Campylobacteriosis in Finland: Passive Surveillance in 2004–2021 and a Pilot Case-Control Study with Whole-Genome Sequencing in Summer 2022
Abstract
1. Introduction
2. Materials and Methods
2.1. Campylobacter Surveillance
2.2. Outbreaks
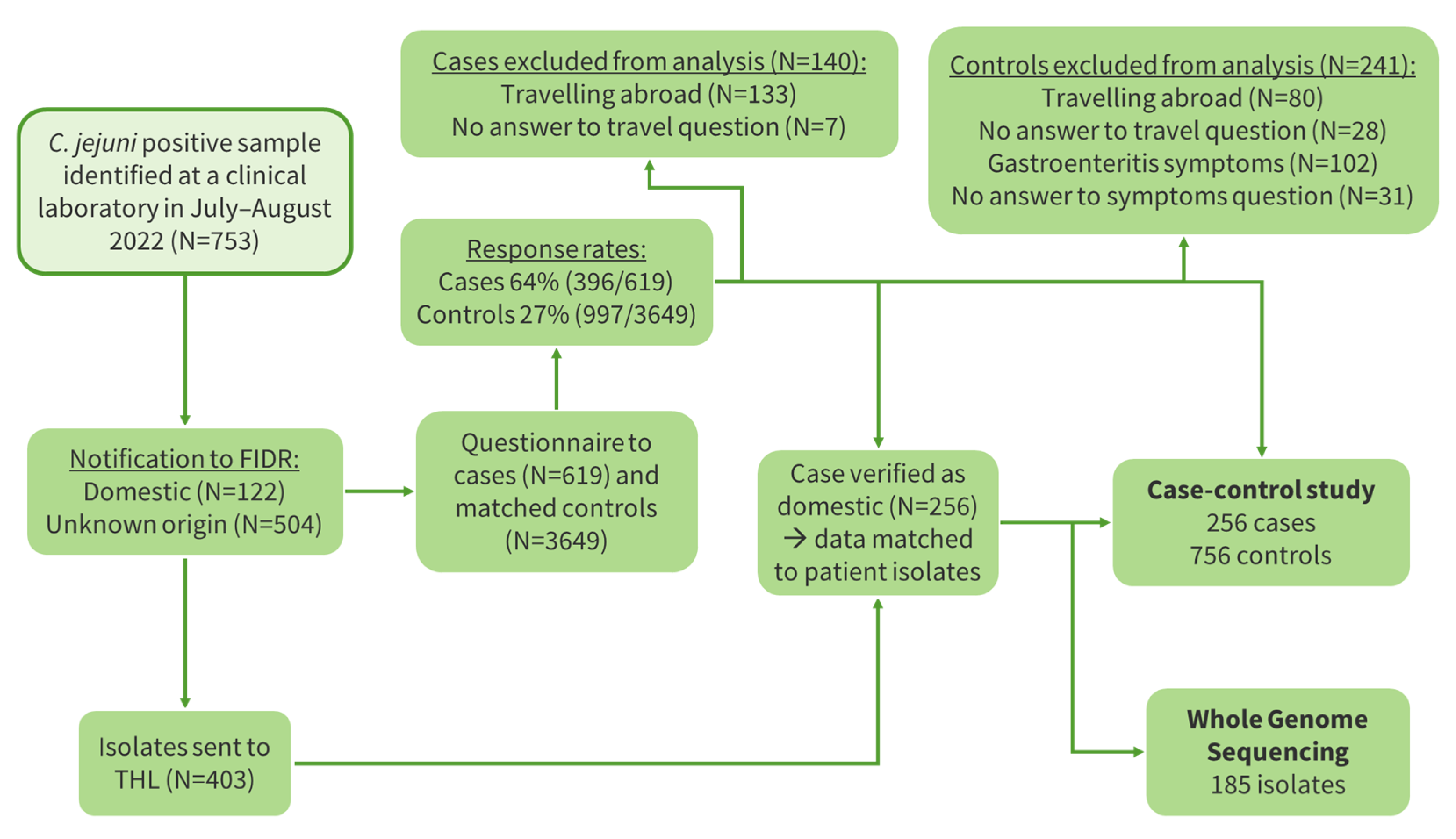
2.3. Case-Control Study
2.4. Microbiological Samples and Methods
2.4.1. Sample Collection and Handling
2.4.2. Whole Genome Sequencing (WGS) and Sequence Analysis
2.5. Source Attribution
2.6. Ethical Approval Statement
3. Results
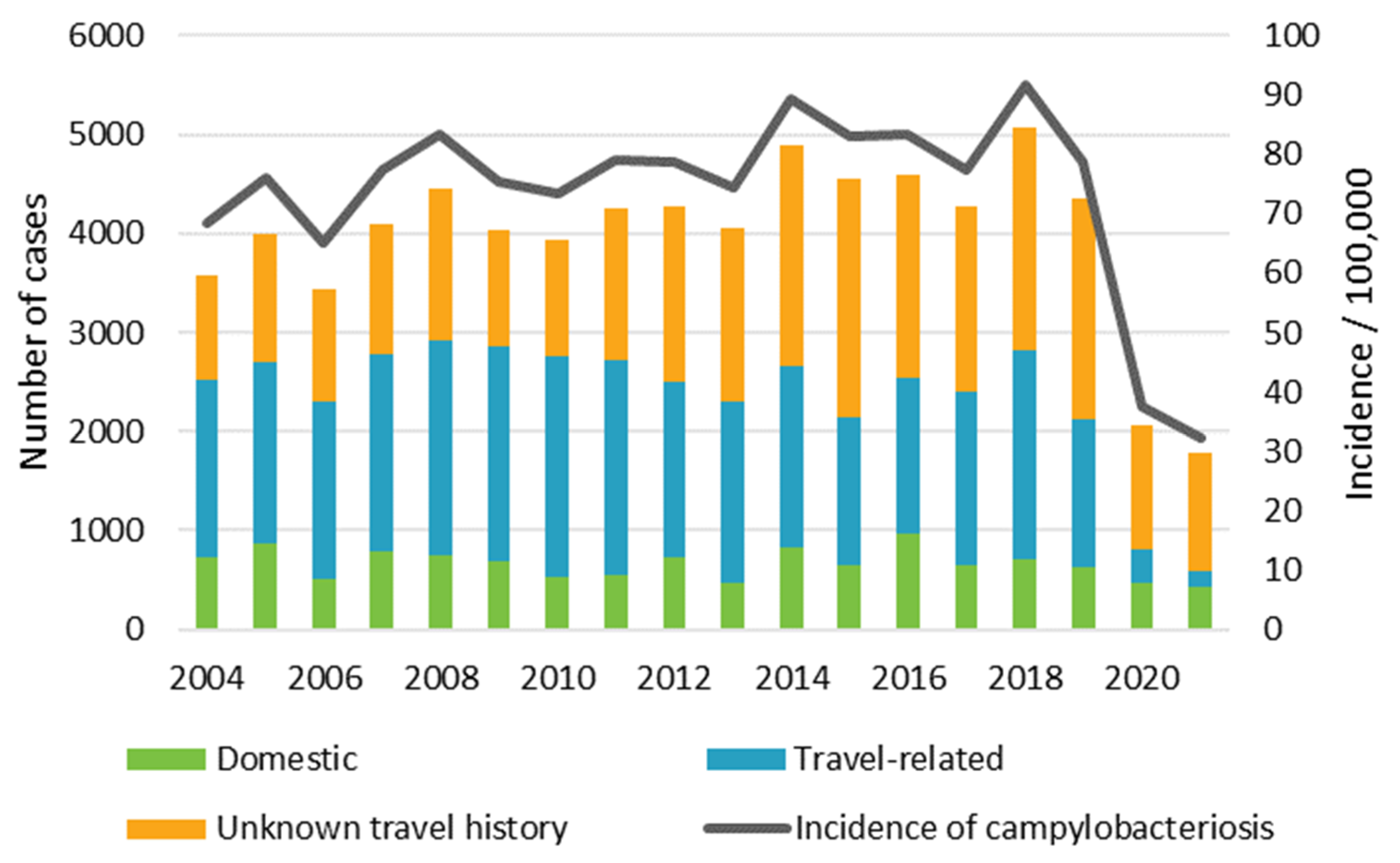
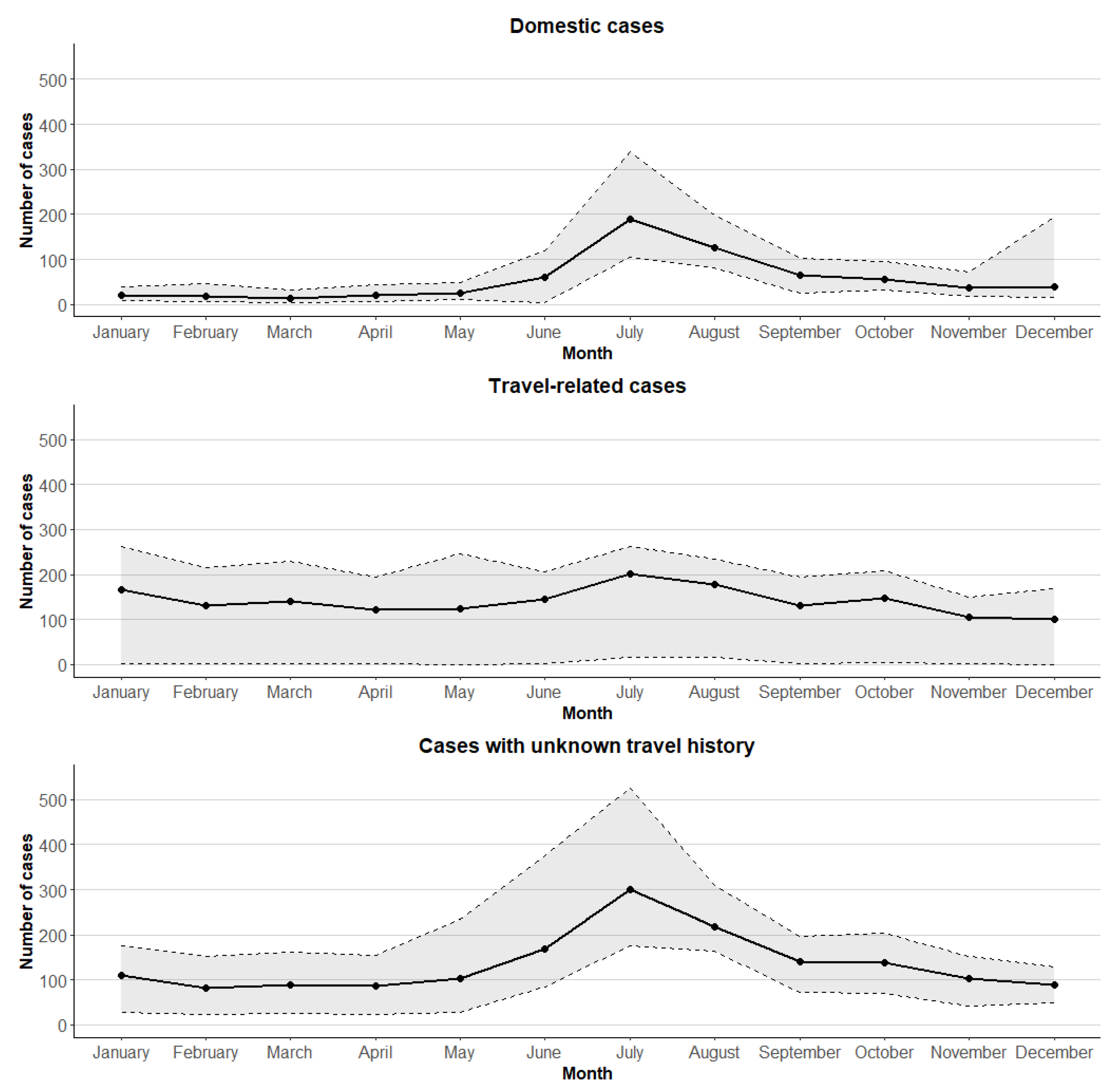
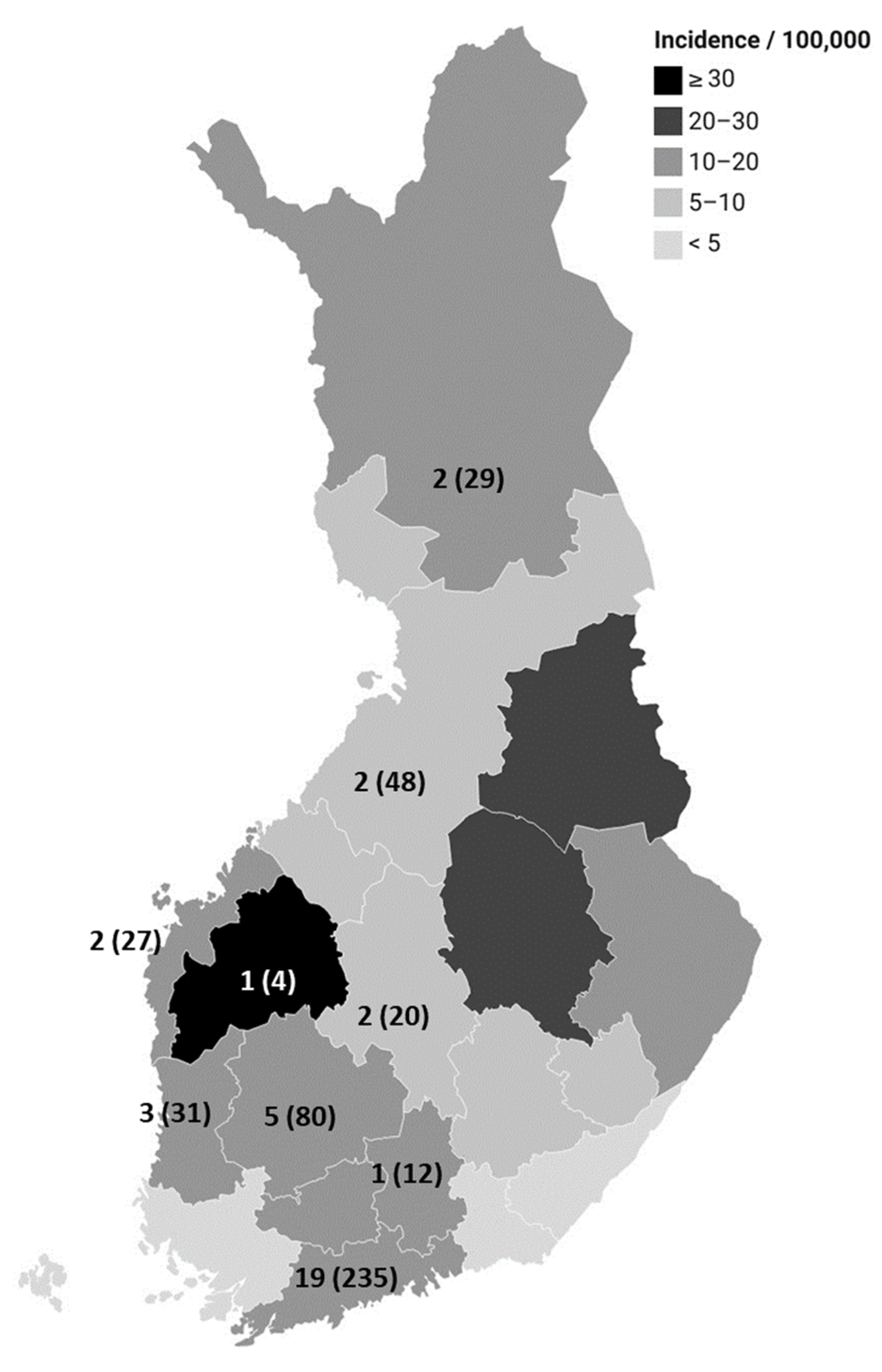
3.1. Campylobacter Surveillance Data from Finnish Infectious Disease Register, 2004–2021
3.2. Food- and Waterborne Outbreaks Caused by Campylobacter, 2010–2021
3.3. Case-Control Study
3.4. Cluster Analysis of Bacterial Isolates
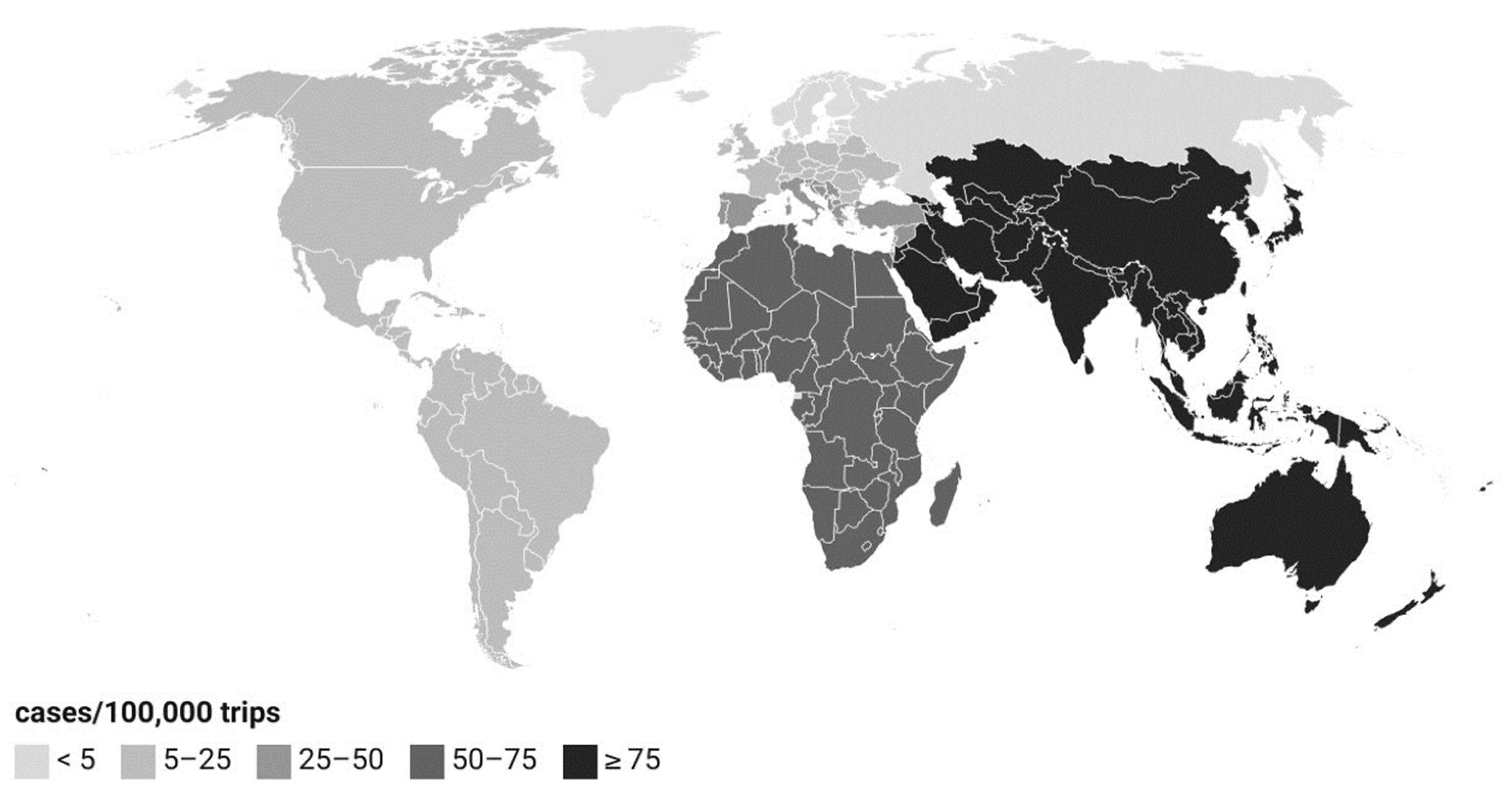
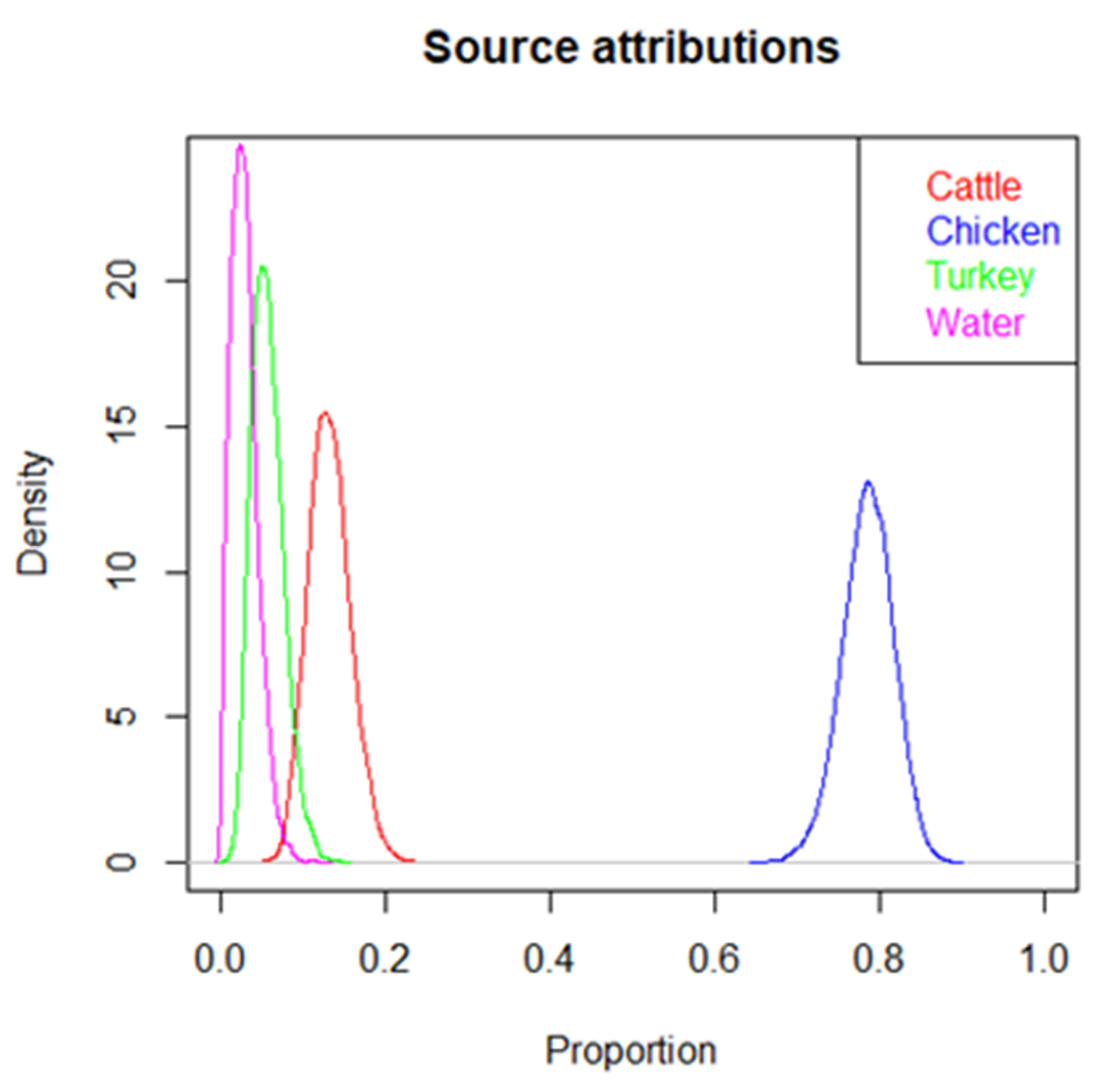
3.5. Source Attribution
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar] [CrossRef]
- Fitzgerald, C. Campylobacter. Clin. Lab. Med. 2015, 35, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Hansson, I.; Sandberg, M.; Habib, I.; Lowman, R.; Engvall, E.O. Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 30–48. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H.; van den Akker, B.; Giglio, S.; Bentham, R. The role of environmental reservoirs in human campylobacteriosis. Int. J. Environ. Res. Public. Health 2013, 10, 5886–5907. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Panel on Biological Hazards. Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA J. 2023, 21, 7867. [Google Scholar] [CrossRef]
- Communicable Diseases Act (21.12.2016/1227). Available online: https://www.finlex.fi/fi/laki/ajantasa/2016/20161227 (accessed on 26 January 2023).
- Official Statistics of Finland: Finnish Travel [e-publication]; Statistics Finland: Helsinki, Finland; ISSN 1798-8837. Available online: https://stat.fi/tilasto/smat (accessed on 7 September 2022).
- Council of State’s Decree on Investigating Food—And Waterborne Outbreaks (1365/2011). Available online: https://www.finlex.fi/fi/laki/alkup/2011/20111365 (accessed on 26 January 2023).
- Hosmer, D.; Lemeshow, S.; Sturdivant, R. Applied Logistic Regression, 3rd ed.; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Decree of the Ministry of Agriculture and Forestry about Zoonoses (316/2021). Available online: https://www.finlex.fi/fi/laki/alkup/2021/20210316 (accessed on 8 September 2023).
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: http://data.europa.eu/eli/reg/2005/2073/2020-03-08 (accessed on 8 September 2023).
- Commission Regulation (EU) 2017/1495 of 23 August 2017 Amending Regulation (EC) No 2073/2005 as regards Campylobacter in Broiler Carcases. Available online: http://data.europa.eu/eli/reg/2017/1495/oj (accessed on 8 September 2023).
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Dingle, K.E.; Colles, F.M.; Wareing, D.R.; Ure, R.; Fox, A.J.; Bolton, F.E.; Bootsma, H.J.; Willems, R.J.; Urwin, R.; Maiden, M.C. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef]
- Gupta, A.; Jordan, I.K.; Rishishwar, L. stringMLST: A fast k-mer based tool for multilocus sequence typing. Bioinformatics 2017, 33, 119–121. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Llarena, A.K.; Ribeiro-Gonçalves, B.F.; Nuno Silva, D.; Halkilahti, J.; Machado, M.P.; Da Silva, M.S.; Jaakkonen, A.; Isiro, J.; Hämäläinen, C.; Joenperä, J.; et al. INNUENDO: A cross-sectoral platform for the integration of genomics in the surveillance of food-borne pathogens. EFSA Support. Publ. 2018, 15, 1498E. [Google Scholar] [CrossRef]
- Finnish Food Safety Authority Evira. Risk Assessment of Campylobacter spp. in Finland. Available online: https://www.ruokavirasto.fi/globalassets/tietoa-meista/julkaisut/julkaisusarjat/tutkimukset/riskiraportit/risk-assessment-of-campylobacter-spp_2_2016.pdf (accessed on 10 February 2023).
- Liu, F.; Lee, S.A.; Xue, J.; Riordan, S.M.; Zhang, L. Global epidemiology of campylobacteriosis and the impact of COVID-19. Front. Cell Infect. Microbiol. 2022, 12, 979055. [Google Scholar] [CrossRef]
- Llarena, A.K.; Taboada, E.; Rossi, M. Whole-genome sequencing in epidemiology of Campylobacter jejuni infections. J. Clin. Microbiol. 2017, 55, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Joensen, K.G.; Kiil, K.; Gantzhorn, M.R.; Nauerby, B.; Engberg, J.; Holt, H.M.; Nielsen, H.L.; Petersen, A.M.; Kuhn, K.G.; Sandø, G.; et al. Whole-genome sequencing to detect numerous Campylobacter jejuni outbreaks and match patient isolates to sources, Denmark, 2015-2017. Emerg. Infect. Dis. 2020, 26, 523–532. [Google Scholar] [CrossRef] [PubMed]
- SVA (National Veterinary Institute, Sweden). Surveillance of Infectious Diseases in Animals and Humans in Sweden 2022. SVA:s Rapportserie 89 1654-7098. Available online: https://www.sva.se/media/mbcf44gi/campylobacteriosis.pdf (accessed on 7 June 2023).
- Suominen, K.; Jaakola, S.; Salmenlinna, S.; Simola, M.; Wallgren, S.; Hakkinen, M.; Suokorpi, A.; Rimhanen-Finne, R. Invasive listeriosis in Finland: Surveillance and cluster investigations, 2011–2021. Epidemiol. Infect. 2023, 151, e118. [Google Scholar] [CrossRef]
- Schönberg-Norio, D.; Mattila, L.; Lauhio, A.; Katila, M.L.; Kaukoranta, S.S.; Koskela, M.; Pajarre, S.; Uksila, J.; Eerola, E.; Sarna, S.; et al. Patient-reported complications associated with Campylobacter jejuni infection. Epidemiol. Infect. 2010, 138, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Pires, S.; Jakobsen, L.S.; Ellis-Iversen, J.; Pessoa, J.; Ethelberg, S. Burden of disease estimates of seven pathogens commonly transmitted through foods in Denmark, 2017. Foodborne Pathog. Dis. 2020, 17, 322–339. [Google Scholar] [CrossRef]
- Lagerweij, G.R.; Pijnacker, R.; Friesema, I.H.M.; Mughini-Gras, L.; Franz, E. Disease Burden of Food-Related Pathogens in the Netherlands, 2019; RIVM Letter Report 2020-0117; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Kuhn, K.G.; Nygård, K.M.; Löfdahl, M.; Trönnberg, L.; Rimhanen-Finne, R.; Sunde, L.S.; Guzman-Herrador, B.; Ethelberg, S. Campylobacteriosis in the Nordic countries from 2000 to 2015: Trends in time and space. Scand. J. Public Health 2020, 48, 862–869. [Google Scholar] [CrossRef]
- Green, M.S.; Schwartz, N.; Peer, V. Sex differences in campylobacteriosis incidence rates at different ages—A seven country, multi-year, meta-analysis. A potential mechanism for the infection. BMC Infect. Dis. 2020, 20, 625. [Google Scholar] [CrossRef]
- Natural Resources Institute Finland. Number of Poultry 2023 (Provisional). Available online: https://www.luke.fi/en/statistics/number-of-livestock/number-of-poultry-2023-provisional (accessed on 11 October 2023).
- Poulsen, M.N.; Pollak, J.; Sills, D.L.; Casey, J.A.; Rasmussen, S.G.; Nachman, K.E.; Cosgrove, S.E.; Stewart, D.; Schwartz, B.S. Residential proximity to high-density poultry operations associated with campylobacteriosis and infectious diarrhea. Int. J. Hyg. Environ. Health 2018, 221, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Natural Resources Institute Finland. Nautojen Lukumäärä 1.5.2023 (Ennakko). Available online: https://www.luke.fi/fi/tilastot/kotielainten-lukumaara/nautojen-lukumaara-152023-ennakko (accessed on 11 October 2023).
- Kuhn, K.G.; Nielsen, E.M.; Mølbak, K.; Ethelberg, S. Epidemiology of campylobacteriosis in Denmark 2000–2015. Zoonoses Public Health 2018, 65, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.A.; Ashraf, M.A.; Mustafa, B.E.; Raza, M.M. Epidemiological trends of foodborne Campylobacter outbreaks in the United States of America, 1998–2016. Food Microbiol. 2021, 97, 103751. [Google Scholar] [CrossRef] [PubMed]
- Finnish Food Authority. Elintarviketurvallisuus Suomessa 2022. Available online: https://www.ruokavirasto.fi/globalassets/tietoa-meista/julkaisut/julkaisusarjat/julkaisuja/ruokaviraston_julkaisuja_2_2023_elintarviketurvallisuus_suomessa_2022.pdf (accessed on 4 September 2023).
- Llarena, A.K.; Kivistö, R. Human campylobacteriosis cases traceable to chicken meat—Evidence for disseminated outbreaks in Finland. Pathogens 2020, 9, 868. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Panel on Biological Hazards. Scientific Opinion on Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010, 8, 1437. [Google Scholar] [CrossRef]
- Schönberg-Norio, D.; Takkinen, J.; Hänninen, M.L.; Katila, M.L.; Kaukoranta, S.S.; Mattila, L.; Rautelin, H. Swimming and Campylobacter infections. Emerg. Infect. Dis. 2004, 10, 1474–1477. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Pijnacker, R.; Coipan, C.; Mulder, A.C.; Fernandes Veludo, A.; de Rijk, S.; van Hoek, A.H.A.M.; Buij, R.; Muskens, G.; Koene, M.; et al. Sources and transmission routes of campylobacteriosis: A combined analysis of genome and exposure data. J. Infect. 2021, 82, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Lake, R.J.; Campbell, D.M.; Hathaway, S.C.; Ashmore, E.; Cressey, P.J.; Horn, B.J.; Pirikahu, S.; Sherwood, J.M.; Baker, M.G.; Shoemack, P.; et al. Source attributed case-control study of campylobacteriosis in New Zealand. Int. J. Infect. Dis. 2021, 103, 268–277. [Google Scholar] [CrossRef]
- de Haan, C.P.; Kivistö, R.I.; Hakkinen, M.; Corander, J.; Hänninen, M.L. Multilocus sequence types of Finnish bovine Campylobacter jejuni isolates and their attribution to human infections. BMC Microbiol. 2010, 10, 200. [Google Scholar] [CrossRef]
- Kuhn, K.G.; Nielsen, E.M.; Mølbak, K.; Ethelberg, S. Determinants of sporadic Campylobacter infections in Denmark: A nationwide case-control study among children and young adults. Clin. Epidemiol. 2018, 10, 1695–1707. [Google Scholar] [CrossRef]
- Llarena, A.K.; Sivonen, K.; Hänninen, M.L. Campylobacter jejuni prevalence and hygienic quality of retail bovine ground meat in Finland. Lett. Appl. Microbiol. 2014, 58, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Grau, F.H. Campylobacter jejuni and Campylobacter hyointestinalis in the intestinal tract and on the carcasses of calves and cattle. J. Food Prot. 1988, 51, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, T. Review of Campylobacter spp. in drinking and environmental waters. J. Microbiol. Methods 2013, 95, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.G.; Nygård, K.M.; Guzman-Herrador, B.; Sunde, L.S.; Rimhanen-Finne, R.; Trönnberg, L.; Jepsen, M.R.; Ruuhela, R.; Wong, W.K.; Ethelberg, S. Campylobacter infections expected to increase due to climate change in Northern Europe. Sci. Rep. 2020, 10, 13874. [Google Scholar] [CrossRef]
- Kovanen, S.; Rossi, M.; Pohja-Mykrä, M.; Nieminen, T.; Raunio-Saarnisto, M.; Sauvala, M.; Fredriksson-Ahomaa, M.; Hänninen, M.L.; Kivistö, R. Population genetics and characterization of Campylobacter jejuni isolates from western jackdaws and game birds in Finland. Appl. Environ. Microbiol. 2019, 85, e02365-18. [Google Scholar] [CrossRef]
- Sauvala, M.; Woivalin, E.; Kivistö, R.; Laukkanen-Ninios, R.; Laaksonen, S.; Stephan, R.; Fredriksson-Ahomaa, M. Hunted game birds—Carriers of foodborne pathogens. Food Microbiol. 2021, 98, 103768. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L. The role of gastric acid in preventing foodborne disease and how bacteria overcome acid conditions. J. Food Prot. 2003, 66, 1292–1303. [Google Scholar] [CrossRef]
- Hafiz, R.A.; Wong, C.; Paynter, S.; David, M.; Peeters, G. The risk of community-acquired enteric infection in proton pump inhibitor therapy: Systematic review and meta-analysis. Ann. Pharmacother. 2018, 52, 613–622. [Google Scholar] [CrossRef]
- Pottegård, A.; Broe, A.; Hallas, J.; de Muckadell, O.B.; Lassen, A.T.; Lødrup, A.B. Use of proton-pump inhibitors among adults: A Danish nationwide drug utilization study. Ther. Adv. Gastroenterol. 2016, 9, 671–678. [Google Scholar] [CrossRef]
- Steffen, R.; Hill, D.R.; DuPont, H.L. Traveler’s diarrhea: A clinical review. JAMA 2015, 313, 71–80. [Google Scholar] [CrossRef]
- Pella, J.; Masuda, M. Bayesian methods for analysis of stock mixtures from genetic characters. Fish. Bull. 2001, 99, 151–167. [Google Scholar]



| Exposure | Odds Ratio (95% CI a) | p-Value |
|---|---|---|
| Eating undercooked broiler or turkey meat | 20 (6.0–69) | <0.001 |
| Medication to treat gastric acidity | 2.7 (1.6–4.5) | <0.001 |
| Swimming in natural waters or drinking untreated water | 2.4 (1.5–4.0) | <0.001 |
| Eating minced beef | 2.0 (1.1–3.5) | 0.018 |
| Contact with wild birds or their feces | 2.8 (1.1–7.1) | 0.028 |
| Handling animal feces or manure | 1.8 (1.1–3.2) | 0.030 |
| MLST | No. of Clusters | Clusters: No. of Patient Isolates | Clusters: No. of Animal/Food Isolates | No. of Cases in the Cluster Who Had Eaten Broiler or Turkey Meat | |
|---|---|---|---|---|---|
| Clonal Complex | Sequence Type (ST) | ||||
| ST-45 complex | 45 | 7 | 7 | 1 broiler isolate | 4/7 |
| 4 | 1 turkey isolate | 4/4 | |||
| 3 | 1 broiler isolate | 3/3 | |||
| 3 | 0 | ||||
| 2 | 5 broiler isolates | 2/2 | |||
| 2 | 0 | ||||
| 2 | 0 | ||||
| 230 | 1 | 8 | 4 broiler isolates | 6/8 | |
| 11 | 1 | 2 | 0 | ||
| 1701 | 1 | 2 | 0 | ||
| 2406 | 1 | 2 | 0 | ||
| ST-677 complex | 677 | 3 | 3 | 0 | |
| 2 | 0 | ||||
| 2 | 0 | ||||
| 6514 | 2 | 2 | 0 | ||
| 2 | 0 | ||||
| ST-21 complex | 50 | 1 | 9 | 1 broiler isolate | 9/9 |
| 19 | 1 | 2 | 0 | ||
| ST-1287 complex | 945 | 1 | 2 | 0 | |
| ST-257 complex | 2254 | 1 | 3 | 0 | |
| ST-48 complex | 918 | 1 | 2 | 1 mink strain | |
| does not belong to a clonal complex | 2274 | 1 | 2 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suominen, K.; Häkkänen, T.; Ranta, J.; Ollgren, J.; Kivistö, R.; Perko-Mäkelä, P.; Salmenlinna, S.; Rimhanen-Finne, R. Campylobacteriosis in Finland: Passive Surveillance in 2004–2021 and a Pilot Case-Control Study with Whole-Genome Sequencing in Summer 2022. Microorganisms 2024, 12, 132. https://doi.org/10.3390/microorganisms12010132
Suominen K, Häkkänen T, Ranta J, Ollgren J, Kivistö R, Perko-Mäkelä P, Salmenlinna S, Rimhanen-Finne R. Campylobacteriosis in Finland: Passive Surveillance in 2004–2021 and a Pilot Case-Control Study with Whole-Genome Sequencing in Summer 2022. Microorganisms. 2024; 12(1):132. https://doi.org/10.3390/microorganisms12010132
Chicago/Turabian StyleSuominen, Kristiina, Tessa Häkkänen, Jukka Ranta, Jukka Ollgren, Rauni Kivistö, Päivikki Perko-Mäkelä, Saara Salmenlinna, and Ruska Rimhanen-Finne. 2024. "Campylobacteriosis in Finland: Passive Surveillance in 2004–2021 and a Pilot Case-Control Study with Whole-Genome Sequencing in Summer 2022" Microorganisms 12, no. 1: 132. https://doi.org/10.3390/microorganisms12010132
APA StyleSuominen, K., Häkkänen, T., Ranta, J., Ollgren, J., Kivistö, R., Perko-Mäkelä, P., Salmenlinna, S., & Rimhanen-Finne, R. (2024). Campylobacteriosis in Finland: Passive Surveillance in 2004–2021 and a Pilot Case-Control Study with Whole-Genome Sequencing in Summer 2022. Microorganisms, 12(1), 132. https://doi.org/10.3390/microorganisms12010132






