeXP Assay
2.7. Application for Detecting Clinical Samples
3. Results
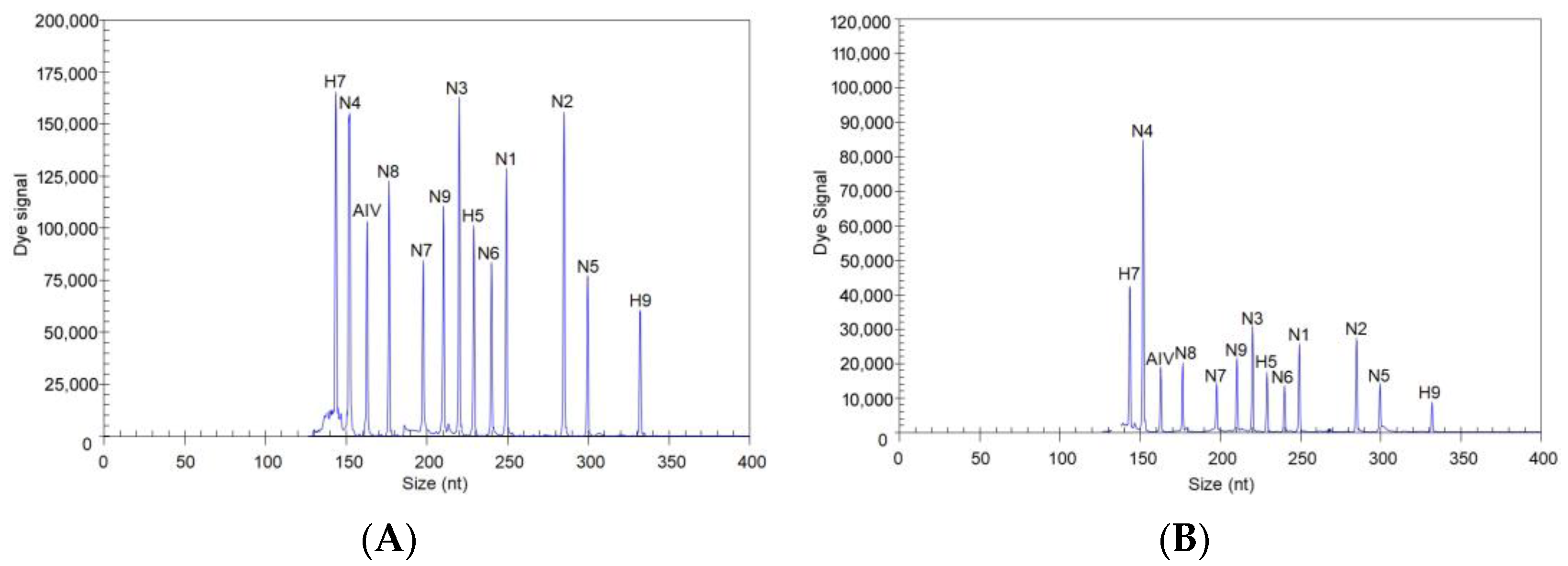
3.1. Screening Results for the 13 Pairs of Primers
3.2. Evaluation of the Specificity of the GeXP Assay
3.3. Evaluation of the Sensitivity of the GeXP Assay
3.4. Detection of AIVs in Clinical Samples Using the GeXP Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoon, S.W.; Webby, R.J.; Webster, R.G. Evolution and ecology of influenza A viruses. Curr. Top. Microbiol. Immunol. 2014, 385, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A Distinct Lineage of Influenza a Virus from Bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Verhagen, J.H.; Mostafa, A.; Wille, M.; Li, R.; Graaf, A.; Jarhult, J.D.; Ellstrom, P.; Zohari, S.; Lundkvist, A.; et al. Global patterns of avian influenza A (H7): Virus evolution and zoonotic threats. FEMS Microbiol. Rev. 2019, 43, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, R.; Saad, M.D.; Davis, C.T.; Swayne, D.E.; Wang, D.; Wong, F.Y.K.; McCauley, J.W.; Peiris, J.S.M.; Webby, R.J.; Fouchier, R.A.M.; et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev. Med. Virol. 2020, 30, e2099. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.; Klimov, A.; Katz, J.; Regnery, H.; Lim, W.; Hall, H.; Perdue, M.; Swayne, D.; Bender, C.; Huang, J.; et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 1998, 279, 393–396. [Google Scholar] [CrossRef]
- Bui, C.H.T.; Kuok, D.I.T.; Yeung, H.W.; Ng, K.C.; Chu, D.K.W.; Webby, R.J.; Nicholls, J.M.; Peiris, J.S.M.; Hui, K.P.Y.; Chan, M.C.W. Risk Assessment for Highly Pathogenic Avian Influenza A(H5N6/H5N8) Clade 2.3.4.4 Viruses. Emerg. Infect. Dis. 2021, 27, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Harfoot, R.; Webby, R.J. H5 influenza, a global update. J. Microbiol. 2017, 55, 196–203. [Google Scholar] [CrossRef]
- Yin, X.; Deng, G.; Zeng, X.; Cui, P.; Hou, Y.; Liu, Y.; Fang, J.; Pan, S.; Wang, D.; Chen, X.; et al. Genetic and biological properties of H7N9 avian influenza viruses detected after application of the H7N9 poultry vaccine in China. PLoS Pathog. 2021, 17, e1009561. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, W.; Sun, Y.; Guo, Y.; Wu, Y.; Pu, J. Effect of the Interaction between Viral PB2 and Host SphK1 on H9N2 AIV Replication in Mammals. Viruses 2022, 14, 1585. [Google Scholar] [CrossRef]
- Kageyama, T.; Fujisaki, S.; Takashita, E.; Xu, H.; Yamada, S.; Uchida, Y.; Neumann, G.; Saito, T.; Kawaoka, Y.; Tashiro, M. Genetic analysis of novel avian A(H7N9) influenza viruses isolated from patients in China, February to April 2013. Eurosurveillance 2013, 18, 20453. [Google Scholar] [CrossRef]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Carnaccini, S.; Perez, D.R. H9 Influenza Viruses: An Emerging Challenge. Cold Spring Harb. Perspect. Med. 2020, 10, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Lejarazu Leonardo, R.; Rojo Rello, S.; Sanz Munoz, I. Diagnostic challenges in influenza. Enferm. Infecc. Microbiol. Clin. 2019, 37 (Suppl. 1), 47–55. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Pang, Y.S.; Liu, J.; Deng, X.; Tang, X.; Sun, J.; Khan, M.I. A multiplex RT-PCR for detection of type A influenza virus and differentiation of avian H5, H7, and H9 hemagglutinin subtypes. Mol. Cell Probes 2006, 20, 245–249. [Google Scholar] [CrossRef]
- Liu, J.; Yao, L.; Zhai, F.; Chen, Y.; Lei, J.; Bi, Z.; Hu, J.; Xiao, Q.; Song, S.; Yan, L.; et al. Development and application of a triplex real-time PCR assay for the simultaneous detection of avian influenza virus subtype H5, H7 and H9. J. Virol. Methods 2018, 252, 49–56. [Google Scholar] [CrossRef]
- Zhang, S.; Shin, J.; Shin, S.; Chung, Y.J. Development of reverse transcription loop-mediated isothermal amplification assays for point-of-care testing of avian influenza virus subtype H5 and H9. Genom. Inform. 2020, 18, e40. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xie, Z.; Huang, J.; Xie, Z.; Xie, L.; Zhang, M.; Li, M.; Wang, S.; Li, D.; Zeng, T.; et al. Simultaneous Differentiation of the N1 to N9 Neuraminidase Subtypes of Avian Influenza Virus by a GeXP Analyzer-Based Multiplex Reverse Transcription PCR Assay. Front. Microbiol. 2019, 10, 1271. [Google Scholar] [CrossRef]
- Yang, M.; Luo, L.; Nie, K.; Wang, M.; Zhang, C.; Li, J.; Ma, X. mGenotyping of 11 human papillomaviruses by mul-tiplex PCR with a GeXP analyzer. J. Med. Virol. 2012, 84, 957–963. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, Z.X.; Liu, J.B.; Pang, Y.S.; Deng, X.W.; Xie, Z.Q.; Xie, L.J.; Fan, Q.; Luo, S.S. Epidemiological surveillance of low pathogenic avian influenza virus (LPAIV) from poultry in Guangxi Province, Southern China. PLoS ONE 2013, 8, e77132. [Google Scholar] [CrossRef]
- Hoffmann, E.; Stech, J.; Guan, Y.; Webster, R.G.; Perez, D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001, 146, 2275–2289. [Google Scholar] [CrossRef]
- Sutton, T.C. The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses. Viruses 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Charostad, J.; Rezaei Zadeh Rukerd, M.; Mahmoudvand, S.; Bashash, D.; Hashemi, S.M.A.; Nakhaie, M.; Zandi, K. A comprehensive review of highly pathogenic avian influenza (HPAI) H5N1: An imminent threat at doorstep. Travel. Med. Infect. Dis. 2023, 55, 102638. [Google Scholar] [CrossRef] [PubMed]
- Abdelwhab, E.M.; Veits, J.; Mettenleiter, T.C. Prevalence and control of H7 avian influenza viruses in birds and humans. Epidemiol. Infect. 2014, 142, 896–920. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Qin, K. Human-infecting influenza A (H9N2) virus: A forgotten potential pandemic strain? Zoonoses Public. Health 2020, 67, 203–212. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Song, W.; Pang, Q.; Miao, Z. Avian influenza virus H9N2 seroprevalence and risk factors for infection in occupational poultry-exposed workers in Tai’an of China. J. Med. Virol. 2016, 88, 1453–1456. [Google Scholar] [CrossRef]
- Bhat, S.; James, J.; Sadeyen, J.R.; Mahmood, S.; Everest, H.J.; Chang, P.; Walsh, S.K.; Byrne, A.M.P.; Mollett, B.; Lean, F.; et al. Coinfection of Chickens with H9N2 and H7N9 Avian Influenza Viruses Leads to Emergence of Reassortant H9N9 Virus with Increased Fitness for Poultry and a Zoonotic Potential. J. Virol. 2022, 96, e0185621. [Google Scholar] [CrossRef]
| Virus | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Gene | GenBank Accession Number |
|---|---|---|---|---|
| AIV | AGGTGACACTATAGAATA AGGCTCTCATGGAGTGGCTA | GTACGACTCACTATAGGGA TGGACAAAGCGTCTACGCTG | M | OQ830456.1 |
| AIV-H5 | AGGTGACACTATAGAATA ATACACCCTCTCACCATCGG | GTACGACTCACTATAGGGA TTGCTGTGGTGGTACCCATA | HA | OQ546777.1 |
| AIV-H7 | AGGTGACACTATAGAATA AGAATACAGATTGACCCAGTSAA | GTACGACTCACTATAGGGA CCCATTGCAATGGCHAGAAG | HA | MK453329.1 |
| AIV-H9 | AGGTGACACTATAGAATA ATGGCAAYCCTTCYTGTGA | GTACGACTCACTATAGGGA TTGTGTATTGGGCGTCYTG | HA | OR528241.1 |
| AIV-N1 | AGGTGACACTATAGAATA GGTGTTTGGATCGGRAGAAC | GTACGACTCACTATAGGGA TCAACCCAGAARCAAGGTC | NA | OP373692.1 |
| AIV-N2 | AGGTGACACTATAGAATA TTGGGTGTTCCGTTTCA | GTACGACTCACTATAGGGA CCATCCGTCATTACTAC | NA | OQ954751.1 |
| AIV-N3 | AGGTGACACTATAGAATA TTCCCAATAGGAACAGCYCCAGT | GTACGACTCACTATAGGGA TTCTCCATGATTTRATGGAGTC | NA | MK978938.1 |
| AIV-N4 | AGGTGACACTATAGAATA CAGAYAAGGAYTCAAATGGTGT | GTACGACTCACTATAGGGA CATGGTACAGTGCAATTCCT | NA | MT421389.1 |
| AIV-N5 | AGGTGACACTATAGAATA GTGAGGTCATGGAGAAAGCA | GTACGACTCACTATAGGGA TGGYCTATTCATTCCRTTCCA | NA | LC339605.1 |
| AIV-N6 | AGGTGACACTATAGAATA CACTATAGATCCYGARATGATGACC | GTACGACTCACTATAGGGA GGAGTCTTTGCTAATWGTCCTTCCA | NA | MT375537.1 |
| AIV-N7 | AGGTGACACTATAGAATA GACAGRACWGCTTTCAGAGG | GTACGACTCACTATAGGGA GTTGCGTTGTCATTATTTCC | NA | MN253549.1 |
| AIV-N8 | AGGTGACACTATAGAATA AGGGAATACAATGAAACAGT | GTACGACTCACTATAGGGA TGCAAAACCCTTAGCATCACA | NA | MT421085.1 |
| AIV-N9 | AGGTGACACTATAGAATA CGCCCTGATAAGCTGGCCACT | GTACGACTCACTATAGGGA ACAGGCCTTCTGTTGTACCA | NA | KP418553.1 |
| GeXP universal primer | AGGTGACACTATAGAATA | GTACGACTCACTATAGGGA |
| Type of Chicken Samples | Number of Positive Samples | GeXP Assay | Real-Time RT-PCR | Sequencing | ||
|---|---|---|---|---|---|---|
| Three Methods for H5, H7 and H9 Real-Time RT-PCR | Nine Methods for N1–N9 Real-Time RT-PCR | HA Gene | NA Gene | |||
| Swab samples from LBMs | 8 | H9, N2, AIV | H9 | N2 | H9 | N2 |
| 2 | N1, AIV | None a | N1 | - b | N1 | |
| 9 | N2, AIV | None a | N2 | - b | N2 | |
| 5 | N6, AIV | None a | N6 | - b | N6 | |
| 3 | N8, AIV | None a | N8 | - b | N8 | |
| Tissue samples from challenged SPF chickens | 3 c | H5, N2, AIV | H5 | N2 | H5 | N2 |
| 3 c | H5, N2, AIV | H5 | N2 | H5 | N2 | |
| 3 c | H5, N2, AIV | H5 | N2 | H5 | N2 | |
| 3 d | H7, N9, AIV | H7 | N9 | H7 | N9 | |
| 3 d | H7, N9, AIV | H7 | N9 | H7 | N9 | |
| 3 d | H7, N9, AIV | H7 | N9 | H7 | N9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Xie, Z.; Li, M.; Li, D.; Zhang, M.; Ruan, Z.; Xie, L.; Wang, S.; Fan, Q.; Zhang, Y.; et al. Simultaneous Differential Detection of H5, H7, H9 and Nine NA Subtypes of Avian Influenza Viruses via a GeXP Assay. Microorganisms 2024, 12, 143. https://doi.org/10.3390/microorganisms12010143
Luo S, Xie Z, Li M, Li D, Zhang M, Ruan Z, Xie L, Wang S, Fan Q, Zhang Y, et al. Simultaneous Differential Detection of H5, H7, H9 and Nine NA Subtypes of Avian Influenza Viruses via a GeXP Assay. Microorganisms. 2024; 12(1):143. https://doi.org/10.3390/microorganisms12010143
Chicago/Turabian StyleLuo, Sisi, Zhixun Xie, Meng Li, Dan Li, Minxiu Zhang, Zhihua Ruan, Liji Xie, Sheng Wang, Qing Fan, Yanfang Zhang, and et al. 2024. "Simultaneous Differential Detection of H5, H7, H9 and Nine NA Subtypes of Avian Influenza Viruses via a GeXP Assay" Microorganisms 12, no. 1: 143. https://doi.org/10.3390/microorganisms12010143
APA StyleLuo, S., Xie, Z., Li, M., Li, D., Zhang, M., Ruan, Z., Xie, L., Wang, S., Fan, Q., Zhang, Y., Huang, J., & Zeng, T. (2024). Simultaneous Differential Detection of H5, H7, H9 and Nine NA Subtypes of Avian Influenza Viruses via a GeXP Assay. Microorganisms, 12(1), 143. https://doi.org/10.3390/microorganisms12010143







