Safety Analysis of Extended Platelet Shelf-Life with Large-Volume Delayed Sampling on BACT/ALERT® VIRTUO® in Australia
Abstract
:1. Introduction
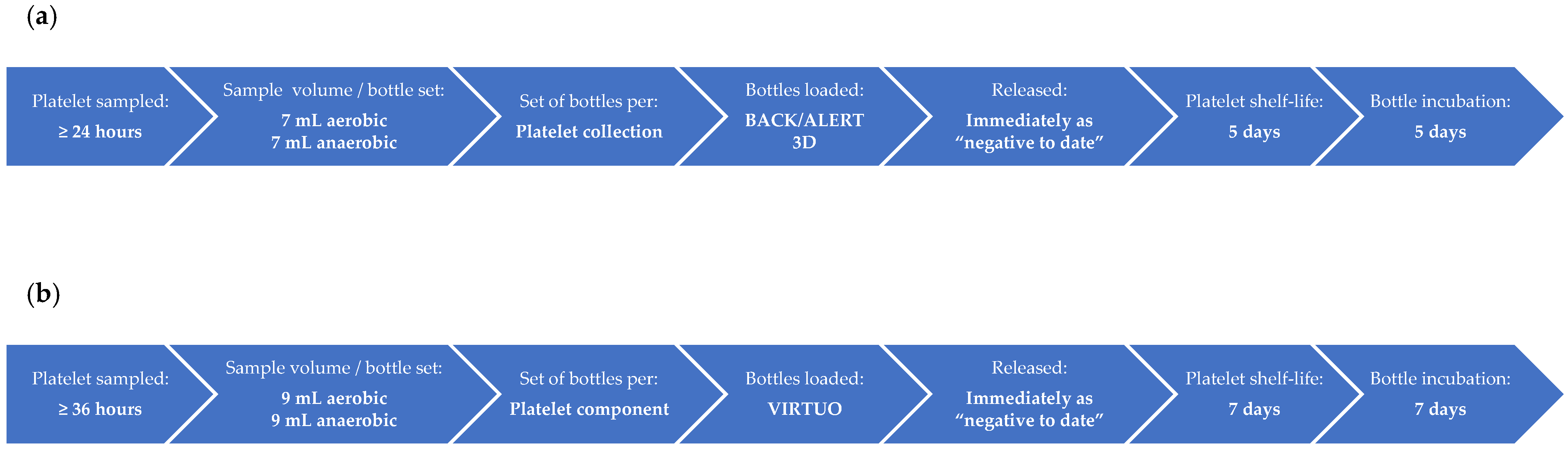
2. Materials and Methods
3. Results
3.1. Total Number of Platelet Donations Screened and the Rates for Initial Machine-Positive, False-Positive, Confirmed Positive, and Indeterminate
3.1.1. Comparison of BACT/ALERT 3D and BACT/ALERT VIRTUO
3.1.2. BACT/ALERT VIRTUO following R3 Software Upgrade
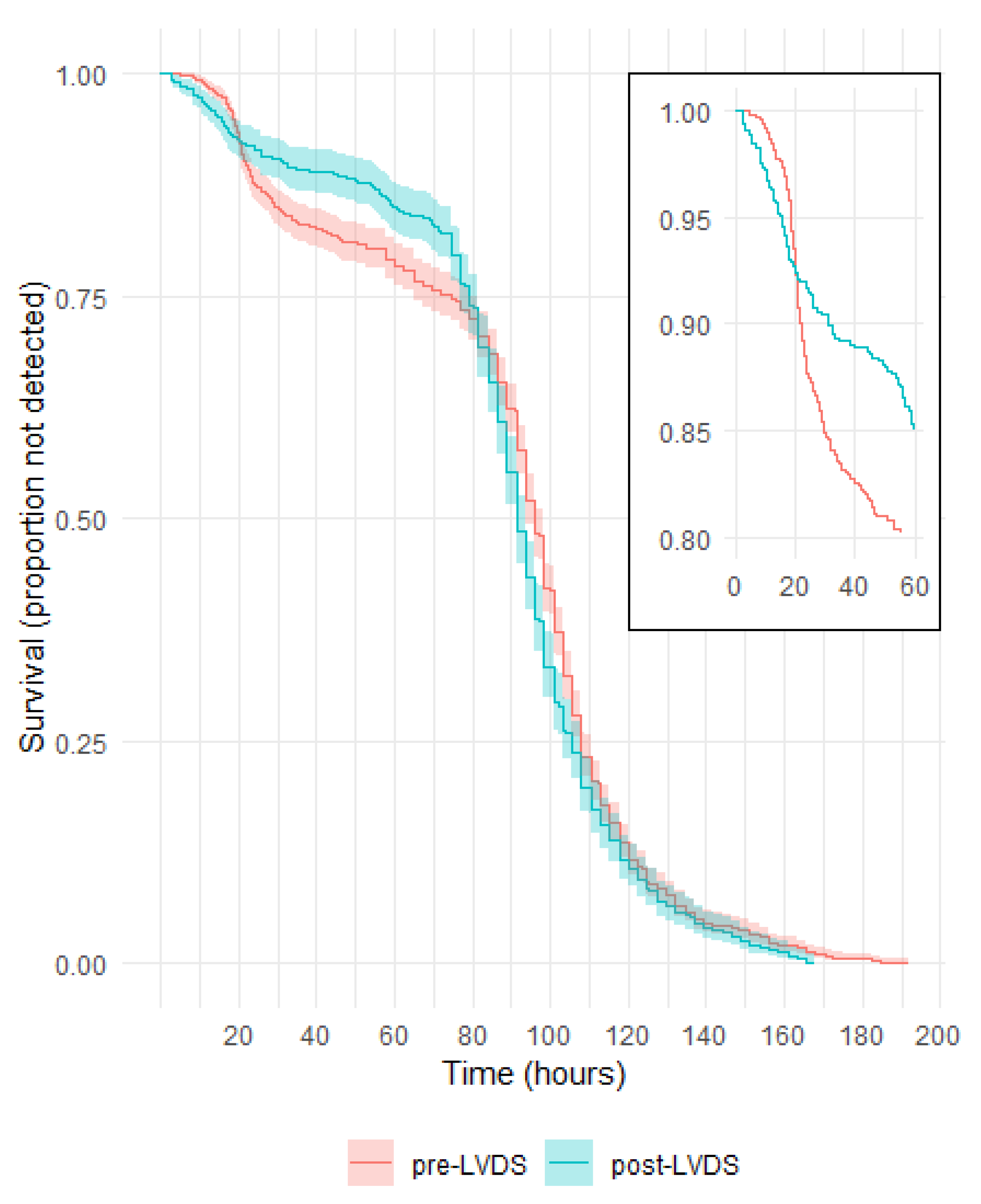
3.2. Time to Detection in Culture Bottles with a Positive Gram Stain or Culture
3.3. Commonly Identified Organisms
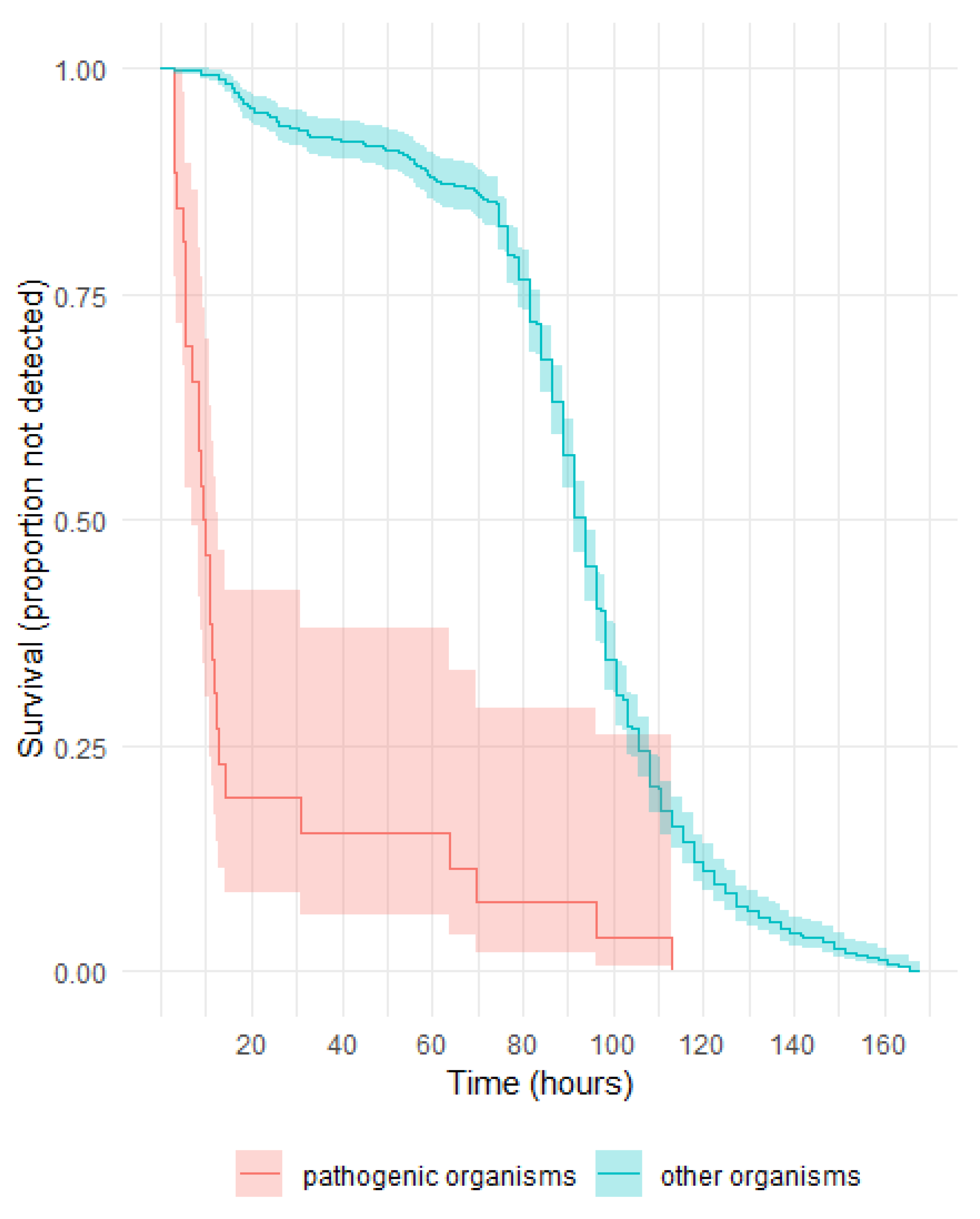
3.4. Pathogenic Potential of Organisms Identified in Post LVDS Platelet Components and Their Time to Detection
| Organism | <6 | ≥6–<12 | ≥12–<18 | ≥18–<24 | ≥24–<48 | ≥48–<72 | ≥72–<96 | ≥96–<120 | ≥120 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pathogenic | ||||||||||
| Skin | Staphylococcus aureus | 6 | 1 | |||||||
| Streptococcus dysgalactiae | 1 | |||||||||
| Streptococcus pyogenes | 1 | |||||||||
| Staphylococcus lugdunensis | 1 | |||||||||
| Environment | Bacillus cereus | 3 | 1 | |||||||
| Gut | Bacteroides spp. | 2 | 2 | |||||||
| Escherichia coli | 1 | |||||||||
| Enterococcus hirae | 1 | |||||||||
| Enterococcus faecalis | 1 | |||||||||
| Campylobacter fetus | 1 | |||||||||
| Throat/lungs | Streptococcus pneumoniae | 1 | ||||||||
| Skin/urine | Staphylococcus saprophyticus | 1 | ||||||||
| Opportunistic | ||||||||||
| Gut/environment | Serratia marcescens | 2 | ||||||||
| Low pathogenicity | ||||||||||
| Skin | Cutibacterium spp. | 1 | 5 | 246 | 197 | 74 | ||||
| Coagulase negative staphylococci 1 | 1 | 12 | 4 | 13 | 29 | 3 | 2 | 1 | ||
| Micrococcus spp. | 1 | 1 | 1 | |||||||
| Streptococcus sanguinis 2 | 2 | |||||||||
| Corynebacterium spp. | 1 | 1 | ||||||||
| Lactococcus spp. | 1 | |||||||||
| Kocuria spp. | 1 | |||||||||
| Streptococcus cristatus | 1 | |||||||||
| Dermacoccus nishinomiyaensis | 1 | |||||||||
| Cutibacterium spp./Micrococcus spp. 3 | 1 | |||||||||
| Unidentified Gram-positive bacilli | 1 | |||||||||
| Environment | Bacillus spp./Paenibacillus spp. 4 | 1 | 6 | 1 | 2 | 5 | 2 | 1 | ||
| Pseudomonas oryzihabitans | 1 | |||||||||
| Environment/skin | Bacillus thuringiensis/Herbaspirillium huttiense/S. epidermidis 3 | 1 | ||||||||
| Skin/gut | Streptococcus gallolyticus 2 | 1 | ||||||||
| Gut | Eucobacterium callanderi | 1 |
Pathogenic Organisms and Their Time to Detection in Aerobic and Anaerobic Culture Bottles
3.5. Frequency of Platelet Component Expiry: 5-Day vs. 7-Day Shelf-Life
3.6. Incidence of TTBI Cases Reported to Lifeblood following the Implementation of BCS in April 2008
4. Discussion
4.1. Principal Findings
4.2. Strengths and Weaknesses of the Study
4.3. Strengths and Weaknesses in Relation to Other Studies, Discussing Particularly any Differences in Results
4.4. Meaning of the Study: Possible Mechanisms and Implications for Clinicians or Policymakers
4.5. Unanswered Questions and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thyer, J.; Perkowska-Guse, Z.; Ismay, S.L.; Keller, A.J.; Chan, H.T.; Dennington, P.M.; Bell, B.; Kotsiou, G.; Pink, J.M. Bacterial testing of platelets—Has it prevented transfusion-transmitted bacterial infections in Australia? Vox Sang. 2018, 113, 13–20. [Google Scholar] [CrossRef]
- McDonald, C.P. Bacterial risk reduction by improved donor arm disinfection, diversion and bacterial screening. Transfus. Med. 2006, 16, 381–396. [Google Scholar] [CrossRef]
- Brecher, M.E.; Blajchman, M.A.; Yomtovian, R.; Ness, P.; AuBuchon, J.P. Addressing the risk of bacterial contamination of platelets within the United States: A history to help illuminate the future. Transfusion 2013, 53, 221–231. [Google Scholar] [CrossRef] [PubMed]
- White, S.K.; Schmidt, R.L.; Walker, B.S.; Metcalf, R.A. Bacterial contamination rate of platelet components by primary culture: A systematic review and meta-analysis. Transfusion 2020, 60, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Ramirez-Arcos, S.; McDonald, C.; ISBT Transfusion-Transmitted Infectious Disease Bacterial Working Party Bacterial Subgroup. The international experience of bacterial screen testing of platelet components with automated microbial detection systems: An update. Vox Sang. 2022, 117, 647–655. [Google Scholar] [CrossRef]
- McDonald, C.; Allen, J.; Brailsford, S.; Roy, A.; Ball, J.; Moule, R.; Vasconcelos, M.; Morrison, R.; Pitt, T. Bacterial screening of platelet components by National Health Service Blood and Transplant, an effective risk reduction measure. Transfusion 2017, 57, 1122–1131. [Google Scholar] [CrossRef]
- Ramirez-Arcos, S.; Evans, S.; McIntyre, T.; Pang, C.; Yi, Q.-L.; DiFranco, C.; Goldman, M. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020, 60, 2918–2928. [Google Scholar] [CrossRef]
- Delage, G.; Bernier, F.; Beaudoin, J.; Thibault, S.; Dion, J.; Gagne, L. Improved bacterial culture of platelet product: Preliminary results after implementation of a two-bottle system with 48-hour sampling [abstract]. Transfusion 2016, 56 (Suppl. S4), 28A. [Google Scholar]
- Borosak, M.; Wood, E. Bacterial Pre-Release Testing of Platelets—The Australian Red Cross Blood Service Clinical Experience. Transfus. Med. Hemother. 2011, 38, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Daane, L.; Adamik, M.; Ullery, M.; Ahmed, M.; Yang, J. Rapid detection of bacterial contaminants in platelet concentrates using the next generation BACT/ALERT® VIRTUO® Microbial Detection System: Improved safety and operational efficiency. Ann. Blood 2021, 6, 40. [Google Scholar] [CrossRef]
- Corean, J.; White, S.K.; Schmidt, R.L.; Walker, B.S.; Fisher, M.A.; Metcalf, R.A. The incremental benefit of anaerobic culture for controlling bacterial risk in platelets: A systematic review and meta-analysis. Vox Sang. 2021, 116, 397–404. [Google Scholar] [CrossRef]
- Delage, G.; Bernier, F. Bacterial culture of platelets with the large volume delayed sampling approach: A narrative review. Ann. Blood 2021, 6, 30. [Google Scholar] [CrossRef]
- Barrett, B.B.; Andersen, J.W.; Anderson, K.C. Strategies for the avoidance of bacterial contamination of blood components. Transfusion 1993, 33, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Pink, J.M.; MacCallum, S.; Ribeiro, A.; Wylie, B.R. Platelet transfusion-related sepsis. Aust. N. Z. J. Med. 1993, 23, 717. [Google Scholar] [CrossRef] [PubMed]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Earnshaw, S.; Beyhaghi, H.; McDade, C.; Purser, M.; Marriott, R.; Daane, L.; Le Coent, V.; Yang, J.; Toback, S. Clinical and economic impacts of large volume delayed sampling and pathogen reduction technology platelet processing strategies in the United States. Transfusion 2021, 61, 2885–2897. [Google Scholar] [CrossRef]
- Seed, C.R.; Kiely, P.; Keller, A.J. Residual risk of transfusion transmitted human immunodeficiency virus, hepatitis B virus, hepatitis C virus and human T lymphotrophic virus. Intern. Med. J. 2005, 35, 592–598. [Google Scholar] [CrossRef] [PubMed]
| Platelet Collection | Total Number Screened | Initial Machine-Positive | False-Positive | Confirmed Positive | Indeterminate |
|---|---|---|---|---|---|
| Pooled | 362,051 | 0.45 (1627) | 0.15 (549) | 0.13 (461) | 0.17 (617) |
| Apheresis | 107,588 | 0.52 (558) | 0.38 (407) | 0.05 (52) | 0.09 (99) |
| Total | 469,639 | 0.47 (2185) | 0.20 (956) | 0.11 (513) | 0.15 (716) |
| Platelet Collection | Total Number Screened | Initial Machine-Positive | False-Positive | Confirmed Positive | Indeterminate |
|---|---|---|---|---|---|
| Pooled | 204,811 | 0.38 (783) | 0.11 (225) | 0.14 (279) | 0.14 (279) |
| Apheresis | 44,147 | 0.38 (167) | 0.17 (76) | 0.05 (24) | 0.15 (67) |
| Total | 248,958 | 0.38 (950) | 0.12 (301) | 0.12 (303) | 0.14 (346) |
| Pre-LVDS | Post LVDS | ||
|---|---|---|---|
| Cutibacterium spp. including C. acnes | 72.1 | Cutibacterium spp. including C. acnes | 80.7 |
| Coagulase-negative staphylococci | 15.9 | Coagulase-negative staphylococci | 10.5 |
| Micrococcus spp. | 3.0 | Bacillus spp./Paenibacillus spp. | 3.5 |
| Bacillus spp./Paenibacillus spp. | 2.4 | Staphylococcus aureus | 1.1 |
| Streptococcus spp. | 1.6 | Streptococcus spp. | 1.1 |
| Corynebacterium spp. | 1.4 | Bacteroides spp. | 0.6 |
| Kocuria spp. | 0.9 | Micrococcus spp. | 0.6 |
| Organism | Platelet Collection | Aerobic Culture Bottle | Anaerobic Culture Bottle | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| S. aureus | Apheresis double | 6.6 | ND | - | 7.2 | ND | - |
| 8.6 | ND | - | 9.1 | 9.2 | - | ||
| 11.3 | ND | - | 11.7 | ND | - | ||
| Pooled | 9.4 | - | - | 8.4 | - | - | |
| 10.6 | - | - | 11.4 | - | - | ||
| 10.8 | - | - | 11.5 | - | - | ||
| ND | - | - | 12.1 | - | - | ||
| B. cereus | Pooled | 3.0 | - | - | 2.8 | - | - |
| 3.2 | - | - | 3.2 | - | - | ||
| 5.2 | - | - | 5.5 | - | - | ||
| ND | - | - | 9.7 | - | - | ||
| Bacteroides spp. | Apheresis double | ND | ND | - | 69.6 | ND | - |
| Pooled | ND | - | - | 63.7 | - | - | |
| ND | - | - | 96.0 | - | - | ||
| ND | - | - | 112.8 | - | - | ||
| S. marcescens | Pooled | 2.8 | - | - | 2.8 | - | - |
| 5.4 | - | - | 5.9 | - | - | ||
| E. coli | Apheresis triple | 3.4 | 3.4 | 3.4 | 2.7 | 3.0 | 3.5 |
| S. pyogenes | Pooled | 5.9 | - | - | 5.2 | - | - |
| S. dysgalactiae | Pooled | 6.0 | - | - | 4.7 | - | - |
| S. saprophyticus | Apheresis double | 12.9 | ND | - | 20.6 | ND | - |
| S. lugdunensis | Pooled | 14.9 | - | - | 14.0 | - | - |
| C. fetus | Pooled | ND | - | - | 35.7 | - | - |
| E. hirae | Pooled | 8.8 | - | - | 8.1 | - | - |
| E. faecalis | Pooled | 9.7 | - | - | 9.4 | - | - |
| S. pneumoniae | Pooled | ND | - | - | 11.7 | - | - |
| Calendar Year | Count | Implicated Organism | Component | Recipient Outcome | BCS Result |
|---|---|---|---|---|---|
| 2016 | 2 | S. aureus | Double apheresis platelet | Major morbidity in both recipients | Negative |
| 2017 | 0 | None | |||
| 2018 | 1 | Yersinia enterocolitica | Red cell | Recovered | Negative 1 |
| 2019 | 2 | S. aureus Group G Streptococcus | Triple apheresis platelet Pooled platelet | Recovered Death | Negative |
| Negative | |||||
| 2020 | 0 | None | |||
| 2021 | 0 | None | |||
| 2022 | 0 | None | |||
| 2023 | 0 2 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, A.; Das, A.; Chaw, K.; Dennington, P.M.; Styles, C.E.; Gosbell, I.B. Safety Analysis of Extended Platelet Shelf-Life with Large-Volume Delayed Sampling on BACT/ALERT® VIRTUO® in Australia. Microorganisms 2023, 11, 2346. https://doi.org/10.3390/microorganisms11092346
Cheng A, Das A, Chaw K, Dennington PM, Styles CE, Gosbell IB. Safety Analysis of Extended Platelet Shelf-Life with Large-Volume Delayed Sampling on BACT/ALERT® VIRTUO® in Australia. Microorganisms. 2023; 11(9):2346. https://doi.org/10.3390/microorganisms11092346
Chicago/Turabian StyleCheng, Anthea, Anindita Das, Khin Chaw, Peta M. Dennington, Claire E. Styles, and Iain B. Gosbell. 2023. "Safety Analysis of Extended Platelet Shelf-Life with Large-Volume Delayed Sampling on BACT/ALERT® VIRTUO® in Australia" Microorganisms 11, no. 9: 2346. https://doi.org/10.3390/microorganisms11092346
APA StyleCheng, A., Das, A., Chaw, K., Dennington, P. M., Styles, C. E., & Gosbell, I. B. (2023). Safety Analysis of Extended Platelet Shelf-Life with Large-Volume Delayed Sampling on BACT/ALERT® VIRTUO® in Australia. Microorganisms, 11(9), 2346. https://doi.org/10.3390/microorganisms11092346






