Impact of Donepezil Supplementation on Alzheimer’s Disease-like Pathology and Gut Microbiome in APP/PS1 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection
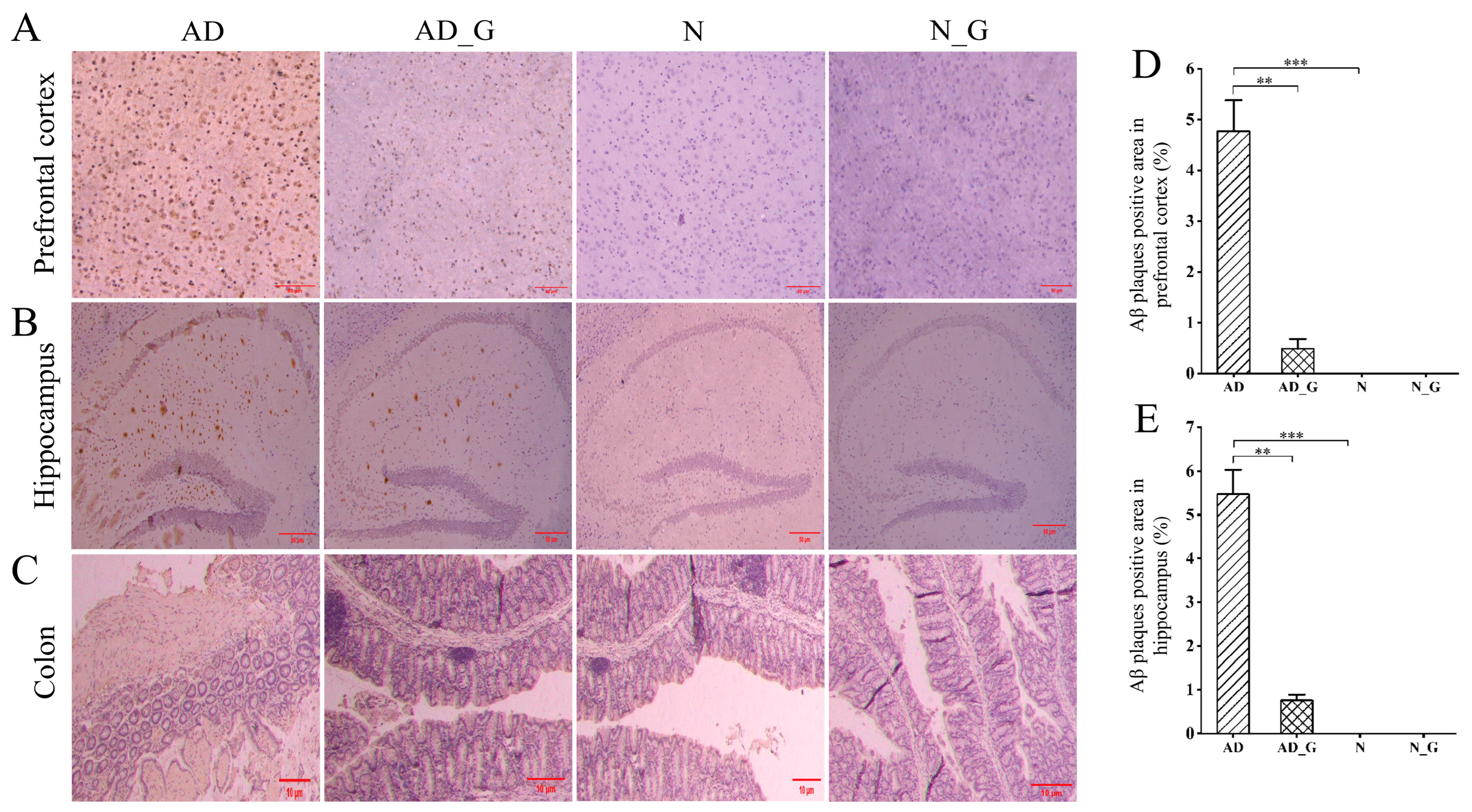
2.3. Aβ Immunohistochemical Test of Brain Sections and HE Staining of Colon Tissue
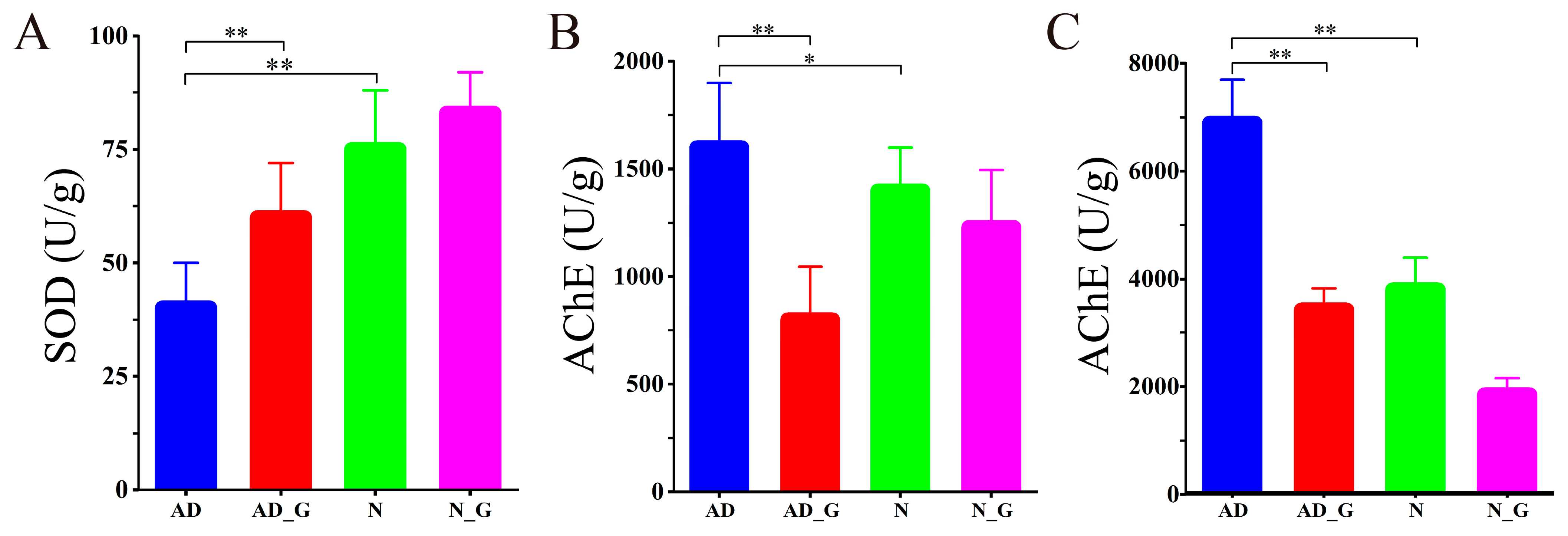
2.4. Determination of Superoxide Dismutase (SOD) and AChE Activities in the Brain and Colon
2.5. PCR Amplification and Product Purification
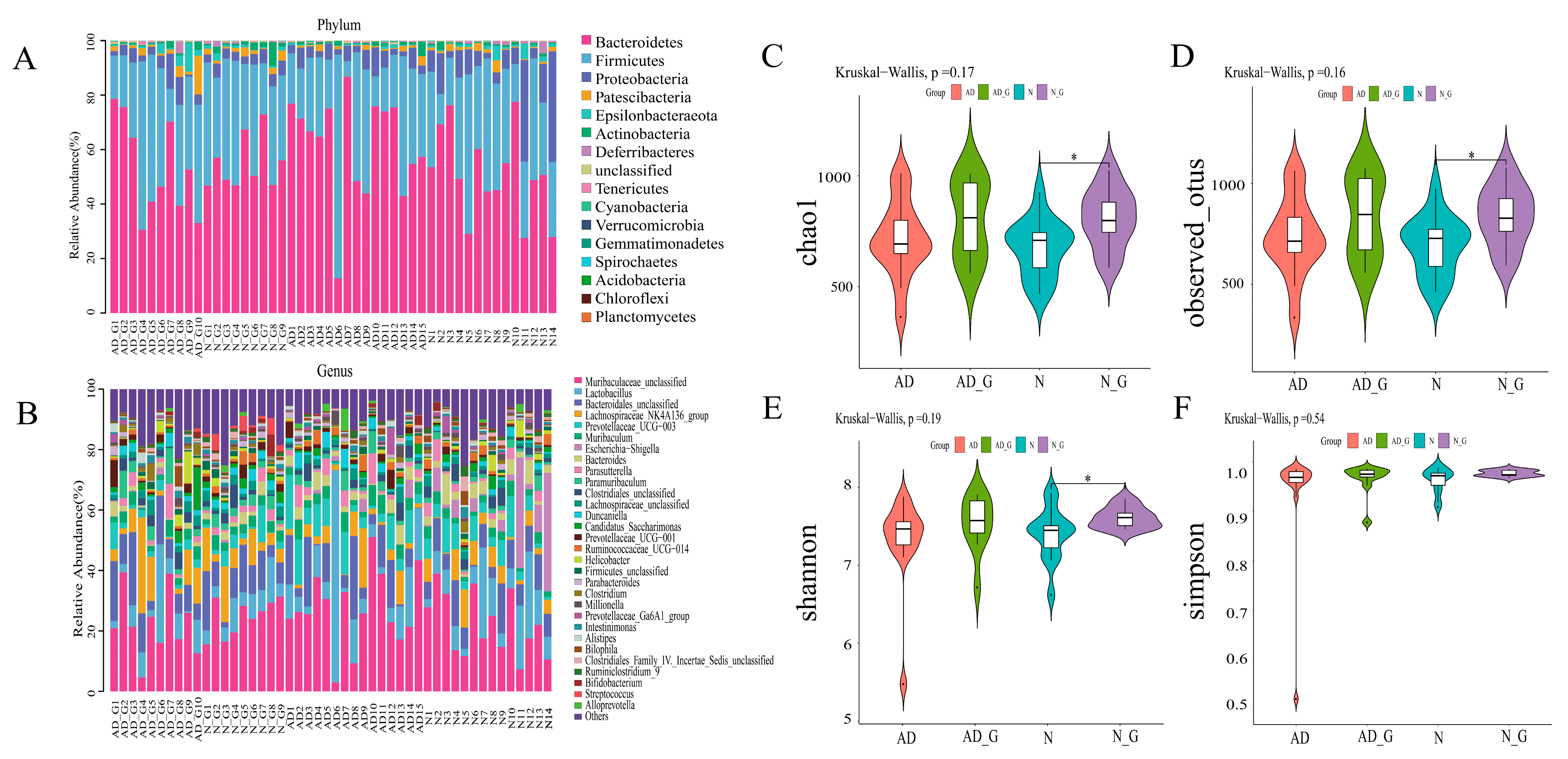
2.6. 16S rRNA Sequencing Analysis
2.7. Molecular Docking
2.8. Statistical Analysis
3. Results
3.1. Effects of DH on the Pathological Performance of the Brain and Colon in AD Mice
3.2. SOD and AChE Activities in Brain and Colon
3.3. Microbial Community Composition and Diversity Analysis of Gut Microbiota
3.4. Analysis of Differential Flora of Gut Microbiota
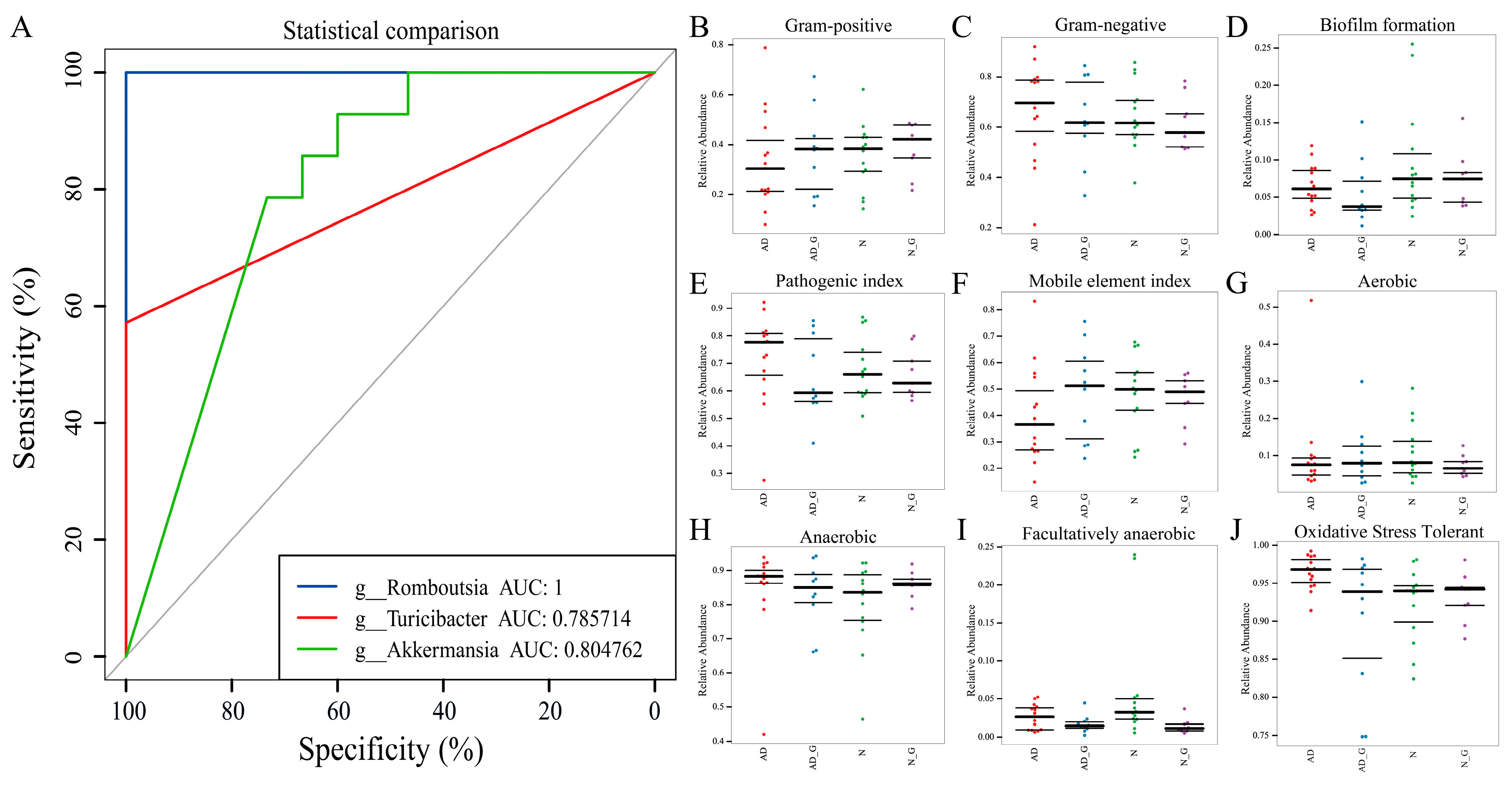
3.5. Analysis of Disease Diagnostic Model and Phenotypic Prediction
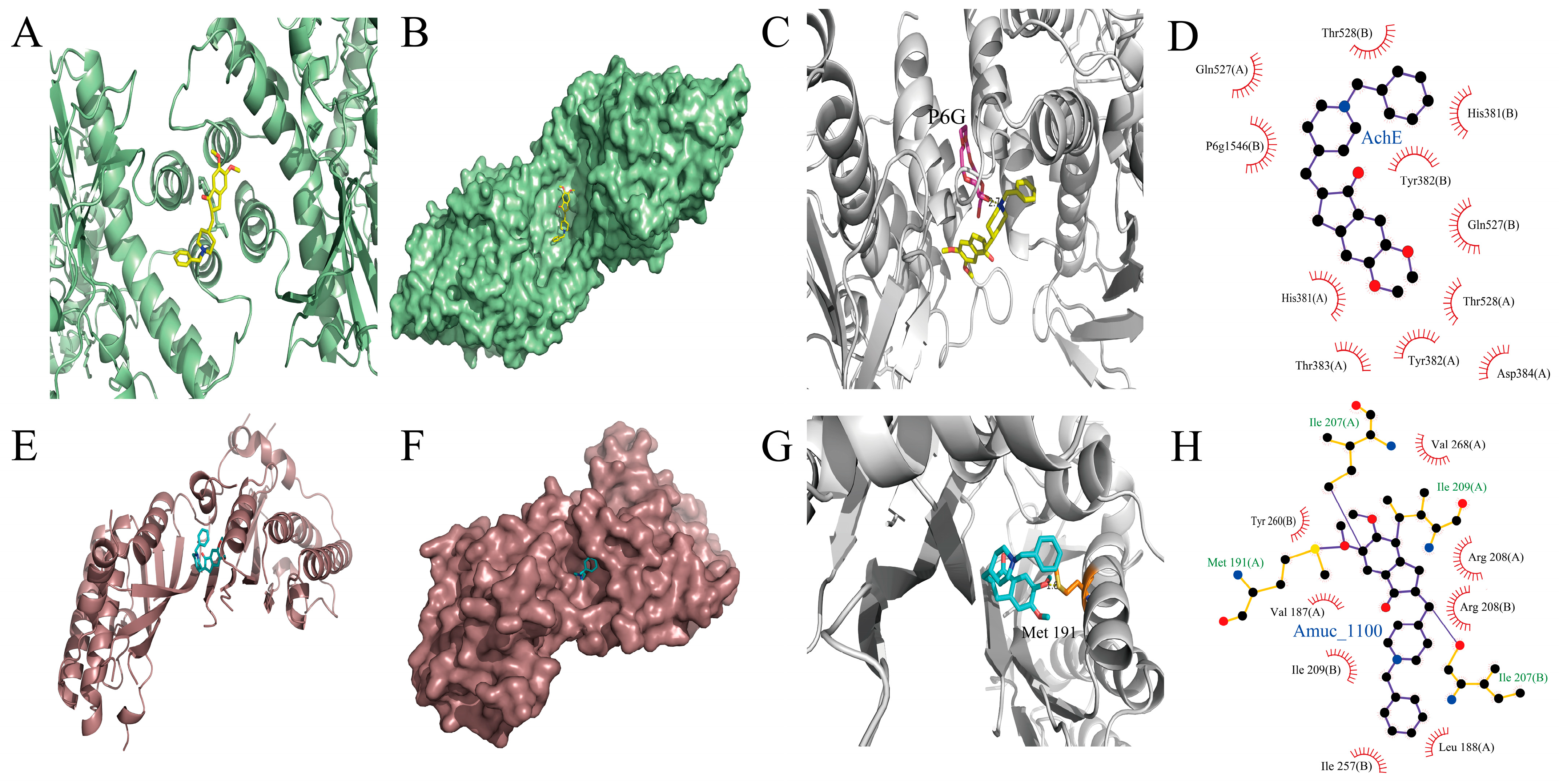
3.6. Interactions of DH with AchE and Amuc_1100
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, F.; Joshi, A.; Devkota, H.P.; Subramaniyan, V.; Kumarasamy, V.; Arora, J. Dietary glucosinolates derived isothiocyanates: Chemical properties, metabolism and their potential in prevention of Alzheimer’s disease. Front. Pharmacol. 2023, 14, 1214881. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Zuliani, G. Frontier on Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 7748. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Kosyreva, A.M.; Sentyabreva, A.V.; Tsvetkov, I.S.; Makarova, O.V. Alzheimer’s Disease and Inflammaging. Brain Sci. 2022, 12, 1237. [Google Scholar] [CrossRef]
- Kalani, K.; Chaturvedi, P.; Chaturvedi, P.; Kumar Verma, V.; Lal, N.; Awasthi, S.K.; Kalani, A. Mitochondrial mechanisms in Alzheimer’s disease: Quest for therapeutics. Drug Discov. Today 2023, 28, 103547. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Beschea Chiriac, S.I.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules 2020, 10, 40. [Google Scholar] [CrossRef]
- Volpato, D.; Holzgrabe, U. Designing Hybrids Targeting the Cholinergic System by Modulating the Muscarinic and Nicotinic Receptors: A Concept to Treat Alzheimer’s Disease. Molecules 2018, 23, 3230. [Google Scholar] [CrossRef]
- Jean, L.; Brimijoin, S.; Vaux, D.J. In vivo localization of human acetylcholinesterase-derived species in a β-sheet conformation at the core of senile plaques in Alzheimer’s disease. J. Biol. Chem. 2019, 294, 6253–6272. [Google Scholar] [CrossRef]
- Sugimoto, H. Donepezil hydrochloride: A treatment drug for Alzheimer’s disease. Chem. Rec. 2001, 1, 63–73. [Google Scholar] [CrossRef]
- Thompson, S.B.N.; Macdonald, J.; Coates, T.D. Improving Visual Memory with Aricept (Donepezil Hydrochloride, E2020) in Mild-to-Moderate Alzheimer’s Disease. Clin. Gerontol. 2001, 24, 55–73. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Zhao, J.; Wang, L. Effect of Resveratrol Combined with Donepezil Hydrochloride on Inflammatory Factor Level and Cognitive Function Level of Patients with Alzheimer’s Disease. J. Healthc. Eng. 2022, 2022, 9148650. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Choudhary, M.; Joshi, D.K.; Mahdi, A.A.; Hasan, M.; Mitra, K. ISDN2012_0195: Influence of age on aluminum induced neurobehavioral, neurochemical and ultrastructural changes in rat brain: Protective role of donepezil HCl. Int. J. Dev. Neurosci. 2012, 30, 677. [Google Scholar] [CrossRef]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.-B.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.-W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Shukla, P.K.; Delotterie, D.F.; Xiao, J.; Pierre, J.F.; Rao, R.; McDonald, M.P.; Khan, M.M. Alterations in the Gut-Microbial-Inflammasome-Brain Axis in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 779. [Google Scholar] [CrossRef]
- Yadav, P.; Lee, Y.-H.; Panday, H.; Kant, S.; Bajwa, N.; Parashar, R.; Jha, S.K.; Jha, N.K.; Nand, P.; Lee, S.-S.; et al. Implications of Microorganisms in Alzheimer’s Disease. Curr. Issues Mol. Biol. 2022, 44, 4584–4615. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and Mechanisms of Gut Microbiota in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef]
- Obrenovich, M.; Tabrez, S.; Siddiqui, B.; McCloskey, B.; Perry, G. The Microbiota–Gut–Brain Axis–Heart Shunt Part II: Prosaic Foods and the Brain–Heart Connection in Alzheimer Disease. Microorganisms 2020, 8, 493. [Google Scholar] [CrossRef]
- Wang, S.-s.; Li, X.-h.; Liu, P.; Li, J.; Liu, L. The relationship between Alzheimer’s disease and intestinal microflora structure and inflammatory factors. Front. Aging Neurosci. 2022, 14, 972982. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Parikh, I.; Green, S.J.; Chlipala, G.; Mohney, R.P.; Keaton, M.; Bauer, B.; Hartz, A.M.S.; Lin, A.-L. Age Drives Distortion of Brain Metabolic, Vascular and Cognitive Functions, and the Gut Microbiome. Front. Aging Neurosci. 2017, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, L.; Chen, S.; Zhou, H.; Fan, Y.; Lin, L.; Li, J.; Xu, J.; Chen, Y.; Ma, Y.; et al. Gut Microbiome Alterations Precede Cerebral Amyloidosis and Microglial Pathology in a Mouse Model of Alzheimer’s Disease. BioMed Res. Int. 2020, 2020, 8456596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Zhao, X.; Sui, S.; Wang, Q.; Shi, G.; Xu, H.; Zhang, X.; He, Y.; Gu, J. Intestinal Microflora Changes in Patients with Mild Alzheimer’s Disease in a Chinese Cohort. J. Alzheimer’s Dis. 2022, 88, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, C.; Zhang, F. Immunity orchestrates a bridge in gut-brain axis of neurodegenerative diseases. Ageing Res. Rev. 2023, 85, 101857. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-L.; Liu, D.; Liu, Y.-J.; Ji, Y.-L.; Liu, G.-S.; Wang, J.-L.-T.; Wang, B.; Wang, H. Phlorizin alleviates cholinergic memory impairment and regulates gut microbiota in d-galactose induced mice. Exp. Gerontol. 2022, 165, 111863. [Google Scholar] [CrossRef]
- Ortega-Santos, C.P.; Al-Nakkash, L.; Whisner, C.M. Exercise and/or Genistein Do Not Revert 24-Week High-Fat, High-Sugar Diet-Induced Gut Microbiota Diversity Changes in Male C57BL/6J Adult Mice. Microorganisms 2022, 10, 2221. [Google Scholar] [CrossRef]
- Cao, J.; Amakye, W.K.; Qi, C.; Liu, X.; Ma, J.; Ren, J. Bifidobacterium Lactis Probio-M8 regulates gut microbiota to alleviate Alzheimer’s disease in the APP/PS1 mouse model. Eur. J. Nutr. 2021, 60, 3757–3769. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.-H.; Kim, M.-S.; Ahn, K.-S.; Kim, M. Effect of Lactobacillus dominance modified by Korean Red Ginseng on the improvement of Alzheimer’s disease in mice. J. Ginseng Res. 2022, 46, 464–472. [Google Scholar] [CrossRef]
- Rigsby, R.E.; Parker, A.B. Using the PyMOL application to reinforce visual understanding of protein structure. Biochem. Mol. Biol. Educ. 2016, 44, 433–437. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Fairley, A.; Stewart, C.J.; Cassidy, A.; Woodside, J.V.; McEvoy, C.T. Diet Patterns, the Gut Microbiome, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 88, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Benakis, C.; Martin-Gallausiaux, C.; Trezzi, J.-P.; Melton, P.; Liesz, A.; Wilmes, P. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 2020, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Vora, A.; Kaur, G.; Akhtar, J. Dysbiosis and Alzheimer’s disease: Role of probiotics, prebiotics and synbiotics. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Ko, B.-S.; Ryuk, J.A.; Park, S. Tetragonia tetragonioides Protected against Memory Dysfunction by Elevating Hippocampal Amyloid-β Deposition through Potentiating Insulin Signaling and Altering Gut Microbiome Composition. Int. J. Mol. Sci. 2020, 21, 2900. [Google Scholar] [CrossRef] [PubMed]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar]
- Qiang, W.; Yau, W.-M.; Lu, J.-X.; Collinge, J.; Tycko, R. Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature 2017, 541, 217–221. [Google Scholar] [CrossRef]
- Bian, Z.; Yamashita, T.; Shi, X.; Feng, T.; Yu, H.; Hu, X.; Hu, X.; Bian, Y.; Sun, H.; Tadokoro, K.; et al. Accelerated accumulation of fibrinogen peptide chains with Aβ deposition in Alzheimer’s disease (AD) mice and human AD brains. Brain Res. 2021, 1767, 147569. [Google Scholar] [CrossRef]
- Zheng, W.; Song, H.; Luo, Z.; Wu, H.; Chen, L.; Wang, Y.; Cui, H.; Zhang, Y.; Wang, B.; Li, W.; et al. Acetylcholine ameliorates colitis by promoting IL-10 secretion of monocytic myeloid-derived suppressor cells through the nAChR/ERK pathway. Proc. Natl. Acad. Sci. USA 2021, 118, e2017762118. [Google Scholar] [CrossRef]
- Ramirez, V.T.; Godinez, D.R.; Brust-Mascher, I.; Nonnecke, E.B.; Castillo, P.A.; Gardner, M.B.; Tu, D.; Sladek, J.A.; Miller, E.N.; Lebrilla, C.B.; et al. T-cell derived acetylcholine aids host defenses during enteric bacterial infection with Citrobacter rodentium. PLoS Pathog. 2019, 15, e1007719. [Google Scholar] [CrossRef]
- Karisetty, B.C.; Bhatnagar, A.; Armour, E.M.; Beaver, M.; Zhang, H.; Elefant, F. Amyloid-β Peptide Impact on Synaptic Function and Neuroepigenetic Gene Control Reveal New Therapeutic Strategies for Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 577622. [Google Scholar] [CrossRef]
- Conte-Daban, A.; Ambike, V.; Guillot, R.; Delsuc, N.; Policar, C.; Hureau, C. A Metallo Pro-Drug to Target CuII in the Context of Alzheimer’s Disease. Chem.–A Eur. J. 2018, 24, 5095–5099. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, R.; Sharma, N.; Khurana, N. Ameliorative effect of myrcene in mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 911, 174529. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liao, W.; Chen, K.; Qin, J.; Tang, H. Synthesis of derivatives of cleistopholine and their anti-acetylcholinesterase and anti-β-amyloid aggregation activity. Bioorg. Chem. 2018, 76, 228–236. [Google Scholar] [CrossRef]
- Li, H.; Shang, Z.; Liu, X.; Qiao, Y.; Wang, K.; Qiao, J. Clostridium butyricum Alleviates Enterotoxigenic Escherichia coli K88-Induced Oxidative Damage Through Regulating the p62-Keap1-Nrf2 Signaling Pathway and Remodeling the Cecal Microbial Community. Front. Immunol. 2021, 12, 771826. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ran, L.; Yang, Y.; Gao, X.; Peng, M.; Liu, S.; Sun, L.; Wan, J.; Wang, Y.; Yang, K.; et al. Deferasirox alleviates DSS-induced ulcerative colitis in mice by inhibiting ferroptosis and improving intestinal microbiota. Life Sci. 2023, 314, 121312. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yao, L.; Meng, T.; Li, C.; Wang, L. Rhodomyrtus tomentosa (Ait.) Hassk fruit phenolic-rich extract mitigates intestinal barrier dysfunction and inflammation in mice. Food Chem. 2022, 393, 133438. [Google Scholar] [CrossRef]
- Kim, S.Y.; Chae, C.W.; Lee, H.J.; Jung, Y.H.; Choi, G.E.; Kim, J.S.; Lim, J.R.; Lee, J.E.; Cho, J.H.; Park, H.; et al. Sodium butyrate inhibits high cholesterol-induced neuronal amyloidogenesis by modulating NRF2 stabilization-mediated ROS levels: Involvement of NOX2 and SOD1. Cell Death Dis. 2020, 11, 469. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Mao, B.; Guo, W.; Liu, X.; Cui, S.; Zhang, Q.; Zhao, J.; Tang, X.; Zhang, H. Potential Probiotic Properties of Blautia producta Against Lipopolysaccharide-Induced Acute Liver Injury. Probiotics Antimicrob. Proteins 2023, 15, 785–796. [Google Scholar] [CrossRef]
- Larsen, O.F.A.; Claassen, E. The mechanistic link between health and gut microbiota diversity. Sci. Rep. 2018, 8, 2183. [Google Scholar] [CrossRef] [PubMed]
- Annalisa, N.; Alessio, T.; Claudette, T.D.; Erald, V.; Antonino, D.L.; Nicola, D.D. Gut Microbioma Population: An Indicator Really Sensible to Any Change in Age, Diet, Metabolic Syndrome, and Life-Style. Mediat. Inflamm. 2014, 2014, 901308. [Google Scholar] [CrossRef]
- Farooq, R.K.; Alamoudi, W.; Alhibshi, A.; Rehman, S.; Sharma, A.R.; Abdulla, F.A. Varied Composition and Underlying Mechanisms of Gut Microbiome in Neuroinflammation. Microorganisms 2022, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Carrano, A.; Hoozemans, J.J.M.; van der Vies, S.M.; van Horssen, J.; de Vries, H.E.; Rozemuller, A.J.M. Neuroinflammation and Blood-Brain Barrier Changes in Capillary Amyloid Angiopathy. Neurodegener. Dis. 2012, 10, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, Y.; Ma, W.; Yu, H.; Lu, Y.; Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Xiao, R. 27-Hydroxycholesterol contributes to cognitive deficits in APP/PS1 transgenic mice through microbiota dysbiosis and intestinal barrier dysfunction. J. Neuroinflamm. 2020, 17, 199. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Fasina, O.B.; Wang, J.; Mo, J.; Osada, H.; Ohno, H.; Pan, W.; Xiang, L.; Qi, J. Gastrodin From Gastrodia elata Enhances Cognitive Function and Neuroprotection of AD Mice via the Regulation of Gut Microbiota Composition and Inhibition of Neuron Inflammation. Front. Pharmacol. 2022, 13, 814271. [Google Scholar] [CrossRef]
- Raplee, I.; Walker, L.; Xu, L.; Surathu, A.; Chockalingam, A.; Stewart, S.; Han, X.; Rouse, R.; Li, Z. Emergence of nosocomial associated opportunistic pathogens in the gut microbiome after antibiotic treatment. Antimicrob. Resist. Infect. Control 2021, 10, 36. [Google Scholar] [CrossRef]
- Kaiyrlykyzy, A.; Kozhakhmetov, S.; Babenko, D.; Zholdasbekova, G.; Alzhanova, D.; Olzhayev, F.; Baibulatova, A.; Kushugulova, A.R.; Askarova, S. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 2022, 12, 15115. [Google Scholar] [CrossRef]
- Herp, S.; Brugiroux, S.; Garzetti, D.; Ring, D.; Jochum, L.M.; Beutler, M.; Eberl, C.; Hussain, S.; Walter, S.; Gerlach, R.G.; et al. Mucispirillum schaedleri Antagonizes Salmonella Virulence to Protect Mice against Colitis. Cell Host Microbe 2019, 25, 681–694.e8. [Google Scholar] [CrossRef]
- Hang, Z.; Cai, S.; Lei, T.; Zhang, X.; Xiao, Z.; Wang, D.; Li, Y.; Bi, W.; Yang, Y.; Deng, S.; et al. Transfer of Tumor-Bearing Mice Intestinal Flora Can Ameliorate Cognition in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2022, 86, 1287–1300. [Google Scholar] [CrossRef]
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 2018, 18, 83–90. [Google Scholar] [CrossRef]
- Jo, J.-K.; Lee, G.; Nguyen, C.D.; Park, S.-E.; Kim, E.-J.; Kim, H.-W.; Seo, S.-H.; Cho, K.-M.; Kwon, S.J.; Kim, J.-H.; et al. Effects of Donepezil Treatment on Brain Metabolites, Gut Microbiota, and Gut Metabolites in an Amyloid Beta-Induced Cognitive Impairment Mouse Pilot Model. Molecules 2022, 27, 6591. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.; Barale, S.; Dhanavade, M.; Sonawane, K. Structural significance of Neprylysin from Streptococcus suis GZ1 in the degradation of Aβ peptides, a causative agent in Alzheimer’s disease. Comput. Biol. Med. 2021, 136, 104691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.-F.; Huang, L.; et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Gong, C.; Shanmugam, R.; Lin, H.; Zhang, L.; Lee, J.-K. The Emerging Biotherapeutic Agent: Akkermansia. Indian J. Microbiol. 2022, 62, 1–10. [Google Scholar] [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes 2020, 10, 12. [Google Scholar] [CrossRef]
- Acharya, K.D.; Friedline, R.H.; Ward, D.V.; Graham, M.E.; Tauer, L.; Zheng, D.; Hu, X.; de Vos, W.M.; McCormick, B.A.; Kim, J.K.; et al. Differential effects of Akkermansia-enriched fecal microbiota transplant on energy balance in female mice on high-fat diet. Front. Endocrinol. 2022, 13, 1010806. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, D.; Zhu, H.; Zhu, J.; Weng, S.; Dong, L.; Liu, T.; Hu, Y.; Shen, X. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe−/− mice. Atherosclerosis 2018, 268, 117–126. [Google Scholar] [CrossRef]
- von Martels, J.Z.H.; Sadaghian Sadabad, M.; Bourgonje, A.R.; Blokzijl, T.; Dijkstra, G.; Faber, K.N.; Harmsen, H.J.M. The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. Anaerobe 2017, 44, 3–12. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021, 12, 3597–3610. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, L.; Cantoni, C.; Rotondo, E.; Galimberti, D. The Gut Microbiome–Brain Crosstalk in Neurodegenerative Diseases. Biomedicines 2022, 10, 1486. [Google Scholar] [CrossRef]
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut microbiota changes in the extreme decades of human life: A focus on centenarians. Cell. Mol. Life Sci. 2018, 75, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Arboleya, S.; Fernández-Navarro, T.; de los Reyes-Gavilán, C.G.; Gonzalez, S.; Gueimonde, M. Age-Associated Changes in Gut Microbiota and Dietary Components Related with the Immune System in Adulthood and Old Age: A Cross-Sectional Study. Nutrients 2019, 11, 1765. [Google Scholar] [CrossRef]
- Nicholson, K.; Bjornevik, K.; Abu-Ali, G.; Chan, J.; Cortese, M.; Dedi, B.; Jeon, M.; Xavier, R.; Huttenhower, C.; Ascherio, A.; et al. The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Jung, J.; Lee, Y.-u.; Kim, K.-h.; Kang, S.; Kang, G.-h.; Chu, H.; Kim, S.-Y.; Lee, S. Comparison of Metabolites and Gut Microbes between Patients with Parkinson’s Disease and Healthy Individuals–A Pilot Clinical Observational Study (STROBE Compliant). Healthcare 2022, 10, 302. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, M.; Kong, M.; Sui, S.; Wang, Q.; He, Y.; Gu, J. Impact of Donepezil Supplementation on Alzheimer’s Disease-like Pathology and Gut Microbiome in APP/PS1 Mice. Microorganisms 2023, 11, 2306. https://doi.org/10.3390/microorganisms11092306
Li Y, Wu M, Kong M, Sui S, Wang Q, He Y, Gu J. Impact of Donepezil Supplementation on Alzheimer’s Disease-like Pathology and Gut Microbiome in APP/PS1 Mice. Microorganisms. 2023; 11(9):2306. https://doi.org/10.3390/microorganisms11092306
Chicago/Turabian StyleLi, Yuan, Mengyao Wu, Mengmeng Kong, Shaomei Sui, Qi Wang, Yan He, and Jinsong Gu. 2023. "Impact of Donepezil Supplementation on Alzheimer’s Disease-like Pathology and Gut Microbiome in APP/PS1 Mice" Microorganisms 11, no. 9: 2306. https://doi.org/10.3390/microorganisms11092306
APA StyleLi, Y., Wu, M., Kong, M., Sui, S., Wang, Q., He, Y., & Gu, J. (2023). Impact of Donepezil Supplementation on Alzheimer’s Disease-like Pathology and Gut Microbiome in APP/PS1 Mice. Microorganisms, 11(9), 2306. https://doi.org/10.3390/microorganisms11092306





